Introduction
Conidial microfungi contain a large diversity of species that are widely distributed across most ecological niches. The majority of conidial microfungi correspond with the asexual states of ascomycetes or, rarely, of basidiomycetes (Kendrick, 2001). Reproduction in these fungi occurs mainly through the production of mitotic spores called conidia, which are borne from conidiogenous cells supported on differentiated cells named conidiophores. Conidiophores are usually solitary although sometimes form aggregations called conidiomata. Acervuli, pycnidia, sporodochia, and synnemata are characteristic conidiomata. In the synnematous microfungi, the conidia are supported by a more or less compacted group of long, erect and sometimes fused conidiophores, which can form conspicuous stipes or fascicules. In the taxonomic system developed by Saccardo, synnematous species were placed in the family Stilbellaceae (or Stilbaceae). This classification system was followed for a long time, yet has currently been abandoned, since it is well documented that it does not represent phylogenetically natural groups (Seifert et al., 2011). However, for identification purposes, grouping the synnematous species is still a common practice that has been the subject of many studies and treaties as those of Gusmão and Grandi (1997), Jong and Morris (1968), Morris (1963; 1966; 1967), Rong and Botha (1993) and Seifert (1990).
Records of synnematous microfungi from Mexico are scattered in the literature. Considering the variety of natural environments in Mexico, a significant diversity of synnematous species likely remains to be discovered. So, in the present paper, the objectives are to describe and record 18 species of saprobic synnematous microfungi previously unknown in Mexico, and to present an account of the synnematous microfungi registered for the Mexican mycobiota up to date. For the latter objective, only species published in refereed journals and documented with herbarium material deposited in registered collections are considered.
Materials and methods
Samples of plant debris consisting of leaf litter, dead branches, palm rachides and petioles, were collected in different tropical and semitropical localities of the state of Veracruz. In the laboratory, plant material was treated following Castañeda-Ruiz et al. (2016). Plant debris was regularly examined under a stereomicroscope to detect fungal sporulation. Permanent slides were prepared with polyvinyl alcohol-glycerol as a mounting medium. The material was examined and photographed using a Nikon 80i phase microscope. Photomicrographs and measurements were captured using a Nikon DS-L3 camera adapted to a Nikon 80i microscope. Fungal species were determined through their conidial ontogeny, conidiogenous events and morphological features. All specimens consisting of permanent slides were deposited in the fungal collection of the Instituto de Ecología A. C. Herbarium (XAL). For the geographical distribution research, the following databases were consulted:
Cybernome (http://www.cybertruffle.org.uk/cybernome/eng), Cybertruffle’s Robigalia (http://www.cybertruffle.org.uk/robigalia/eng/), Global Biodiversity Information Facility (GBIF: http://www.gbif.org/), Landcare Research Manaaki Whenua, Virtual Fungal Dried Reference Collection of New York Botanic Garden and USDA Fungal Databases.
Descriptions
Arthrobotryum stilboideum Ces., 1854. Hedwigia 1: Tab. 4, Fig. 1.
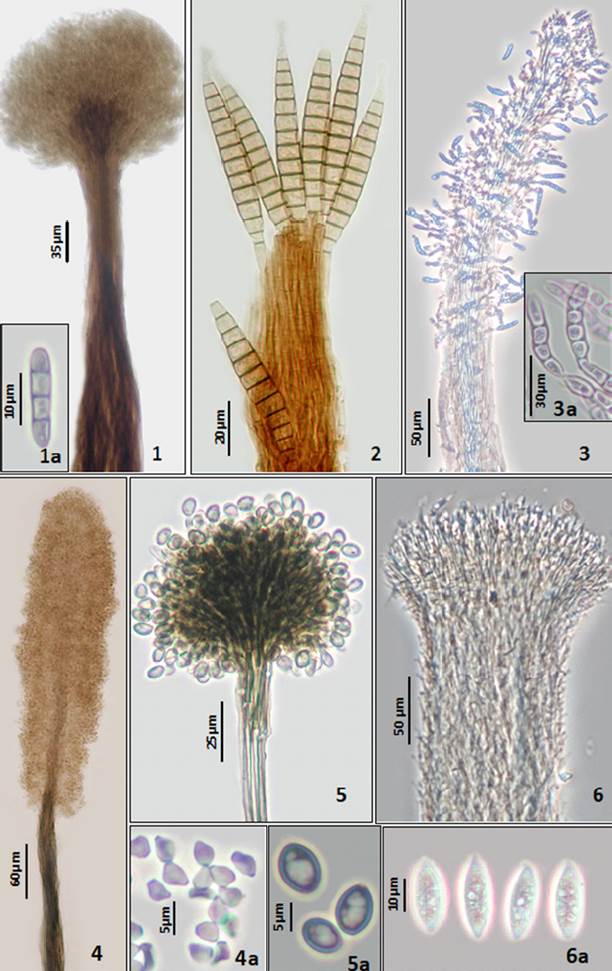
Plate 1 Figures 1-6 1. Arthrobotryum stilboideum. Synnema. 1a. Conidia ellipsoidal. 2. Bactrodesmium longisporum. Synnema with subulate conidia. 3. Blastocatena pulneyensis. Synnema. 3a. Conidia distoseptate. 4. Cephalotrichum microsporum. Synnema. 4a. Conidia obovoid aseptate. 5. Cephalotrichum purpureofuscum. Synnema. 5a. Conidia ovoid aseptate. 6. Didymostilbe capsici. Synnema. 6a. Conidia ellipsoidal, aseptate with granular cytoplasm.
Synnemata scattered or in small groups, cylindrical, dark brown, up to 1,000 μm long × 40-65 μm wide, with a globose or subglobose pale orange head composed of spores embedded in mucilage. Conidiophores threads adhering closely to each other along their length, dark brown, paler above. Conidiogenous cells holoblastic, integrated and terminal, pale brown. Conidia ellipsoidal or cylindrical, rounded at the apex, pale brown, smooth, 1-3 transverse septa, 11-13 × 2.5-3.5 μm.
Taxonomic summary
Veracruz, Texolo, Mpio. Xicochimalco, E. Hernández, 2/12/1995, CB659-2, on dead branches.
Geographical distribution and substrate known: Arthrobotryum stilboideum is a lignicolous fungus, registered in several countries of Europe (Ellis, 1971), India (Chavan & Bhambure, 1975; Kapoor & Munjal, 1966), Taiwan (Chang, 1989), South Africa (GBIF: http://www.gbif.org/) and Iran (Gharizadeh et al., 2007). The present collection is the first record for this species in the Neotropical region.
Bactrodesmium longisporum M.B. Ellis, 1976. More dematiaceous Hyphomycetes: 68. Plate 1. Fig. 2
≡ Stigmina longispora var. stilboidea (R.F. Castañeda & G.R.W. Arnold) J. Mena & Mercado, 1987. Rep. de Investigación del Instituto de Ecología y Sistemática, Academia de Ciencias de Cuba, Ser. Bot. 17: 10.
≡ Bactrodesmium stilboideum R.F. Castañeda & G.R.W. Arnold, 1985. Revta. Jardín Bot. Nac., Univ. Habana 6(1): 48. ≡ Stigmina longispora (M.B. Ellis) S. Hughes, 1978. N.Z. Jl Bot. 16(3): 353.
Synnemata or sporodochia scattered, superficial, up to 167 μm long, brown to pale brown Conidiophores fasciculate, straight or flexuous, smooth, pale brown up to 90 μm long. Conidiogenous cells holoblastic, integrated and terminal, pale brown. Conidia subulate, truncate at the base, up to 17 transverse septa, pale brown, smooth, 50-80 × 7-8 μm, with the apex often enveloped by a thin spherical sheath.
Taxonomic summary
Veracruz, Las Cañadas, Mpio. Huatusco, M. Reyes, G. Heredia, 21/7/1999, CB806, on dead branches. Veracruz, Agüita Fría, Mpio. San Andrés Tlalnehuayocan, G. Heredia, 07/02/10, CB1705; CB1706; CB1707, on dead wood. Veracruz, San Martín Tuxtla, Mpio. San Andrés Tuxtla, G. Heredia, 05/01/11, CB2016, on dead wood.
Comments: except for the CB806 specimen, which has a typical synnemata, the rest of the material studied have sporodochia. The presence of both conidiomata is common in this species.
Distribution and substrate known: Bactrodesmium longisporum has been widely collected mainly in tropical areas, including Cuba (Castañeda-Ruiz & Günter, 1985; Cybernome: http://www.cybertruffle.org.uk/cybernome/eng), India (Rao & de Hoog, 1986), Peru (Matsushima, 1993), Hong Kong (Wong & Hyde, 2001), Filipinas (Cai et al., 2003), Australia, (Vijaykrishna & Hyde, 2006), Venezuela (Castañeda-Ruiz et al., 2009), Brazil (Barbosa & Gusmão, 2011; Santa Izabel & Gusmão, 2016) and Guatemala (Figueroa et al., 2016). There is also information from Bulgaria (Huseyin et al., 2011), Taiwan (GBIF, http://www.gbif.org/), South Africa and Great Britain (Ellis, 1976). This species has been collected mainly on decaying lignified substrates.
Blastocatena pulneyensisSubram. & Bhat, 1987. Kavaka 15(1-2): 44.
Synnemata scattered, singly or in groups, fertile from the lower middle to the apical region, up to 710 long × 45-70 μm wide at the base. Conidiophores septate, simple, straight or flexuous smooth, hyaline to subhyaline. Conidiogenous cells holoblastic, hood-like, hyaline. Conidia, developing in acropetal chains, obovoid to cylindrical, sometimes curved in the middle, thick-walled, smooth, 3-5-distoseptate, subhyaline, 21-36 × 6-7 μm.
Taxonomic summary
Veracruz, Comapa, Mpio. Comapa, J. Mena-Portales, 16/06/1995, CB434, on a dead branches of palm leaf.
Geographical distribution and substrate known: this species had not been registered since Subramanian and Bhat described the type specimen, collected in South India on dead twigs (Subramaniam & Bhat, 1987). Thereby this is the first record for the Neotropical region.
Cephalotrichum microsporum (Sacc.) P.M. Kirk, 1984. Kew Bull. 38(4): 578.
≡ Doratomyces microsporus (Sacc.) F.J. Morton & G. Sm., 1963. Mycol. Pap. 86: 77.
≡ Graphium graminum Cooke, 1887. Grevillea 16, p. 11. ≡ Stysanus microsporus Sacc., 1878. Michelia 1: 274.
Synnemata thin, straight or flexuous, with long cylindrical fertile heads, brown to dark brown, up to 982 μm long × 23-30 μm wide at the base. Conidiophores septate, straight or flexuous, smooth, dark brown, branched towards the apex. Conidiogenous cells monoblastic, integrated, ampulliform, pale brown. Conidia obovoid with truncate base and rounded or acutely pointed apex, smooth, subhyaline and pale brown, 0-septate, 3-5 × 2-3μm.
Taxonomic summary
Veracruz, Botanical garden F. J. Clavijero, Mpio. Xalapa, G. Heredia, 30/11/2001, CB811, on dead branches.
Geographical distribution and substrate known: Cephalotrichum microsporum has a worldwide distribution, it has been found on a wide variety of substrates as seeds, dung, soil, rot wood, dead leaves and compost (Domsch et al., 1980; Ellis, 1971).
Cephalotrichum purpureofuscum (S. Hughes) S. Hughes, 1958. Can. J. Bot. 36: 744.
≡ Doratomyces purpureofuscus (Fr.) F.J. Morton & G. Sm., 1963. Mycol. Pap. 86: 74.
≡ Stysanus purpureofuscus (Fr.) Hughes, 1953. Can. J. Bot. 31: 615.
≡ Aspergillus purpureofuscus Schwein, 1832. Trans. Am.
Phil. Soc., New Series 4(2): 282.
Synnemata thin, straight or flexuous, with spherical or subspherical fertile heads, dark grey to blackish brown, up to 350 μm long × 8-16 μm wide at the base. Conidiophores septate, straight or flexuous, smooth, dark grey, branched towards the apex. Conidiogenous cells monoblastic, integrated, ampulliform, dark to pale grey. Conidia ovoid to oblong, round at the apex, smooth, pale grey, 0-septate, 5-7 × 3.5-5 μm.
Taxonomic summary
Veracruz, Botanical garden F. J. Clavijero, Mpio. Xalapa, R.M. Arias, 30/11/2001, CB808, CB809, on dead branches.
Geographical distribution and substrate known: Cephalotrichum purpureofuscum is a cosmopolitan species, common on plant debris, also isolated from dung and soil (Domsch et al., 1980; Ellis, 1971; Morton & Smith, 1963).
Coremiella cubispora (Berk. & M.A. Curtis) M.B. Ellis, 1971. Dematiaceous Hyphomycetes: 33.

Plate 2 Figures 7-10 7. Coremiella cubispora. Synnema. 7a. Conidia catenate. 8. Gangliostilbe costaricensis. Synnema. 8a. Conidia obovoid, septate. 9. Roigiella lignicola. Synnema. 9a. Conidia hyaline, allantoid. 10. Drumopama girisa. Synnema. 10a. Conidia hyaline, aseptate.
≡ Cladosporium cubisporum Berk. & M.A. Curtis, 1875, apud Berk. In Grevillea 3: 107.
≡ Coremiella ulmariae (McWeeney) E.W. Mason, Hughes, 1953. Can. J. Bot. 31: 640.
Synnemata scattered, forming a loose coremia, arising singly or in groups, fertile in the upper half, pale gray to mid olivaceous brown, 500-790 μm long, and up to 340 μm wide at the base. Conidiophores septate, simple, straight or flexuous smooth, hyaline to pale gray. Conidiogenous cells fragmenting, hyaline. Conidia, catenate, oblong or cubical, 0-septate, smooth, hyaline, subhyaline, pale mid olivaceous brown together, 4.5-8 × 4-8 μm.
Taxonomic summary
Veracruz, Botanical garden, F. J. Clavijero, Mpio. Xalapa, S. Gómez, 02/ 03/2006, CB902, on rotten wood.
Geographical distribution and substrate known: Coremiella cubispora has been collected in Great Britain, USA (Ellis, 1971), Russia (Tikhomirova, 1989), Kenya (Sutton, 1993), India (Prasher & Singh, 2013) and Malawi (Cybernome, 2017), on submerged and terrestrial vegetable remains. The present collection is the first record for this species in the Neotropical region.
Didymostilbe capsici (Pat.) Seifert, 1985. Stud. Mycol. 27: 135.
≡ Stilbum capsici Pat. 1893. Bull. Soc. Mycol. Fr. 9: 163.
Synnemata scattered, gregarious, or caespitose, cylindrical-capitate, fertile in the upper, white, up to 1,619 μm long × 88-195 μm wide at the base. Conidiophores threads adhering closely to each other along their length, subhyaline. Conidiogenous cells phialidic, cylindrical to clavate, hyaline. Conidia, ellipsoidal to oblong-ellipsoidal, with a truncate or nipple-like base, 0-septate, 15-23 × 5-10 μm, with granular cytoplasm.
Taxonomic summary
Veracruz, Ranch Guadalupe, Mpio. Xalapa. G. Rosas, 13/06/1995, CB403-1; G. Heredia, 30/ 03/2001, CB807, on dead branches.
Geographical distribution and substrate known: Didymostilbe capsici have been collected in Ecuador, Gold Coast (Seifert, 1985), Peru (Matsushima, 1993) and Cuba (Mena-Portales et al., 2017), on dead leaves, petioles and branches.
Drumopama girisa Subram., 1957. Proceedings of the Indian National Science Academy, Part B. Biol. Sciences 46: 333.
Synnemata scattered, erect, dark brown to pale brown, subhyaline towards the tip, up to 910 μm long × 15-42 μm wide at the base. Conidiophores septate, simple, straight, curved, bent or flexuous, smooth, become free above, markedly geniculate, varying lengths. Conidiogenous cells polyblastic, integrated, geniculate, denticulate. Conidia, singly and acrogenously, ovoid to subglobose, with a basal papilla, hyaline, smooth, 0-septate, 10-12 μm diam.
Taxonomic summary
Veracruz, Botanical garden F. J. Clavijero, Mpio. Xalapa, R. M. Arias, 30/ 03/2001, CB776-1, on decaying leaves.
Geographical distribution and substrate known: the type material of Drumopama girisa comes from India (Subramanian, 1957), latter was collected in Taiwan (Chang, 1989), Japan (Chang, 1989) and Cuba (Castañeda & Kendrick, 1990; Mena-Portales et al., 1995), always associated with decaying leaves.
Gangliostilbe costaricensis Mercado, Gené & Guarro, 1997. Nova Hedwigia 64 (3-4): 456.
Synnemata scattered, erect, composed of closely aggregated parallel conidiophores, blackish brown to pale brown towards the tip, up to 850 μm long, and up to 160 μm wide at the base. Conidiophores septate, simple, straight, bent or flexuous, smooth, divergent in the apical region. Conidiogenous cells monoblastic, integrated, terminal. Conidia, singly and acrogenously, broadly obovoid, truncate at the base, smooth, 3-septate, pale brown to brown, 41-48 × 20-25 μm, and 4-6 μm wide at the base.
Taxonomic summary
Veracruz, San Martín Tuxtla Volcano, Mpio. San Andrés Tuxtla, G. Heredia, 5/01/11, CB1940, CB2013, on dead branches.
Comments: synnemata in the Mexican material are longer and wider than those reported by Mercado-Sierra et al. (1997) from Costa Rica (250-450 × 60 μm).
Geographical distribution and substrate known: Gangliostilbe costaricensis has been registered only for the Neotropical region, the type specimen was collected on rotten wood from Costa Rica (Mercado-Sierra et al., 1997), later was found in Brazil (Marques et al., 2008; Santa-Izabel & Gusmão, 2016) also on rotten wood and decaying leaves.
Hyalosynnema multiseptatum Matsush., 1975. Icon. microfung. Matsush. lect. (Kobe): 85.

Plate 3 Figures 11-15 11. Phragmocephala elliptica. Synnema with conidia attached. 12. Hyalosynnema multiseptatum. Synnema. 12a. Conidia cylindrical. 13. Phaeoisaria infrafertilis. Synnema. 13a. Conidia falcate. 13b. Conidiogenous cells with conidia attached. 14. Phaeoisaria clavulata. Synnema. 14a. Conidia sphaerical. 15. Phaeoisaria sparsa. Synnema with denticulate conidiogenous cells.
Synnemata scattered, erect, straw-colored to hyaline, up to 280μm long, and up to 35μm wide at the base. Conidiophores septate, simple, straight, bent or flexuous, smooth, hyaline. Conidiogenous cells monoblastic, integrated, terminal. Conidia, singly and acrogenously, cylindrical, ellipsoidal, truncate at the base, 5-septate, hyaline, 21-28 × 5-6 μm.
Taxonomic summary
Veracruz, Biol. Station “Los Tuxtlas”, Mpio. San Andrés Tuxtla, J. Mena-Portales, 23/11/93, CB137, on dead branches.
Geographical distribution and substrate known: the type material of Hyalosynnema multiseptatum was collected in Iriomote Island, Okinawa (Matsushima, 1975), later it was registered from Peru (Matsushima, 1993) and Taiwan on bark of dead branches (Kirschner et al., 2001).
Menisporopsis anisospora R.F. Castañeda & Iturr., 2001. Cryptog. Mycol. 22 (4): 260.

Plate 4 Figures 16-18 Menisporopsis anisospora. Synnema. 16a. Conidia fusiform with setules. 17. Menisporopsis multisetulata. Synnema. 17a. Conidia fusiform, with setules. 18. Menisporopsis profusa. Synnema. 18a. Conidiogenous cells. 18b. Conidia fusiform, with single setules at each end.
Synnemata erect, composed of closely aggregated parallel conidiophores surrounding a single seta, dark brown to blackish brown, up to 355 (whithout considering the seta lenght) and, up to 64 wide at the base. Conidiophores simple, straight, dark brown at the base, brown to pale brown towards the apex. Seta simple erect, straight, rounded at the apex, 300-386 × 8.5-9.5 μm. Conidiogenous cells phialidic, integrated, terminal. Conidia, aggregated in slimy masses, allantoid, fusiform, vermiform to irregular, 0-septate, hyaline, smooth, 18-30 × 2-6 μm, with 1-3 setulae, apical, eccentric and basal.
Taxonomic summary
Veracruz, San Martín Tuxtla Volcano, Mpio. San Andrés Tuxtla, G. Heredia, 05/01/11, CB1957, CB2015, on rachis of palm.
Geographical distribution and substrate known: this is the first report of M. anisospora since the species was described by Castañeda and Iturriaga from a petiole of decaying leaves collected on Venezuela (Castañeda-Ruiz et al., 2001).
Menisporopsis multisetulata K.M. Tsui, Goh, K.D. Hyde & Hodgkiss, 1999. Mycol. Res. 103 (2): 150.
Synnemata erect, composed of closely aggregated parallel conidiophores surrounding a single seta, dark brown to blackish brown, up to 110 μm long (whithout considering the seta lenght) and, up to 30 μm wide at the base. Conidiophores septate, simple, straight, dark brown at the base, brown to pale brown towards the apex. Seta simple erect, straight, rounded at the apex, up to 300 × 7-8 μm. Conidiogenous cells phialidic, integrated, terminal. Conidia, aggregated in slimy masses, allantoid, 0-septate, hyaline, 12-19 × 2.5-4 μm, with 5-6 setulae on each end.
Taxonomic summary
Veracruz, Biol. Station “Los Tuxtlas”, Mpio. San Andrés Tuxtla, G. Heredia, 08/02/06, CB812, on decaying leaves.
Comments: synnemata and setae in the Mexican material are shorter than those reported by Tsui et al. (1999) from the type material (180-220 μm × 22-40 μm and 500 μm × 6-10 μm respectively).
Geographical distribution and substrate known: this is a scarcely reported species, besides the type specimen collected on submerged wood from Lam Tsuen River from Hong Kong (Tsui et al., 1999), García-García et al. (2013) listed as part of the mycobiota of decomposing fallen leaves of Rinorea guatemalensis, from a rainforest in Tabasco state, Mexico. Since there was not back-up description with herbarium material from the species, the formal registration of Menisporopsis multisetula from Mexico is presented in this contribution. The present collection is the first record for this species in the Neotropical region.
Menisporopsis profusa Piroz. & Hodges, 1973. Can. J. Bot. 51(1): 164.
Synnemata erect, composed of closely aggregated parallel conidiophores surrounding a single seta, dark brown to blackish brown, up to 120 μm long × 13-30 μm wide at the base (without considering the seta length). Conidiophores septate, simple, straight, dark brown at the base, brown to pale brown towards the apex. Seta simple erect, straight, rounded at the apex, up to 356 μm long × 7-9 μm wide. Conidiogenous cells phialidic, integrated, terminal. Conidia, aggregated in slimy masses, cylindrical, curved, aseptate, hyaline, smooth, 10-15 × 2-2.5, bearing at each end a single setula.
Taxonomic summary
Veracruz, San Martín Tuxtla Volcano, Mpio. San Andrés Tuxtla, G. Heredia, 05/01/11, CB1996, on dead branches.
Comments: as in the Brazilian material studied by Cruz et al. (2014), our specimens have longer and wider setae than those reported by Pirozynski and Hodges (150-250 × 4.6-6 μm) for the type material (Pirozynski & Hodges, 1973).
Geographical distribution and substrate known: the type specimen of Menisporopsis profusa was detected on fallen leaves of Persea borbonia from South Carolina, USA (Pirozynski & Hodges, 1973). It has been collected in Malasya (Cybernome, 2017) and Brazil (Marques et al. 2007; Cruz et al., 2014) on decaying leaves, stems and petioles.
Phaeoisaria clavulata (Grove) E.W. Mason & S. Hughes, 1953. Mycol. Pap. 56: 42.
≡ Pachnocybe clavulata Grove, 1885, J. Bot. Lond, 30: 168. ≡ Graphium grovi Sacc., 1886, Syll. Fung. 4: 613.
Synnemata erect, straight or flexuous, dark brown to pale brown, up to 790 μm long × 20-36 μm wide at the base. Conidiophores, straight, splaying out at the apex and along the sides of the upper half or two thirds of each synnema, dark brown at the base, brown to pale brown towards the apex. Conidiogenous cells polyblastic, integrated, terminal, denticles, hyaline. Conidia spherical, hyaline, smooth, 0-septate, 1-2 μm diam.
Taxonomic summary
Veracruz, Tlaltetela, Mpio. Huatusco, G. Heredia, 16/06/1995, CB426-5, on dead branches. Veracruz, Agüita Fría, Mpio. San Andrés Tlalnehuayocan, G. Heredia, 07/02/2010, CB1864, CB1865, CB1869, on dead branches.
Geographical distribution and substrate known: Phaeoisaria clavulata has been widely collected in different European localities (Ellis, 1971; Révay, 1986). There are also reports from Malawi (Sutton, 1993), India (Rao & De Hoog, 1986; Sridhar & Sudheep, 2011), Malasya (Cybernome, 2017) and Pakistan (Abbas et al., 2013). It is considered a lignicolous species. The present collection is the first record for this species in the Neotropical region.
Phaeoisaria infrafertilis B. Sutton & Hodges, 1976. Nova Hedwigia 27 (1-2): 219.
≡ Chryseidea africanaOnofri, 1981. Mycotaxon 31(2): 333
Synnemata erect, straight, dark brown, up to 815 μm long × 20-40 μm wide at the base. Conidiophores, straight, brown to pale brown towards the apex. Conidiogenous cells polyblastic, integrated, terminal, with cylindrical truncate denticles, pale brown to hyaline. Conidia solitary, falcate, apex and base finally obtuse, hyaline to pale brown, smooth, 0-septate, 12.5-18 × 1.5-2 μm.
Taxonomic summary
Veracruz, Tlaltetela, Mpio. Tlaltetela, G. Rosas, 16/ 06/1995, CB459, CB471-1, on fallen leaves. Veracruz, Mesa de Yerba, Mpio. Acajete, G. Heredia, 05/12/2010 CB1912, on dead herbaceous stems. Veracruz, San Martín Tuxtla Volcano, Mpio. San Andrés Tuxtla, G. Heredia, 05/01/11, CB1968, CB1992, on dead leaves.
Comments: synnemata in studied material are longer and wider, and conidia smaller than those reported by Sutton and Hodges (1976) (setae up to 200 × 10 μm; conidia 19.5-22 × 2-3 μm).
Geographical distribution and substrate known: the type specimen was collected in Brazil on Eucalyptus sp. dead leaves (Sutton & Hodges, 1976). There are reports from Ivory Coast (Onofri et al. 1981), Brazil (Conceição & Marques, 2015; Cruz et al., 2007; Grandi, 1996), Mauritius (Dulymamode et al., 2001), Cuba and Venezuela (Castañeda-Ruiz et al., 2002). This species has been frequently found on decaying leaves.
Phaeoisaria sparsa B. Sutton, 1973. Mycol. Pap. 132: 87. Plate 3. Fig. 15
Synnemata erect, straight, dark brown to pale brown, up to 895 μm long × 84 μm wide at the base. Conidiophores, straight, splaying out at the apex, branched towards the apices, brown to pale brown towards the apex. Conidiogenous cells polyblastic, integrated, terminal, with cylindrical truncate denticles, hyaline. Conidia solitary, fusiform, hyaline to pale brown, smooth, 0-septate and 1-septate, 10-16 × 2-3.5 μm.
Taxonomic summary
Veracruz, Biol. Station “Los Tuxtlas”, Mpio. San Andrés Tuxtla, A. Mercado, 20/05/94, CB271-1, on dead branches. Veracruz, Park “El Haya”, Mpio. Xalapa, E. Hernández, 26/03/95, CB328-2, on bark of Quercus. Veracruz, Tlaltetela, Mpio. Tlaltetela, G. Rosas, 16/ 06/1995, CB423-5, on dead branches. Veracruz, Ixhuacán de los Reyes, Mpio. Ixhuacán de los Reyes, A. Mercado, 04/03/1996, CB596-1, on dead branches.
Geographical distribution and substrate known: the type specimen was collected on bark from Canada (Sutton, 1973). There are reports from New Zealand (Hughes, 1978), Malaysia (Ho et al., 2001), Cuba (Cybernome, 2017) and Nepal (GBIF: http://www.gbif.org/). It is mainly a lignic species of terrestrial and aquatic environments.
Phragmocephala elliptica (Berk. & Broome) S. Hughes, 1979. N.Z. Jl. Bot. 17 (2): 164.
Plate 3. Fig. 11
≡ Monotospora elliptica Berkeley et Broome, Ann. & Mag., 1881. Nat. Hist. Ser. 5, 7: 130. (Notices of British Fungi No. 1909).
≡ Brachysporium ellipticum (Berk, et Br.) Massee, 1893. British Fungus Flora 3: 414.
≡ Endophragmia elliptica (Berk, et Br.) Ellis, 1959. Mycol. Papers 72: 20.
≡ Phragmocephala cookei Mason et Hughes, 1951. Naturalist, Leeds 838: 98.
Synnemata fasciculate, straight, dark brown to pale brown, up to 165 μm long × 40-61 μm μm wide at the base. Conidiophores, straight, splaying out at the apex, brown to pale brown towards the apex. Conidiogenous cells monoblastic, integrated, terminal, pale brown. Conidia solitary, acrogenous, ellipsoidal, smooth, unequally colored, 5-septate, cells at each end subhyaline or pale brown, central cells brown to dark brown, 32-43 μm × 18-25 μm wide in the broadest part, 9-11 μm wide at the truncate base.
Taxonomic summary
Veracruz, Mesa de Yerba, Mpio. Acajete, G. Heredia, 05/ 12/2010, CB1883, CB1883-1, CB1883-2, on decaying leaves.
Geographical distribution and substrate known: this species have been collected in several European localities from Great Britain, Belgium, Switzerland (Ellis, 1959), Czechoslovakia (Holubová-Jechová, 1986), Hungary (Révay, 1986) and Russia (Davydkina & Mel’nik, 1989). There are also registers from Japan (Matshushima, 1985), Puerto Rico (Mel'nik, 1998) and Bolivia (Arias et al., 2007). Common on dead stems and leaves.
Roigiella lignicola R.F. Castañeda. 1984. Rev. Jardín Bot. Nac. Univ. Habana 5(1): 64.
Synnemata erect, straight, hyaline, up to 270 μm long × 12-21 μm wide at the base. Conidiophores, straight, flexuous, splaying out at the apex, hyaline. Conidiogenous cells polyblastic, integrated, terminal, with cylindrical truncate denticles, hyaline. Conidia solitary, obovoid, clavate, allantoid, hyaline, smooth, 1-septate, 11-17 × 2-3 μm.
Taxonomic summary
Veracruz, Agüita Fría, Mpio. San Andrés Tlalnehuayocán, G. Heredia, 07/02/10, CB1874, CB1875, on dead branches. Veracruz, San Martín Tuxtla Volcano, Mpio. San Andrés Tuxtla, G. Heredia, Veracruz, 05/01/11, CB1980, on dead branches.
Geographical distribution and substrate known: this is the first record of Roigiella lignicola since it was described from dead branches of Cecropia peltata collected in Cuba (Castañeda-Ruiz, 1984).
Account of the synnematous species recorded for Mexico: 22 species of synnematous hyphomycetes belonging to 18 genera have been previously recorded for Mexico (Table 1). We found information only for the states of Chiapas (1), Tabasco (6), Tamaulipas (1) and Veracruz (17), and almost in all the cases, the records are scarce. Most materials have been collected from rain forest and cloud forest localities. In total, combined with the present contribution, to date 40 species belonging to 28 genera of synnematous microfungi have been documented for Mexico.
Table 1 Synnematous species registered from Mexico.
| Species | Vegetation substrate | State | References |
| Atractilina biseptata R.F. Castañeda | RF/fallen leaves | Ch | Heredia et al., 2000a |
| Arthrobotryum stilboideum Ces. | CF/dead branches | V | This contribution |
| Bactrodesmium longisporum M.B. Ellis | CF/dead branches | V | This contribution |
| Blastocatena pulneyensis Subram. & Bhat | RF/dead rachis of palm | V | This contribution |
| Cephalotrichum microsporum (Sacc.) P.M. Kirk | CF/dead branches | V | This contribution |
| Cephalotrichum purpureofuscum (S. Hughes) S. Hughes | CF/dead branches | V | This contribution |
| Coremiella cubispora (Berk. & M.A. Curtis) M.B. Ellis | CF/rot wood | V | This contribution |
| Dendrographium atrum Massee | RF/dead branches | V | Heredia et al., 1997a |
| Didymostilbe capsici (Pat.) Seifert | CF/dead branches | V | This contribution |
| Didymobotryum verrucosum I. Hino & Katum. | RF/dead bamboo stalk | Tb | Becerra et al., 2008 |
| Drumopama girisa Subram. | CF/decaying leaves | V | This contribution |
| Exophiala calicioides (Fr.) G. Okada & Seifert | RF/dead branches & rotten wood | V | Heredia et al., 1997b |
| Exosporium monanthotaxis Piroz. | RF/dead branches | V | Heredia et al., 1997b |
| Gangliostilbe costaricensis Mercado, Gené & Guarro | CF/dead branches | V | This contribution |
| Graphium penicillioides Corda | RF/fallen leaves | Tb | Heredia et al., 2006 |
| Hyalosynnema multiseptatum Matsush. | RF/dead branches | V | This contribution |
| Melanographium cookei M.B. Ellis | RF/dead branches | V | Heredia et al., 1997a |
| Melanographium selenioides (Sacc. & Paol.) M.B. Ellis | CF/dead stems | V | Delgado-Rodríguez et al., 2006 |
| Menisporopsis anisospora R.F. Castañeda & Iturr. | CF/dead rachis of palm | V | This contribution |
| Menisporopsis novae-zelandiae S. Hughes & W.B. Kendr. | CF/fallen leaves | V | Arias et al., 2010 |
| Menisporopsis multisetulata K.M. Tsui, Goh, K.D. Hyde & Hodgkiss | RF/decaying leaves | V | This contribution |
| Menisporopsis pirozynskii Varghese & V.G. Rao | CF/fallen leaves | V | Heredia et al., 2000b |
| Menisporopsis profusa Piroz. & Hodges | CF/dead branches | V | This contribution |
| Menisporopsis theobromae S. Hughes | RF, CF/dead branches & leaves | Tb, V Tm | Heredia, 1994, Heredia et al., 1997b, Heredia et al., 2006 |
| Neosporidesmium maestrense Mercado & J. Mena | RF/dead branches | V | Heredia et al., 1997b |
| Nodulisporium gregarium (Berk. & M.A. Curtis) J.A. Mey. | RF/decaying wood | V | Heredia et al., 1997b |
| Penicillium duclauxii Delacr. | CF/soil forest | V | Heredia et al., 2011 |
| Penicillium vulpinum (Cooke & Massee) Seifert & Samson | CF/soil from forest & coffee plantation | V | Heredia et al., 2011 |
| Phaeoisaria clavulata (Grove) E.W. Mason & S. Hughes | CF/dead branches | V | This contribution |
| Phaeoisaria clematidis (Fuckel) S. Hughes | RF/dead branches & leaves | Tb, V | Heredia et al., 1997b, Heredia et al., 2006 |
| Phaeoisaria infrafertilis B. Sutton & Hodges | RF,CF/dead leaves & branches | V | This contribution |
| Phaeoisaria sparsa B. Sutton | RF/dead branches | V | This contribution |
| Phalangispora nawawii Kuthub. | CF/submerged leaves | V | Heredia et al., 2000b |
| Phragmocephala atra (Berk. & Broome) E.W. Mason & S. Hughes | CF/dead stems Musa sp. | V | Mercado-Sierra & Heredia,1994 |
| Phragmocephala elliptica (Berk. & Broome) S. Hughes | CF/decaying leaves | V | This contribution |
| Roigiella lignicola R.F. Castañeda | CF/dead branches | V | This contribution |
| Synnemacrodictys stilboidea (J. Mena & Mercado) W.A. Baker & Morgan-Jones | CF/dead branches | V | Heredia et al., 2000a |
| Thozetella cristata Piroz. & Hodges | RF/dead branches | Tb | Becerra et al., 2007 |
| Tretopileus sphaerophorus (Berk. & M.A. Curtis) S. Hughes & Deighton | RF/dead leaves & branches | Tb, V | Heredia et al., 2000a, Becerra et al., 2008 |
| Virgatospora echinofibrosa Finley | RF/dead branches | V | Heredia et al., 1997b |
State: Ch = Chiapas, Tb = Tabasco, Tm = Tamaulipas, V = Veracruz.
Vegetation: RF = rain forest, CF = cloud forest.
Discussion
All material analyzed in this contribution corresponded to asexual morphs. A limited number of studies have linked synnematous microfungi with their sexual morphs, based on either on direct evidence or by phylogenetic inferences from molecular data (i.e., Lombard et al., 2016; Rossman et al., 2001). Molecular studies of some synnematous species of the genera Vamsapriya Gawas & Bhat, Myrothecium Tode, Pseudocercospora Speg. and Graphium Corda, have led to their association with Xylariales, Hypocreales, Capnodiales and Microascales, respectively (Crous et al., 2006; Dai et al., 2014; Lombard et al., 2016; Okada et al., 1998).
In recent decades, molecular tools have allowed the phylogenetic relationships of many hyphomycetes to be inferred. Even so, in many cases, such as that of the present study, the lack of sufficient biological material from the natural substrates or the inability to obtain strains in artificial cultures, complicate the application of such tools. Thus, in view of the huge diversity and economical importance of hyphomycetes, the use of morphological characters for their identification remains necessary.
Approximately 135 genera of conidial microfungi with synnematous conidiomata have been described (Seifert et al., 2011), from these, 28 have been documented in the Mexican mycobiota. As well as the rest of the saprobic conidial microfungi, synnematous species are still essentially unexplored in Mexico. In the face of the extensive human impact and rapid habitat loss of tropical and semitropical Mexican regions, where it is assumed that there is the greatest fungal diversity (Rossman, 1997), it is urgent to promote studies to explore the diversity of these important microorganisms.











 nueva página del texto (beta)
nueva página del texto (beta)


