Introduction
Given the current scenario of environmental change, efforts to increase the knowledge of the alpha diversity, especially in tropical regions, should be prioritized. Above all, continually updated, so that changes can be characterized, evaluated, and predicted over time, as well as their potential impacts on vulnerable or endangered species (Bickford et al., 2006; Gray, 2000). A strategy that helps determine conservation priorities has been the identification of regions with high species richness and/or a large number of endemic, rare, or endangered species (Ceballos et al., 1998). The specific richness of marine mammals known for Mexico ranges from 45 to 49 species, distributed in the orders Cetartiodactyla, Carnivora, and Sirenia (Medrano-González, 2006; Salinas y Ladrón-de Guevara, 1993; Torres et al, 1995; Urbán & Rojas, 1999). According to Torres et al. (1995), who divided the country into 4 zones, the highest richness occurrs in the western coast of the Baja California Peninsula with 35 species, followed by the Gulf of California with 32, the Mexican Tropical Pacific, and the Gulf of Mexico-Caribbean with 30 species each. The last calculation, made exclusively for the Mexican Pacific, estimated 37 species, of which 5 are pinnipeds, 7 mysticetes, and 25 odontocetes (Rosales-Nanduca et al., 2011). The state of Oaxaca lies in the Mexican South Pacific and has been ranked among the top 3 places with the largest mastofaunistic diversity of the entire country (Briones-Salas, 2012; Ramírez-Pulido et al., 2005). However, a high percentage of the work done in this regard has focused almost exclusively on the study of terrestrial mammals, from the first explorations more than a century ago to the most recent inventories in some areas that had remained unexplored (Briones-Salas & Sánchez- Cordero, 2004). Knowledge about marine mammals in the Central Coast of Oaxaca (CCO), resulting from sporadic studies and with few publications in the period comprised between 2001 and 2008. Bastida-Zavala et al. (2013) presented the most updated list for this period, based on a review of published data. Santos-Moreno (2014) also reviews mammals from Oaxaca and mentions 13 marine mammal species, one carnivore and 12 cetaceans, based on a review by Meraz and Sánchez-Díaz (2008), in wich Physeter macrocephalus and Ziphius cavirostris are dubious records. This revision reports the presence of 11 confirmed species belonging to 10 genera and 3 families: Feresa attenuata, Globicephala macrorhynchus, Grampus griseus, Megaptera novaeangliae, Pseudorca crassidens, Stenella attenuata, S. longirostris, Tursiops truncatus (Salinas & Ladrón-de Guevara, 1993), Orcinus orca (Sánchez-Díaz & Meraz, 2001), Zalophus californianus (Meraz-Hernando, 2003), and Balaenoptera musculus (Lira-Torres, 2007). Additionally, Castillejos-Moguel and Villegas-Zurita (2011) recorded Delphinus delphis. This relatively low species richness (12 species) represents approximately 24.5% of the marine mastofauna distributed in Mexico and 32.4% of the one in the Mexican Pacific. The present study focused on estimating the species richness (α-diversity) and composition of marine mammal occurring off the coast of Oaxaca, aimed to integrate an updated taxonomic list, with information on the conservation status for each species included.
Meterials and methods
The study area is located at the western limit of Gulf of Tehuantepec (GT), comprises the CCO and includes 3 municipalities of the coastal region: Santa María Tonameca, San Pedro Pochutla, and Santa María Huatulco (Fig. 1). Due to its latitudinal position (between 15° and 16° North) and the influence of the warm waters of the Pacific Ocean, it corresponds to a warm climate of intertropical zones with rains in summer, Aw2 (w) IG according to Köppen (modified by García, 2004) and to a subhumid tropical climatic condition (García, 2004). The average annual atmospheric temperature is 26 to 28 °C and varies according to climatic seasons, dry (November-April) and rainy (May-October), the sea surface temperature in winter ranges between 25-28 °C and 29.5 °C on average in summer (Trasviña et al., 1995; Wilkinson et al., 2009). The continental shelf is very narrow between 7 and 15 km wide (Arriaga-Cabrera et al., 1998). In the transitional zone of 2 marine ecoregions, the Mexican Pacific Transition is characterized by a narrow continental shelf very close to the coast that falls steeply to great ocean depths (2,500 to 3,000 m), a complex abyssal plains and a subtropical sea, and the Middle American Pacific, where the wide continental shelf starts and extends south east in to the GT and present a tropical sea (Wilkinson et al., 2009). The coast is mainly influenced by the Costa Rica Coastal Current (CRCC), which carries waters with low salinity along the Central American coast all the way to the south of Mexico (Trasviña et al., 1995). The region is influenced by the oceanographic characteristics and high productivity of the GT, which defines it as a center of high biological activity (Ayala-Duval et al., 1988; Ortega-García et al., 2000). Mesoscale eddies are generated in winter (dry season) (Trasviña & Barton, 2008) directly associated with the Tehuan events, jet winds locally called “Tehuanos, Tehuantepecanos or Nortes” (Trasviña et al., 2003; Reyes-Hernández et al., 2016), although it has also been observed that eddies non-associated to the wind can be produced (Flores-Vidal et al., 2011). The currents and sea surface temperature show temporal variability near the coast of the GT when these events occur in the winter (Velázquez-Muñoz et al., 2011), particularly upwelling in the GT and a notable decrease in sea surface temperature (Trasviña et al., 2003). In this area there is a coastal current of approximately 100 km wide, that flows from westwards, which can be persistent throughout the year causing the entry of a warm water mass to the GT (Velázquez-Muñoz et al., 2011). It has also been shown that the flow of this surface current is interrupted by Tehuano winds of moderate to strong intensity (Reyes-Hernández et al., 2016). It has been suggested that the biological influence on the coastal zone, especially during the summer, can extend much farther from the coast when combined with the CRCC which has a width of 300 to 500 km (Reyes-Hernández et al, 2016; Trasviña & Barton, 2008). The annual recurrence, intensity, and persistence of Tehuantepec and Papagayo anticyclonic eddies suggests that they may be important biological hot spots, which transport nutrient-rich coastal waters and organisms to the ocean interior (Palacios & Bograd, 2005).
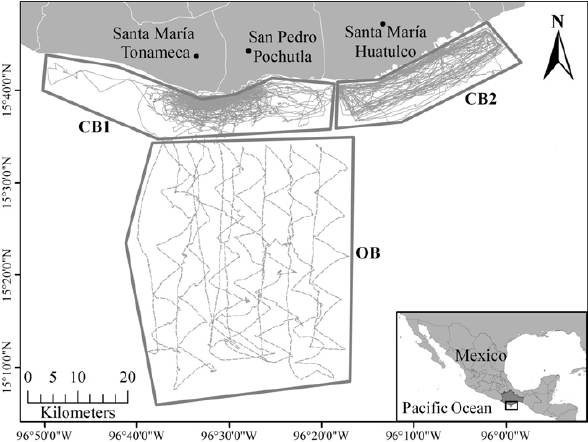
Figure 1 Surveys carried out between December 2011 and April 2015. Continuous lines represent coastal surveys and dotted lines represent oceanic surveys. Coastal block 1 (CB1), Coastal block 2 (CB2) and oceanic block (OB) are in thick and continuous line.
Surveys were carried out from December 11, 2011 to April 30, 2015 (41 months) to record marine mammal sightings in 3 areas of the CCO, divided into blocks called coastal (10 km off the coastline), and oceanic (after 10 km and varies between 50 to 60 km off the coastline) (Fig. 1). The coastal block comprises an area of approximately 225 km2 divided into 2 sub-blocks. Coastal block 1 (CB1) from the northwest to the southeast includes the coastal towns of Santa Elena, Barra de Tonameca, La Ventanilla, El Mazunte, San Agustinillo, Zipolite, Puerto Angel, Estacahuite, La Mina, La Boquilla, Tijera beach and Zapotengo. The surveys in CB1 were carried out from December 11, 2001 to April 30, 2015. Coastal block 2 (CB2) from the northwest to the southeast includes the coastal towns of Barra de Coyula, Playa Coyote, the main bays of Huatulco (San Agustín, Chachacual, Cacaluta, El Maguey, Chahue, Tangolunda, Santa Cruz, Conejo Bay, and El Órgano) and the delta of the Copalita River. The marine polygon of the Huatulco National Park overlaps with this sub-block from the bays San Agustín to Chahué. The surveys in CB2 were carried out from October 26, 2012 to August 27, 2014. The oceanic block is located after 10 km off the coast line in front of the Puerto Angel Bay and Salchi beach, with an approximate area of 406.07 km2. Its southern extreme limit varies between 50 and 60 km off the coastline, the surveys in this block were carried out monthly from December 13, to July 19, 2014. The sampling was systematic with linear transects of variable length, using a 7 m long fiberglass boat with a 60-75 HP outboard engine, with an average survey speed of 10.8 km/hr. The closing mode method was used, in which the vessel leaves the linear transect to approach the sighted groups for a more accurate estimation of the group size and species identity (Buckland et al., 2001; Dawson et al., 2008). In these cases, the course was resumed diagonally at 45° of the nearest point of the transect, using the display of a handheld GPS (Garmin eTrex 20).
To detect individuals, groups, blows, bodies, and breaches of marine mammals, each survey had a minimum of 4 observers, who used the naked eye technique actively looking for the species by doing visual sweeps of 180° from the horizon, towards the front and each side of the boat. To avoid eye fatigue, and, therefore, errors in the probability of detection, pauses of 15 to 20 min and position rotation of observers were done every 2 hours. When a sighting occurred, photographs were taken and geographical position was recorded in situ, with species identification and group size estimation. In those cases where the species identification was uncertain, the photographs taken in situ were used and compared with the identification guides by Carwardine (2002) and Reeves et al. (2002). When navigation conditions were not adequate, the observation effort was suspended (Beaufort ≥ 3, rain and/or periods of poor visibility).
Data were also obtained from specimens of the Marine Mammal Osteological Collection of the UMAR (Coleccción Osteológica de Mamíferos Marinos de la Universidad del Mar; COMMUMAR), which were considered as opportunistic stranding records. These records comprised strandings from 2008 to 2014 along the coast of Oaxaca. The specimens were identified with specialized guides (Carwardine, 2002; Jefferson et al., 2008; Reeves et al., 2002). Additionally, a literature review was carried out to identify which species were registered in the state prior to 2013 and to include them in a corrected and updated taxonomic list of marine mammals off the coast of Oaxaca. In addition, we established whether the species were in any risk category, based on different criteria, such as the Mexican regulation NOM-059-Semarnat-2010 (Semarnat, 2010), the IUCN Red List by the International Union for the Conservation of Nature (http://www.iucnredlist.org), and the appendices to the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) (http://checklist.cites.org/).
Species richness was determined as the total number of species recorded (Pérez-Irineo & Santos-Moreno, 2013), considering all sources of information used, records of sightings in coastal and oceanic blocks, and pre-2008 records. No gray literature records (published in non-arbitrated media) were included in the composition of corrected and updated taxonomic listing of marine mammals of Oaxaca. To evaluate evenness and dominance, only abundances of recorded sightings were considered to eliminate biases derived from difference in sampling methods used for the rest of the records. The Simpson dominance index (λ) was applied with the Pielou correction (Pielou, 1975); the Shannon index (H ') and the Pielou eveness index (J) were also used (Krebs, 1989). Five non-parametric models of species accumulation (Chao1, Chao2, Jacknife1, Jacknife2, and bootstrap) and 2 asymptotic or parametric models (Clench and Linear dependency) were used to evaluate the representativeness of the species richness obtained from surveys (Colwell & Coddintong, 1994; Krebs, 1989). The corresponding accumulation curves of species were constructed from a matrix of abundance. In this case, sampling effort was expressed as the number of surveys (an average of 53.79 km traveled in every survey) carried out in the study area. To reduce the bias produced by data entry order, the matrix was randomized 999 times with the program Primer 6 (Clarke, 1993). Finally, to determine species composition, we considered all the compiled records of marine mammals on the coast of Oaxaca using specialized keys and identification guides to determine the taxonomic level (Carwardine, 2002; Jefferson et al., 2008; Reeves et al., 2002)
Results
The sampling effort consisted of 293 surveys representing 15,709.5 km of which 14,658.9 km corresponded to the CB1 (229 surveys) and CB2 (56 surveys) and 1,050.6 km to the OB (8 surveys). As a result, 1,177 sightings were obtained. Regarding the strandings, 29 stranding records were obtained, of which 23 came from the COMMMUMAR database, and 8 records came from published references. In total, 1,206 marine mammal records were obtained for Oaxaca (Table 1). In 47 sightings, identification was not possible, therefore, the observed individuals were assigned to the corresponding suborder and family (Odontoceti, N = 6; Delphinidae, N = 34, and Balaenopteridae, N = 7).
Table 1 Number of marine mammal sightings recorded in the surveys and strandings recorded in COMMUMAR database from Oaxaca. The published records are shown in parentheses.
| Species | Coastal block sightings |
Oceanic block sightings | Strandings | Totals | Frequency (%) | |
| CB1 | CB2 | OB | ||||
| Balaenoptera musculus | (1) | 1 | 0.08 | |||
| Balaenoptera edeni | 12 | 6 | 2 | 20 | 1.66 | |
| Megaptera novaeangliae | 231 | 33 | 7 | 271 | 22.47 | |
| Delphinus delphis | 43 | 5 | 6 | 54 | 4.48 | |
| Grampus griseus | 4 | 1 | 1 | 6 | 0.50 | |
| Orcinus orca | 8 | 2 | 10 | 0.83 | ||
| Pseudorca crassidens | 2 | 3 | (1) | 6 | 0.50 | |
| Stenella attenuata | 368 | 149 | 8 | 3 (1) | 529 | 43.86 |
| Stenella coeruleoalba | 1(1) | 2 | 0.17 | |||
| Stenella longirostris | 18 | 2 | 2 | 2(1) | 25 | 2.07 |
| Steno bredanensis | 77 | 19 | 3 | 1 | 100 | 8.29 |
| Tursiops truncatus | 85 | 26 | 7 | 4 | 122 | 10.12 |
| Kogia sima | 2 | 2 | 0.17 | |||
| Ziphius cavirostris | 2 | 1 | 3 | 0.25 | ||
| Mesoplodon peruvianus | (1) | 1 | 0.08 | |||
| Arctocephalus australis | (1) | 1 | 0.08 | |||
| Arctocephalus philippii townsendi | 1(1) | 2 | 0.17 | |||
| Eumetopias jubatus | 1 | 1 | 0.08 | |||
| Zalophus californianus | 2 | 1 | 3 | 0.25 | ||
| Delphinidae | 25 | 8 | 1 | 34 | 2.82 | |
| Odontoceti | 6 | 6 | 0.50 | |||
| Balaenopteridae | 5 | 2 | 7 | 0.58 | ||
| Totals | 889 | 256 | 32 | 29 | 1,206 | 100 |
Thirteen species were registered in the surveys in both coastal-blocks (CB1 = 13 and CB2 = 10), 8 in the oceanic and 13 were found in the stranding records. The total number of species recorded using the previous methods was 18. When we added 3 species recorded in the literature (Feresa attenuata, Globicephala macrorhynchus, and Mesoplodon peruvianus), which were not present in the surveys or strandings registered in COMMUMAR, we obtained a richness of 21 species, and the taxonomic composition was divided into 2 orders, 3 suborders, 5 families, and 16 genera (Table 2).
Table 2 Updated taxonomic list of the marine mammals of Oaxaca, Mexico, as a result of the presente study.
| Phylum Chordata Bateson, 1885 | Reference |
| Class Mammalia Linnaeus, 1758 | |
| Order Cetartiodactyla | |
| Suborder Mysticeti Cope, 1891 | |
| Family Balaenopteridae Gray, 1864 | |
| Balaenoptera musculus (Linnaeus, 1758) | Lira-Torres (2007) |
| Balaenoptera edeni Anderson, 1878 | Villegas-Zurita et al. (2016) |
| Megaptera novaeangliae (Borowski, 1781) | Urbán and Aguayo (1987) |
| Suborder Odontoceti Flower, 1869 | |
| Family Delphinidae Gray, 1821 | |
| Delphinus delphis (Linnaeus, 1758) | Castillejos-Moguel and Villegas-Zurita (2011) |
| Feresa attenuata Gray, 1875 | Salinas and Ladrón-de Guevara (1993) |
| Globicephala macrorhynchus Gray, 1843 | Salinas and Ladrón-de Guevara (1993) |
| Grampus griseus (Cuvier, 1812) | Salinas and Ladrón-de Guevara (1993) |
| Orcinus orca (Linnaeus, 1758) | Sánchez-Díaz and Meraz-Hernando (2001) |
| Pseudorca crassidens (Owen, 1846) | Salinas and Ladrón-de Guevara (1993) |
| Stenella attenuata (Gray, 1846) | Salinas and Ladrón-de Guevara (1993) |
| Stenella coeruleoalba (Meyen, 1833) | Wilson et al. (1987) |
| Stenella longirostris (Gray, 1828) | Salinas and Ladrón-de Guevara (1993) |
| Steno bredanensis (Cuvier in Lesson, 1828) | Villegas-Zurita (2015) |
| Tursiops truncatus (Montagu, 1821) | Salinas and Ladrón-de Guevara (1993) |
| Family Kogiidae | |
| Kogia sima (Owen, 1866) | This work |
| Family Ziphiidae | |
| Ziphius cavirostris (Cuvier, 1823) | This work |
| Mesoplodon peruvianus (Reyes, Mead and Van Waerebeek, 1991) | García-Grajales et al. (2017) |
| Order Carnivora Bowdich, 1821 | |
| Suborder Caniformia Kretzoi, 1938 | |
| Family Otariidae (Gray, 1825) Gill, 1866 | |
| Arctocephalus australis (Zimmermann, 1783) | Villegas-Zurita et al. (2016) |
| Arctocephalus philippii townsendi Merriam, 1897 | Villegas-Zurita et al. (2015) |
| Eumetopias jubatus (Schreber, 1776) | This work |
| Zalophus californianus (Lesson, 1828) | Gallo-Reynoso and Solórzano-Velasco (1991) |
Seven of the 18 species recorded in surveys and strandings are new records for Oaxaca; Balaenoptera edeni, Kogia sima, Steno bredanensis, Ziphius cavirostris, Eumetopias jubatus, Arctocephalus philippii townsendi, and A. australis. The latter species constitutes a new record for Mexico. It should be clarified that the records of B. edeni (Villegas-Zurita, Castillejos-Moguel et al., 2016), S. bredanensis (Villegas-Zurita, 2015), A. australis (Villegas-Zurita, Elorriaga-Verplancken et al., 2016), and A. philippii townsendi (Villegas-Zurita et al., 2015) were published as part of this work. Additionally, the species most recently added to the list was Mesoplodon peruvianus by means of a stranding record, considered the first for Oaxaca by García-Grajales et al. (2017). This record was only considered to be added to the taxonomic list in this work as it was not recorded in the navigations or in the strandings of the COMMUMAR database.
Regarding the conservation status for the 21 species found, 19 are protected by the NOM-059-Semarnat-2010, 18 of these are “subject to special protection” (Pr) and 1 is “endangered” (P) (Semarnat, 2010). A. australis and E. jubatus are not included in this protection list because their known distribution does not include the Mexican waters and their records are considered atypical for the country. With regard to the IUCN Red List, 10 species are considered as least concern (LC), 8 are data-deficient (DD), 2 are clasified as near threatened (NT) and 1 as endangered (EN) (IUCN, 2017). As for the CITES appendices, 4 species are listed in Appendix I, 15 in Appendix II, and 2 are not listed (UNEP-WCMC, 2017) (Table 3).
Table 3 Risk categories of the marine mammals found in the coast of Oaxaca, Mexico following Mexican and international (IUCN and CITES) criteria. NOM-059-Semarnat-2010: Pr = subject to special protection, P = endangered. IUCN: DD = insufficient data, EN = endangered, LC = least concern, NT = nearly threatened, VU = vulnerable. CITES: I = appendix I (all the species endangered included). Specimen commercialization for this species is authorized only under exceptional circumstances. II = Appendix II (includes species that are not necessarily threatened with extinction, but their commercialization must be controlled to avoid a utilization in a way that would be incompatible with their survival), NC = not cited in appendices CITES, * = not normally distributed in Mexico.
| Species | NOM-059-Semarnat-2010 | CITES | IUCN |
| D. delphis | Pr | II | LC |
| F. attenuata | Pr | II | DD |
| G. macrorhynchus | Pr | II | DD |
| G. griseus | Pr | II | LC |
| O. orca | Pr | II | DD |
| P. crassidens | Pr | II | DD |
| S. attenuata | Pr | II | LC |
| S. coeruleoalba | Pr | II | LC |
| S. longirostris | Pr | II | DD |
| S. bredanensis | Pr | II | LC |
| T. truncatus | Pr | II | LC |
| K. sima | Pr | II | DD |
| M. peruvianus | Pr | II | DD |
| Z. cavirostris | Pr | II | LC |
| B. edeni | Pr | I | DD |
| B. musculus | Pr | I | EN |
| M. novaeangliae | Pr | I | LC |
| A. australis | * | II | LC |
| A. philippii townsendi | P | I | LC |
| E. jubatus | * | NC | NT |
| Z. californianus | Pr | NC | LC |
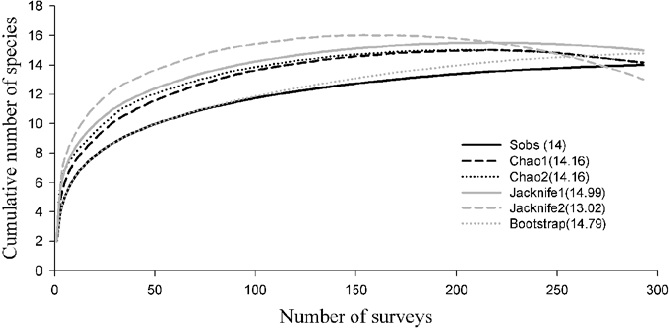
The non-parametric species accumulation models were fitted including only data for the 14 species recorded during our surveys. The models showed that the number of expected species ranged from 13 to 15 (Fig. 2). The Clench model was the one that best fitted the data (R = 0.976 and R2 = 95.347). The asymptote predicted by the model was 14 species. The diversity expressed by the Shannon index was H' = 0.680, the Pielou equitability J = 0.593, and the Simpson dominance λ = 0.050. S. attenuata was the species with the most sighting records.
Discussion
The species richness obtained in this work from sighting records (14 species) is consistent with the number of species predicted by the accumulation curve models. However, this value is considerably increased by adding 4 species recorded from strandings and 3 from the literature, which were not recorded with the methods above mentioned, therefore, the actual value of the alpha diversity for this study was estimated to be 21 species. The results showed that the community of marine mammals in Oaxaca is not strongly dominated in terms of abundance by a few species, since the Simpson dominance index had a relatively low value (5%) and the equitability of Pielou showed an eveness of almost 60%. This could be the result of the particular conditions of the study area.
Odontocetes and mysticetes accounted for 66.6% and 14.3% of the species richness, respectively, altogether contributing with 80.9%. Carnivores, represented exclusively by otariids, contributed with 19% to richness, showing a significant increase from one to 4 species in this group, although A. australis and E. jubatus are still considered extra-limit ocurrences. The number of species recorded in this study represents 42.8% of the richness of marine mammals known for Mexico (49 species), 56.7% for the Mexican Pacific (37 species), as characterized by Rosales-Nanduca et al. (2011) and 70% of these species recorded in the Mexican Tropical Pacific (30 species) covering from Nayarit to Chiapas (Torres et al., 1995). This shows that the coast of Oaxaca and, particularly, the Mexican South Pacific is a region of elevated richness and considerable diversity in its composition of marine mammals. Contrasting this richness of marine mammals in Oaxaca, with the Gulf of California (representing a larger extension compared to our study area), we found that 36 species (grouped into 11 families) have been recorded for that area in an effort of more than 3 decades of research (Niño-Torres et al., 2011). On the other hand, in the Pacific of Guatemala, which is a southern area closer to Oaxaca, 19 species have been registered belonging to 5 families of cetaceans from a literature review of sightings and strandings records over a 50-year period (Cabrera et al., 2014).
When comparing our results with the most recent lists for Oaxaca, which include 12 species proposed by Bastida-Zavala et al. (2013) and the records published by Castillejos-Moguel and Villegas-Zurita (2011), our work evidences a 75% increase in the current richness previously proposed for the state. Our records of K. sima, Z. cavirostris, and E. jubatus, represent the first for Oaxaca. With the exception of B. musculus, G. macrorhynchus, and F. attenuata, we recorded and confirmed the presence of the remaining species listed by Bastida-Zavala et al. (2013). Regarding the presence of P. macrocephalus and Z. cavirostris mentioned by Santos-Moreno (2014), it is important to notice that the records were taken by Meraz and Sánchez-Díaz (2008) from a congress summary (gray bibliography) which makes the records doubful, and given that the sightings were made fom the beach in the first species, and in the second, it was a stranding record, there was not a possibility of confirmation. In another case of sperm wale sightings, also mentioned by Meraz and Sánchez-Díaz (2008), the record was uncorroborated and declared from a personal communication. For this reason we consider them as unconfirmed records. The presence of G. griseus in Oaxaca waters had been only suggested in the list of Salinas and Ladrón-de Guevara (1993), and this work confirmed the species. In contrast, G. macrorhynchus remained unregistered for the coast of Oaxaca and, consequently, it is maintained only as a species with potential distribution in the area. On the other hand, throughout our extensive bibliographic review, we evidenced that S. coeruleolba had not been considered in the lists prior to this work, despite the fact that it was registered for the first time in Oaxaca by Wilson et al. (1987) in a report that lists the worldwide localities where this species were registered. Additionally, a stranding record of this species was obtained from the COMMUMAR database.
During the comparison of the list provided by Bastida-Zavala et al. (2013), we identified 2 inconsistencies and propose the respective corrections. The first record of Z. californianus is currently atributed to Meraz-Hernando (2003), but we found the description of a previous record in La Blanca Island (Roca Blanca) in the vicinity of Zipolite, reported by Gallo-Reynoso and Solórzano- Velasco (1991). A second correction was made for M. novaeangliae attributed to Salinas and Ladrón-de Guevara (1993), as there is a previous mention for the Tehuantepec Isthmus, by Urbán and Aguayo (1987).
On the other hand, Medrano-González (2006) defines tropical species as those having a distribution primarily between the Capricorn and Cancer Tropics. In this sense, the atypical presence of otariids in this tropical zone of the Pacific is highlighted, since this group is considered very sensitive to ocean temperature changes and their effects on the availability of food (Ceballos et al., 2010; Oliveira, 2011), especially for species from different biogeographic affinities as E. jubatus and A. australis. The literature shows that the Steller Sea Lion (E. jubatus) exhibits a distribution confined to the North Pacific, ranging from northern California along the West coast of North America to the Gulf of Alaska and the Aleutian Islands, as well as northern Japan (Loughlin, 2009); therefore, the sightings recorded in the present work are considered as the southernmost for the species, as in the Mexican South Pacific there is only 1 previous record in Colima by Ceballos et al. (2010). The South American fur seal (A. australis) is distributed along the Pacific and Atlantic margins in South America, from Southeast Peru to Southeast Brazil, respectively (Vaz-Ferreira, 1982) and is added to the previous records of atypical distribution, as it is the northernmost for the species and is also the first for Mexico and Oaxaca (Villegas-Zurita, Elorriaga-Verplancken et al., 2016)). In addition, regarding the presence of the Guadalupe Fur Seal (A. philippii townsendi) restricted to Guadalupe Island and with repopulation in the San Benito Archipelago in Baja California, Mexico (Aurioles-Gamboa et al., 2010), the stranding records were considered as the southernmost distribution event of the species and the first for Oaxaca (Villegas-Zurita et al., 2015). The presence of pinnipeds in the Mexican Tropical Pacífic, out of their potential feeding or reproductive areas, is primarily associated with anomalous sea surface temperature (ASST), which are related to La Niña conditions (cold temperatures) and El Niño (warm phase) (Páez-Rosas et al., 2017; Villegas- Zurita, Elorriaga-Verplancken et al., 2016). Since there are no pinniped colonies around the region, this event is considered atypical. Added to this, the area is a transitional zone of 2 marine ecoregions, Mexican Pacific Transition and Middle American Pacific (Wilkinson et al., 2009), due to its complex oceanography, high productivity and biological characteristics it may be a zone of convergence of species.
As a result of our analysis a list of marine mammal species for Oaxaca is presented in Table 2.
It should be noted that due to their low number of sightings, 55.5% of the 18 species recorded are considered rare in the study area, defined in terms of its local abundance, and tipically not be numerically abundant in the majority of comunities in which they were recorded (Cunningham & Lindenmayer, 2005). These include the 4 species of the otariid group (A. australis n = 1, A. philippii townsendi n = 2, E. jubatus n = 1 and Z. californianus n = 3), and the oceanic cetacean group, including one species of mysticete (B. musculus n = 1), and 6 odontocetes (K. sima n = 2, G. griseus n = 5, P. crasidens n = 6, S. coeruleoalba n = 2, M. peruvianus n = 1, and Z. cavirostris n = 3). This fact highlights the importance of the study area in terms of conservation priority because of its high number of rare species. Regarding the analysis of vulnerability related to extinction risk categories, the results show that all species are in some category of risk at national and international levels (Table 3). Particularly, 19 species are protected in NOM-059-Semarnat-2010, of which 18 species are in the lowest risk category (subject to special protection) and only A. philippii townsendi is in the category of highest vulnerability (endangered), in accordance with Appendix I of CITES (includes all species threatened with extinction, trade in specimens of these species except when the purpose of the import is not commercial, for instance for scientific research). However, it is in a lower category (least concern or LC) in the IUCN Red List (Aurioles-Gamboa, 2015). In contrast, B. musculus is in the lowest risk or vulnerability category (subject to special protection) of the NOM-059-Semarnat-2010, but is in a higher risk category (endangered or EN) in the IUCN Red List (Reilly et al., 2008) and in Appendix I of CITES. In the case of A. australis and E. jubatus, these are not considered in the list of protected species by NOM-059-Semarnat-2010, because the natural distribution of both species is not considered to be within the national territory and this only applies to species with distribution in Mexico (Semarnat, 2010). In particular, A. australis is included in the IUCN Red List in the least concern category (LC) and in CITES Appendix II (includes species not necessarily threatened with extinction, but in which trade must be controlled in order to avoid utilization incompatible with their survival). E. jubatus is found in the IUCN Red List under the near threatened (NT) category (Gelatt & Swenney, 2016), but is not included in the CITES appendices.
Our work is relevant since it synthesizes results from the first continuous effort (41 months) and systematic monitoring of marine mammals on the coast of Oaxaca and the Mexican South Pacific.The results of this study are particularly relevant, as they highlight the importance of the Mexican South Pacific region as a possible area of convergence among species with extreme distribution, in response to environment changes, particularly ASST for otariids. We also provide valuable evidence that the central coast of Oaxaca is a priority area for conservation because of its high species richness, considerable diversity in species composition, high numbers of rare species, and its concentration of vulnerable marine mammals. Our findings provide more representative and updated information about the community of marine mammal in Oaxaca and the Mexican South Pacific and will serve for future and more effective conservation strategies and policies for marine mammals and their critical habitats. In addition, this information will serve as a basis (previously nonexist) for detecting future modifications and possible impacts on marine mammals challenged with a panorama of significant and unusual enviromental changes caused by anthropogenic activities.











 text new page (beta)
text new page (beta)