Introduction
Due to the physical characteristics of the marine environment, such as tidal regimes and currents, a sessile lifestyle is possible and favorable (Wahl, 1997). Organisms like bacteria, protists, sponges, cnidarians, molluscs, rotifers, bryozoans, polychaetes, echinoderms, crustaceans, and ascidians can be sessile at some point in their life cycles (Wahl & Mark, 1999). With limited escape capabilities and dispersal mechanisms, they need to have adaptive strategies to improve their survival (Wahl & Mark, 1999). One of these strategies is to associate with other organisms of different taxa. These interspecific interactions are important and necessary in the biological cycle of many species living in terrestrial and aquatic environments (Bronstein et al., 2006), because they provide benefits for at least one of the organisms involved and increase its chances of survival (Hoeksema & Bruna, 2000). Epibiosis is an example of an ecological interspecific interaction between organisms, with epibionts and basibionts more frequently found among marine invertebrates (Wahl, 2010).
Epibionts are organisms that grow and colonize living substrates; consequently, the basibiont is the host (live substrate) that is the basis for colonization of epibionts (Wahl, 1989; Wahl & Mark, 1999). Thus, epibiosis is defined as an interspecific interaction involving at least 2 organisms of different species (Wahl, 1989). However, epibiosis provides direct and indirect relationships, resulting in benefits for both associated individuals and often disadvantages for one of them. Therefore, studying this interaction is important to explore the structure, dynamics, and evolution of a community (Wahl, 1989).
The use of gastropod shells by hermit crabs is an evolutionary adaptation to protect their fragile abdomen, and it enables them to explore different environments (Batista-Leite et al., 2005; Hazlett, 1981, 1989; Worcester & Gaines, 1997) while also benefiting from associations with epibionts.
In the southern region of Brazil, there is still a gap in studies concerning the occurrence and characterization of epibionts associated with gastropod shells occupied by hermit crabs. Ayres-Peres and Mantelatto (2010) analyzed the occurrence of epibionts in shells used by the hermit Loxopagurus loxochelis Moreira, 1901 in the coast northeast of the state of São Paulo; Turra (2003) and Turra et al. (2005) investigated the inlaying of epibionts and the adequacy of shells used by sympatric hermit crabs of the genus Clibanarius Dana, 1852 on the island of Pernambuco, Brazil; and Sant’Anna et al. (2004) studied a symbiosis in Clibanarius vittatus (Bosc, 1802) in São Vicente, São Paulo, Brazil. In this study, we used the hermit crab Isocheles sawayai Forest & Saint Laurent, 1967, on the northern coast of Santa Catarina, Brazil, to characterize the epibionts found in the shells and to evaluate different internal and external infestation types among males, females, and ovigerous females and among different sizes of animals.
Materials and methods
Hermit crabs were collected monthly from July 2010 to June 2011 at 5 sampling sites parallel to the shoreline at 5 depths: 5, 8, 11, 14, and 17 m. The trawls lasted 30 min in each sampling site, using a shrimp boat outfitted with double-rig nets, in adjacent areas in Babitonga Bay (Fig. 1). After collection, all specimens were sorted, frozen, and transported to the laboratory, where they were removed from their shells, counted, and identified according to Melo (1999). The sexes were recognized by verifying the position of the gonopores, and the reproductive condition was determined according to the presence of ovigerous females (Biagi & Mantelatto, 2006). These observations were performed using a stereomicroscope.
Figure 1 Map of the study area, highlighting the sampled stations in an area adjacent to Babitonga Bay, Southern Brazil (source: Grabowski et al., 2014).
The cephalothoracic shield length (CSL) of the crabs was measured with a digital caliper (0.01 mm). The shells were weighed (0.01 g precision) under the same conditions (Gofas, 2014; Rios, 1994), and were inspected for the presence of internal and external epibionts. The species of macroinvertebrates comprising this community of epibionts were identified to the lowest possible level. Every shell used by a hermit crab that presented internal or external epibionts was considered infested, and the presence of bryozoan colonies was considered as 1 record. The hermit crabs were grouped into 9 size classes, from 3 to 11mm (3 = 3.00 - 3.99 mm; 4 = 4.00 - 4.9 mm; 5 = 5.00 - 5.99 mm; 6 = 6.00 - 6.99 mm; 7 = 7.00 - 7.90 mm; 8=8.00-8.99mm;9=9.00-9.99mm;10=10.00- 10.99 mm; 11 = 11.00 - 11.99 mm), in order to compare the presence and absence of epibionts with the hermit crabs’ sizes.
The Student’s t-test (shell of the same species of gastropod) was used to detect differences in the infestation of internal and external epibionts in gastropod shells and also to compare differences in weight between shells with epibionts and shells without epibionts. Analysis of variance (Anova) and the Tukey post hoc test were used to compare differences in the infestation among demographic groups (males, females, and ovigerous females). Tests for homoscedasticity (Levene tests) and normality (Shapiro- Wilk tests) were first performed as pre-requisites for the statisticaltest. The variables with out normal distribution were log-transformed prior to analysis (Zar, 1999).
Results
In total, 575 individuals of I. sawayai were collected, including 156 females (27%), 103 ovigerous females (18%), and 316 males (55%), which occupied shells of 10 species of gastropods: Buccinanops gradates (Deshayes, 1844), Dorsanum moniliferum (Valenciennes, 1834), Leucozonia nassa (Gmelin, 1791), Olivancillaria urceus (Roding, 1798), Olivancillaria vesica (Gmelin, 1791), Phalium granulatum (Born, 1778), Pisania auritula (Link, 1807), Polinices hepaticus (Roding, 1798), Siratus tenuivaricosus (Dautzenberg, 1927), and Stramonita haemastoma (Linnaeus, 1767). Stramonita haemastoma and O. urceus had the most used shells (202 and 148, respectively). Therefore, the statistical analyses were carried out only with the shells of these 2 species of gastropods. The epibionts found in the shells used by I. sawayai were barnacles, polychaete worm tubes, bivalves, anemones, sponges, and bryozoans (Fig. 2).
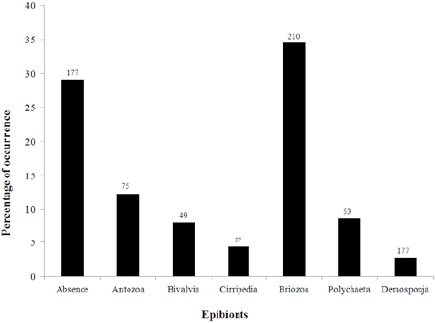
Figure 2 Percentage of epibionts found in gastropod shells used by Isocheles sawayai in the region adjacent to Babitonga Bay- SC (absolute number of individuals above of the bars).
We observed differences in the prevalence of external and internal epibiont infestations (t test, p < 0.05), with a higher infestation rate inside the shell. Regarding the demographic groups, ovigerous females used shells with a lower percentage of infestation (40%), followed by males (70%) and females (80%) (Anova, Tukey post hoc, p < 0.05) (Fig. 3). However, ovigerous females had a higher proportion of anemones in comparison to non-ovigerous females (Fig. 4).
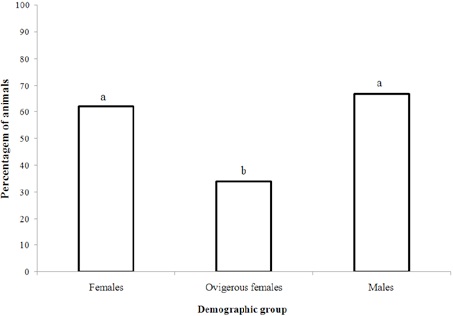
Figure 3 Demographic groups of Isocheles sawayai using shells with presence of epibionts during the study period in the region adjacent to Babitonga Bay- SC. Similar letters represent similarities among demographic groups (Tukey, p < 0.05).
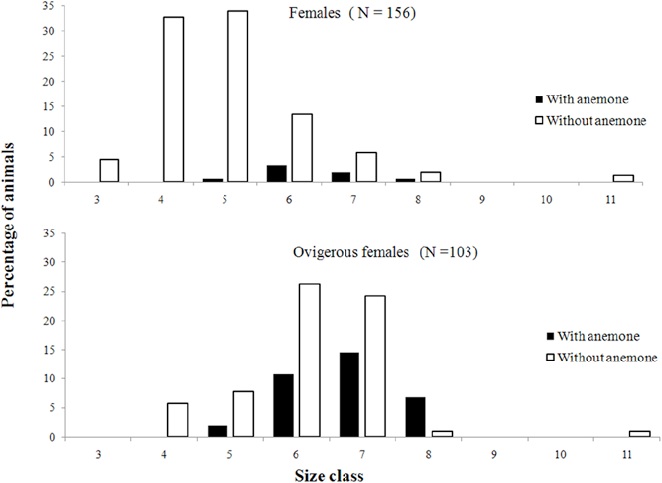
Figure 4 Proportion of females of Isocheles sawayai with and without anemones according to reproductive stage (ovigerous females and non-ovigerous females) in different carapace width classes (mm) during the study period in the region adjacent to Babitonga Bay- SC.
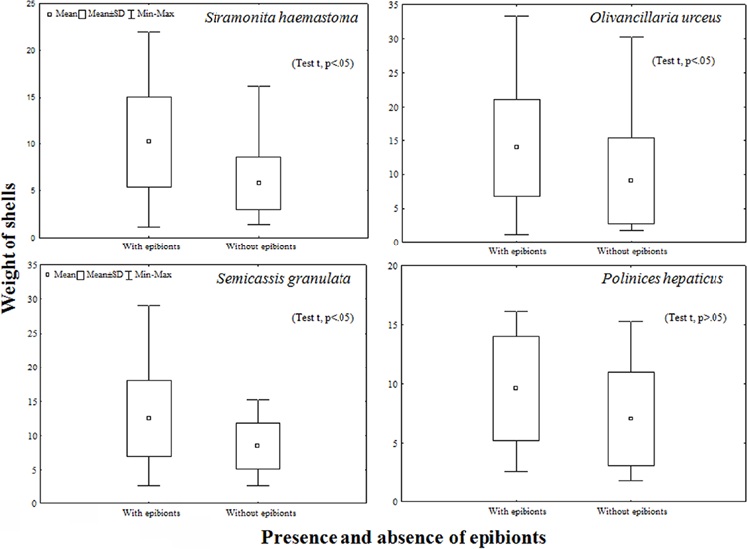
The presence of epibionts affects the average weight of shells of 3 (Student’s t-test, p < 0.05) of the 4 busiest shells, with the exception of Polinices hepaticus, which had mostly bryozoan epibionts (Student’s t-test, p > 0.05) (Fig. 5). However, regarding the presence of epibionts in the busiest shells (S. haemastoma, O. urceus), increased with increasing of CSL (Fig. 6).
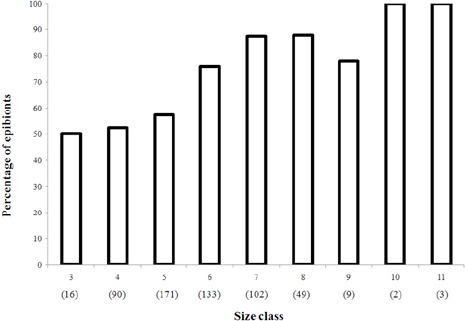
Figure 5 Mean values and standard deviation of the weight of the shells with and without epibionts of the 4 species with more abundant shells used by the hermit crabs during the study period in the region adjacent to Babitonga Bay- SC.
Discussion
In unconsolidated benthic environments, hermit crab shells and decapod crustacean carapaces are among the few solid surfaces available, which makes them very important for attachment, survival, and dispersion of various sessile animals (Acuña et al., 2003; Conover, 1978). Bryozoans were the most abundant epibionts in our study. A similar pattern of occupation of shells by 2 species of hermit crabs was found on the Brazilian (Ayres-Peres & Mantelatto, 2010; Pereira et al., 2009) and Japanese coasts (Oba et al., 2008). As this taxon does not affect shell weight (Ayres-Peres & Mantelatto, 2010), it may be advantageous for the crab to inhabit a shell with bryozoans. In addition, bryozoans belonged to the category of “improvers” of the shell structure, because they produce calcareous colonies that can modify the morphology of the shells greatly, granting greater protection to the hermit against predators through the increased resistance of the shells or even providing camouflage (Ayres-Peres & Mantelatto, 2010). Therefore, although no species is an obligate symbiont, the high incidence of bryozoans on hermit crabs suggests a beneficial relationship between them in non-consolidated environments and, consequently, this association favors a cooperative relationship, which is an act performed by an individual that increases the fitness of another (Bergmüller et al., 2007)
Another pattern found was the constant presence of epibionts in larger animals, which could be age and molting frequency-related (Davis & White, 1994). This hypothesis has been explored by Costa et al. (2010) in Arenaeus cribrarius where they found a low epibiont presence and suggested that this condition is due to the frequent molting of the host, because the juvenile stage has a characteristic rapid growth with short inter-molt periods. Consequently, after ecdysis hermit crabs seek larger shells; otherwise their growth may be limited. As discussed in the literature, when hermit crabs use a very small shell, their growth is affected or even restrained (Fantucci et al., 2008; Ziegler & Forward, 2006). Thus, the larger the hermit crab is, the longer the inter-molt period; in other words, the hard substrate habitable by epibionts remains in movement for more time (Nogueira-Junior et al., 2006). The low frequency of epibionts found in smaller animals supports this hypothesis. In general, mature crustaceans, hermit crabs, or other taxa that undergo molting less frequently shelter a greater diversity and density of epibiont species (Dick et al., 1998; Fernández et al., 1998; Mori & Manconi, 1990).
On the other hand, ovigerous females occupied shells with fewer epibionts. According to Wait and Shoeman (2012), shell selection is a process that involves individual and sexual preferences in different dimensions, seeking the best protection for the hermit and also sufficient space for the development of embryos. Therefore the presence of epibionts causes a possible competition for space, especially when epibionts are found internally and in large numbers (conditions found in the present study), further altering shell weight (Sant’Anna et al., 2004). In addition, heavy shells can negatively affect reproduction and growth (Bertness, 1981; Osorno et al., 1998). Thus, the hypothesis that best adjusts to the use of shells with less internal infestation by ovigerous females is related to the physiological need of the species to maximize reproductive success.
Mantelatto et al. (2002), studying Paguristes tortugae Schmitt, 1933, found a positive correlation between fecundity and the internal volume of the shell, suggesting that the larger the volume, the greater the fecundity of the female. In the present study, this might explain the low prevalence of internal epibionts in the shells occupied by ovigerous females, because internal epibionts limit the space available for oviposition and development of fertilized eggs, and some polychaete epibionts of the genus Polydora use hermit crab eggs as food (Williams, 2002). However, although ovigerous females occupied shells with lower infestation of epibionts, anemones should be highlighted, as they were found mostly on the outside of the shells used by ovigerous females. A similar pattern was found in the spider crabs Libinia ferreirae Brito Capello, 1871 and Libinia spinosa Milne-Edwards, 1834 (Acuña et al., 2003; Cordeiro & Costa, 2002; Nogueira-Junior et al., 2006; Winter & Masunari, 2006) and the mud crab Scylla serrata (Forskal, 1775) (Jeffries et al., 1992), where ovigerous females had a higher occurrence of epibionts in their carapaces (anemones and cirripeds) than males and non-ovigerous females. According to the authors, ovigerous females lose the habit of burying themselves when carrying their embryos, in order to oxygenate the eggs. This fact would help to establish larger infestations in this category, due to the greater probability of contact with the epibionts, a situation that we believe also occurs in I. sawayai. This species has the habit of remaining half-buried, with only its antennae protruding from the substrate to obtain food particles in suspension and to avoid predators (Fantucci et al., 2009; Stanski et al., 2016).
Another hypothesis that should be considered for hermit crabs concerns the longer use of the shell by ovigerous females, because in this condition they do not molt, facilitating the acquisition of epibionts. Furthermore, Bach and Herrnkind (1980) inferred that the higher relative frequencies of the association between ovigerous females of Pagurus pollicaris Say, 1817 and anemones are related to higher predation pressure, because it is believed that females reduce their mobility when they are ovigerous, and thus the use of anemones for their defense can reduce the likelihood of being preyed upon.
The advantage of this association for anemones is not the use of the food remains of the host, because I. sawayai is a filter feeder (Stanski & Castilho, 2016), but the availability of a biological substrate that provides greater mobility with ability to migrate to various habitats and thus explore new resources, increasing their food availability and providing protection (Winter & Masunari, 2006). On the other hand, the main advantage that anemones provide to hermit crabs is a chemical defense mechanism against predators (Winter & Masunari, 2006).
Therefore, our results suggest that the presence of epibionts in shells can be advantageous to the survival of the hermit crab, mainly as a defense and camouflage strategy. On the other hand, epibionts can also have a negative impact if they become too numerous as to increase significantly the shell weight. Although many studies are still needed to elucidate all the fine details of the interaction between epibionts and hermit crabs, and many questions remain, it is clear that this interaction is a rich example of how evolutionary processes can interact together in the variation of life histories of the species.











 nova página do texto(beta)
nova página do texto(beta)