Introduction
The southeastern (SE) reefs of the Dominican Republic are located along the coast of Hispaniola, in the Caribbean Sea. These reefs are distributed along a semi-continuous fringing reef dominated by the rocky and coral substrate and are fringing reefs with some small and dispersed coral patches (Geraldes, 2003). The region forms part of the Southeastern Reefs Marine Sanctuary (SMASE), covering an area of 7,855.31 km2. In 2009, this region was declared a Protected Marine Area by Dominican Government Decree 571-09 to conserve the natural habitat and unique environment that exists along the continental shelf on the SE part of the island (SINAP, 2014). Additionally, this region is regarded as an area of great importance due to its biodiversity, its variety of marine and coastal ecosystems (mangrove, sea grasses, rocky/sandy beaches, coral reefs), and its economic value, which originates from coastal and touristic development (Vargas, 2011).
Several studies of fish fauna have been conducted in the area; Williams et al. (1983) provided the first checklist of 172 marine fishes of the south coast of Dominican Republic. Another study reported the abundance of fishes in shallow (algal/seagrass) habitats (León et al., 1996). Bustamante et al. (1998) and Chiappone et al. (1998) provided information about reef fish assemblages. More recently, Schmitt et al. (2002) listed a total of 125 species in 36 families of reef fishes in 4 reefs (Dominicus Reef, La Raya, Rubén, and El Toro) of the SE Dominican Republic. However, little data has been gathered about the overall marine biodiversity, and there is a lack of information about the fish fauna in particular.
In this work, we generated an updated checklist of fish fauna associated with the coral formations of the SMASE. The checklist was based on field surveys and including the reefs of Catalina Island (La Pared) and adjacent to the municipality of Bayahibe (Coralina, Atlantic Princess, Magallán, and Pepito I and II) for the first time.
Materials and methods
The SMASE is formed by a reef system located near the SE part of the island Hispaniola (SINAP, 2014), between 18°22’9” - 18°11’4” N, 68°51’9” - 68°38’15” W (Fig. 1). This region is part of the Central Province of the Greater Caribbean; the area is located between Saona Island in the Cotubanama National Park and east of Puerto Rico, where the Mona Passage is located (Geraldes & Vega, 2004). This area is characterized by shallow sand banks, meadows of sea grasses, and coral reefs. To the southeast of Saona Island, there are fringing reefs with a deeper and wider shelf, where beds are mixed with low-lying hard corals and sea grasses (Chiappone, 2001; Vega et al., 1997).
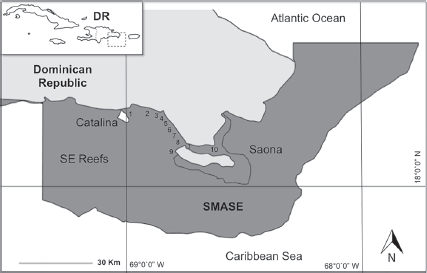
Figure 1 Geographic location of the SMASE in the Dominican Republic. Each number corresponds to 1 of the 10 selected reefs under study (Table 1).
The study site was a 35 Km section of the coastline located between the eastern portion of Catalina Island and the southern part of Saona Island. Expeditions were carried out from 2013-2016. A total of 360 visual surveys were conducted (daytime, between 9 a.m. and 1 p.m.) via the Roving Diver Technique (RDT), in the RDT method trained SCUBA divers swim around the reef site approximately 30-45 minutes recording all fish species observed (REEF, 2008). In this manner, 10 fringing reefs on the SE coast were selected for sampling by geographical location, the east-west location, reef type and depth (< 15 m of depth) (Table 1).
Table 1 Sites of study (visual surveys) of fish associated with coral reefs in the SMASE. Geographical coordinates (decimal degrees) and depths (m) are included.
| Reef | Latitude (N) | Longitude (W) | Depth |
| 1. La Pared | 18º15’28.8” | 68º46’55.2” | 6 |
| 2. Coralina | 18º22’12” | 68º50’49.2” | 4 |
| 3. Atlantic Princess | 18º22’8.4” | 68º51’7.2” | 13 |
| 4. Magallán | 18º21’43.2” | 68º50’45.6” | 13 |
| 5. Pepito I | 18º20’42” | 68º49’58.8” | 14 |
| 6. Pepito II | 18º20’34.8” | 68º49’48” | 14 |
| 7. Dominicus Reef | 18º20’34.8” | 68º49’58.8” | 13 |
| 8. El Peñón | 18º15’10.8” | 68º46’44.4” | 13 |
| 9. Punta Cacón | 18º10’37.2” | 68º47’31.2” | 14 |
| 10. Cayo Ratón | 18º11’42” | 68º38’13.2” | 4 |
The individuals observed were identified following the Cervigón et al. (1992), Humann and Deloach (2002) and Robertson et al. (2016) identification guides and were systematically ordered according to FishBase criteria (Froese & Pauly, 2016). For interpretation of the biogeographic affinities of the species, analysis of the following regions in Eschmeyer and Fricke’s catalog of fishes (2016) was used: WA = Western Atlantic, EA = Eastern Atlantic, CIRC = Circumtropical, and IS = Invasive species (De Freitas & Lotufo, 2014).
Results
A total of 150 species of reef fishes from 86 genera and 47 families were observed in the transects (Table 2).
Table 2 Taxonomic list of fish fauna observed in the SMASE coral reefs of La Pared (LP), Coralina (C), Atlantic Princess (AP), Magallán (M), Pepito I (PI), Pepito II (PII), Dominicus Reef (DR), El Peñón (EP), Punta Cacón (PC), and Cayo Ratón (CR). An * indicates a new record for the area.
| Order, family and species | |
| Order Anguiliformes | |
| Family Congridae | |
| Hetecoconger longissimus (Günther, 1870) | C, LP, PII |
| Family Muraenidae | |
| Echidna catenata (Bloch, 1795) | EP, PC |
| Gymnothorax funebris (Ranzani, 1839) | AP, PI, PII |
| Gymnothorax miliaris (Kaup, 1856) | EP, PII |
| Gymnothorax moringa (Cuvier, 1829) | EP, LP |
| Gymnothorax vincinus (Castelnau, 1855) | EP |
| Family Ophichthidae | |
| Myrichthys breviceps (Richardson, 1848) | DR |
| Order Aulopiformes | |
| Family Synodontidae | |
| Synodus foetens (Linnaeus, 1766) | EP, CR, PII |
| Synodus intermedius (Spix & Agassiz, 1829) | EP, PC, DR, C, AP, LP, M, PI, PII |
| Order Beloniformes | |
| Family Belonidae | |
| Ablennes hians (Valenciennes, 1846) | C, M |
| Family Hemiramphidae | |
| *Hemiramphus balao (Lesueur, 1821) | EP, C, AP |
| *Hemiramphus brasiliensis (Linnaeus, 1758) | EP, PC, DR, AP, M, PI |
| Order Beryciformes | |
| Family Holocentridae | |
| Myripristis jacobus (Cuvier, 1829) | EP, PC, DR, C, AP, CR, PI |
| Sargocentron coruscum (Poey, 1860) | EP, PC, DR, AP, LP, M, PI, PII |
| Order Lophiiformes | |
| Family Antennariidae | |
| Antennarius multiocellatus (Valenciennes, 1837) | EP |
| Order Myliobatiformes | |
| Family Dasyatidae | |
| Dasyatis americana (Hildebrand & Schroeder, 1928) | EP, PII |
| Family Myliobatidae | |
| Aetobatus narinari (Euphrasen, 1790) | DR, M, PI, PII |
| Family Urotrygonidae | |
| Urobatis jamaicensis (Cuvier, 1816) | EP, PC, CR |
| Order Orectolobiformes | |
| Family Ginglymostomatidae | |
| Ginglymostoma cirratum (Bonnaterre, 1788) | PC, CR |
| Order Perciformes | |
| Family Acanthuridae | |
| Acanthurus bahianus (Castelnau, 1855) | EP, PC, DR, C, AP, LP, CR, M, PI |
| Acanthurus chirurgus (Bloch, 1787) | EP, DR, C, LP, CR |
| Acanthurus coeruleus (Bloch & Schneider, 1801) | EP, PC, DR, C, AP, LP, CR, M, PI |
| Family Apogonidae | |
| Astrapogon stellatus (Cope, 1867) | PC, C, CR |
| Family Carangidae | |
| Caranx bartholomaei (Cuvier, 1833) | PI, PII |
| Caranx ruber (Bloch, 1793) | EP, PC, DR, C, AP, LP, CR, PII |
| *Seriola dumerili (Risso, 1810) | M |
| Trachinotus blochii (Lacepède, 1801) | EP, PC, CR, PI, PII |
| Family Chaenopsidae | |
| Acanthemblemaria spinosa (Metzelaar, 1919) | EP, PC, DR, AP, LP, CR, M, PI |
| *Emblemariopsis sp. (Longley, 1927) | DR |
| Family Chaetodontidae | |
| Chaetodon aculeatus (Poey, 1860) | PC, DR, C, AP, PII |
| Chaetodon capistratus (Linnaeus, 1758) | CR, PII |
| Chaetodon ocellatus (Bloch, 1787) | EP, PC, DR, C, AP, LP, CR, M, PI, PII |
| Chaetodon sedentarius (Poey, 1860) | EP, PC, DR |
| Chaetodon striatus (Linnaeus, 1758) | EP, PC, DR, C, AP, LP, CR, M, PI |
| Family Cirrhitidae | |
| Amblycirrhitus pinos (Mowbray, 1927) | C, CR |
| Family Echeneidae | |
| Echeneis naucrates (Linnaeus, 1758) | PI, PII |
| Family Gobiidae | |
| Coryphopterus personatus (Jordan & Thompson, 1905) | EP, PC, DR, AP, LP, M, PI, PII |
| Elacatinus prochilos (Böhlke & Robins, 1968) | EP, PC, DR, C, AP, LP |
| Family Haemulidae | |
| Anisotremus surinamensis (Bloch, 1791) | AP |
| Anisotremus virginicus (Linnaeus, 1758) | DR, AP, PII |
| Haemulon album (Cuvier, 1830) | EP, DR, C, AP, LP, CR, M, PI, PII |
| Haemulon aurolineatum (Cuvier, 1830) | PC, AP, PII |
| Haemulon carbonarium (Poey, 1860) | EP, PC, DR, AP, PI |
| Haemulon chrysargyreum (Günther, 1859) | EP, C, CR, PII |
| Haemulon flavolineatum (Desmarest, 1823) | EP, DR, C, AP, LP, M |
| Haemulon macrostomum (Günther, 1859) | EP, PC, DR, C, AP, LP, CR, M, PI, PII |
| Haemulon melanurum (Linnaeus, 1758) | EP, DR, LP |
| *Haemulon parra (Desmarest, 1823) | EP, DR |
| Haemulon plumieri (Lacepède, 1801) | EP, DR, PII |
| Haemulon sciurus (Shaw, 1803) | EP, DR, AP, CR, PII |
| Family Kyphosidae | |
| Kyphosus sp. (Lacepède, 1801) | EP, PC, DR |
| Family Labridae | |
| Bodianus rufus (Linnaeus, 1758) | CR, PII |
| Clepticus parrae (Bloch & Schneider, 1801) | EP, PC, DR, AP, LP, M, PI |
| Halichoeres bivitattus (Bloch, 1791) | EP, PC, DR, C, AP, CR, PII |
| Halichoeres garnoti (Valenciennes, 1839) | EP, C, LP, PII |
| Halichoeres radiatus (Linnaeus, 1758) | EP, PC, DR, C, AP, LP, CR, PI, PII |
| Lachnolaimus maximus (Walbaum, 1792) | EP, C |
| Thalassoma bifasciatum (Bloch, 1791) | EP, PC, DR, C, AP, LP, CR, M, PI |
| Family Labrisomidae | |
| Malacoctenus triangulatus (Springer, 1959) | EP |
| Family Lutjanidae | |
| Lutjanus analis (Cuvier, 1828) | PC, AP |
| Lutjanus apodus (Walbaum, 1792) | EP, PC, DR, C, AP, CR |
| *Lutjanus cyanopterus (Cuvier, 1828) | EP |
| Lutjanus griseus (Linnaeus, 1758) | EP, PC, CR |
| Lutjanus jocu (Bloch & Schneider, 1801) | EP, C, AP |
| Lutjanus mahogoni (Cuvier, 1828) | EP, DR, C, AP, M, PI, PII |
| Lutjanus synagris (Linnaeus, 1758) | EP, DR, AP, M, PII |
| Ocyurus chrysurus (Bloch, 1791) | EP, PC, DR, C, AP, LO, DR, PI |
| Family Malacanthidae | |
| Malacanthus plumieri (Bloch, 1786) | EP, PC, PI, PII |
| Family Mullidae | |
| Mulloidichtys martinicus (Cuvier, 1829) | EP, PC, DR, AP, LP, PI, PII |
| Pseudupeneus maculatus (Bloch, 1793) | EP, PC, DR, AP, CR, PI, PII |
| Family Opistognathidae | |
| *Ophioblennius macclurei (Silvester, 1915) | C, LP, CR |
| Opistognathus aurifrons (Jordan & Thompson, 1905) | EP, DR |
| Family Pomacanthidae | |
| Holacanthus ciliaris (Linnaeus, 1758) | EP, DR |
| Holacanthus tricolor (Bloch, 1795) | CR |
| Holocentrus adscensionis (Osbeck, 1765) | EP, PC, DR, CR, M, PI |
| Holocentrus rufus (Walbaum, 1792) | EP, AP, CR |
| Neoniphon marianus (Cuvier, 1829) | EP |
| Pomacanthus arcuatus (Linnaeus, 1758) | CR |
| Pomacanthus paru (Bloch, 1787) | CR |
| Family Pomacentridae | |
| Abudefduf saxatilis (Linnaeus, 1758) | EP, C, AP, LP, CR, PII |
| Chromis cyanea (Poey, 1860) | EP, DR, CR |
| Chromis multilineata (Guichenot, 1853) | DR |
| Microspathodon chrysurus (Cuvier, 1830) | EP, PC, DR, C, AP, LP, CR, M, PI, PII |
| *Stegastes adustus (Troschel, 1865) | |
| Stegastes diencaeus (Jordan & Rutter, 1897) | EP, PC, DR, C, AP, LP, CR, M, PI, PII |
| EP, PC, DR, C, AP, LP, CR, M, PI | |
| Stegastes leucostictus (Müller & Troschel, 1848) | EP, PC, DR, C, AP, LP, CR, M, PI |
| Stegastes partitus (Poey, 1868) | EP, PC, DR, C, AP, LP, CR, M, PI |
| Stegastes planifrons (Cuvier, 1830) | EP, PC, DR, C, AP, LP, CR, M, PI |
| Stegastes variabilis (Castelnau, 1855) | EP, PC, DR, C, AP, LP, CR, M, PI |
| Family Priacanthidae | |
| Heteropriacanthus cruentatus (Lacepède, 1801) | EP, PC, DR, PI, PII |
| Priacanthus arenatus (Cuvier, 1829) | EP |
| Family Scaridae | |
| Scarus guacamaia (Cuvier, 1829) | EP |
| Scarus iseri (Bloch, 1789) | EP, PC, DR, C, AP, LP, CR, M, PII |
| Scarus taeniopterus (Desmarest, 1831) | EP, PC, DR, C, AP, LP, M, PI, PII |
| Scarus vetula (Bloch & Schneider, 1801) | LP, M |
| Sparisoma atomarium (Poey, 1861) | EP, PC, DR |
| Sparisoma aurofrenatum (Valenciennes, 1840) | EP, PC, DR, C, AP, LP, CR, M, PI |
| Sparisoma chrysopterum (Bloch & Schneider, 1801) | EP, PC, DR, C, CR, PII |
| Sparisoma rubripinne (Valenciennes, 1840) | EP, C |
| Sparisoma viride (Bonnaterre, 1788) | EP, PC, DR, AP, LP, CR, M, PI, PII |
| Family Sciaenidae | |
| Equetus punctatus (Bloch & Schneider, 1801) | AP, CR, M, PII |
| Pareques acuminatus (Bloch & Schneider, 1801) | EP, PC, DR, PI |
| Family Scombridae | |
| Scomberomorus regalis (Bloch, 1793) | C, AP, LP |
| Family Serranidae | |
| Cephalopholis cruentata (Lacepède, 1802) | EP, PC, DR, AP, LP, DR, M, PI, PII |
| Cephalopholis fulva (Linnaeus, 1758) | EP, PC, DR, AP, PII |
| Epinephelus adscensionis (Osbeck, 1765) | EP, PC |
| Epinephelus guttatus (Linnaeus, 1758) | EP, PC, DR, C, AP, LP, CR, M, PI |
| Epinephelus striatus (Bloch, 1792) | EP, PC, DR AP, LP, PII |
| Hypoplectrus indigo (Poey, 1851) | EP |
| Hypoplectrus puella (Cuvier, 1828) | EP, PC, DR, C, PI, PII |
| Hypoplectrus unicolor (Walbaum, 1792) | EP, PC, DR, C, AP, LP, M, PI |
| *Mycteroperca bonaci (Poey, 1860) | DR |
| Mycteroperca tigris (Valenciennes, 1833) | EP, AP |
| Paranthias furcifer (Valenciennes, 1828) | PC, AP |
| Rypticus saponaceus (Bloch & Schneider, 1801) | EP, PC, DR, C, PII |
| Serranus baldwini (Evermann & Marsh, 1899) | EP, PII |
| Serranus tabacarius (Cuvier, 1829) | EP, PC, DR |
| Serranus tigrinus (Bloch, 1790) | EP, PC, DR, AP, LP M, PI |
| Family Sparidae | |
| Calamus bajonado (Bloch & Schneider, 1801) | EP, PC, DR |
| Calamus calamus (Valenciennes, 1830) | EP |
| Calamus penna (Valenciennes, 1830) | EP, PII |
| Calamus pennulata (Guichenot, 1868) | EP |
| Family Sphyraenidae | |
| Sphyraena barracuda (Walbaum, 1792) | EP, CR, PII |
| Order Pleuronectiformes | |
| Family Bothidae | |
| Bothus lunatus (Linnaeus, 1758) | EP, DR, C, AP, M, PII |
| Order Scorpaeniformes | |
| Family Dactylopteridae | |
| Dactylopterus volitans (Linnaeus, 1758) | EP, PC, DR, C, AP, LP, CR, M, PI |
| Family Scorpaenidae | |
| *Pterois volitans (Linnaeus, 1758) | EP, PC, DR, C, AP, LP, CR, M, PI |
| Scorpaena plumieri (Bloch, 1789) | EP, PC, DR |
| Order Syngnathiformes | |
| Family Aulostomidae | |
| Aulostomus maculatus (Valenciennes, 1837) | EP, PC, DR, C, AP, LP, CR, M, PI |
| Family Syngnathidae | |
| *Cosmocampus elucens (Poey, 1868) | DR |
| *Hippocampus reidi (Ginsburg, 1933) | EP, PC, DR, AP, M |
| * Halicampus crinitus (Jenyns, 1842) | DR |
| Order Tetraodontiformes | |
| Family Balistidae | |
| Balistes vetula (Linnaeus, 1758) | EP, CR, PII |
| Canthidermis sufflamen (Mitchill, 1815) | PC, PII |
| Melichthys niger (Bloch, 1786) | EP, PC, DR, PII |
| *Balistes capriscus (Gmelin, 1789) | PC, DR |
| Family Diodontidae | |
| Diodon holocanthus (Linnaeus, 1758) | EP, PC, DR, LP, PII |
| Diodon hystrix (Linnaeus, 1758) | EP, PC, DR, C, AP, LP, PII |
| Family Monacanthidae | |
| Aluterus schoepfii (Walbaum, 1792) | C, PII |
| Aluterus scriptus (Osbeck, 1765) | EP, PC, DR, AP, M, PI, PII |
| Cantherhines macrocerus (Hollard, 1853) | EP, DR |
| Cantherhines pullus (Ranzani, 1842) | EP |
| Monacanthus tuckeri (Bean, 1906) | M |
| Family Ostraciidae | |
| Lactophrys bicaudalis (Linnaeus, 1758) | EP, DR, AP, PII |
| Lactophrys trigonus (Linnaeus, 1758) | EP, PII |
| Lactophrys triqueter (Linnaeus, 1758) | EP, PC, DR, C, AP, LP, PI |
| Family Tetraodontidae | |
| Acanthostracion polygonia (Poey, 1876) | EP, PC, DR, AP, PII |
| Acanthostracion quadricornis (Linnaeus, 1758) | EP, PC, DR |
| Canthigaster rostrata (Bloch, 1786) | EP, PC, DR, C, AP, LP, PI |
| Chilomycterus antennatus (Cuvier, 1816) | EP, PC, DR, C, LP, CR, M, PI, PII |
The greatest number of species counted in the surveys belonged to the order Perciformes, with 105 species (70%), followed by the Tetraodontiformes with 18 (12.1%) and the Anguilliformes with 7 (4.7%). Determination of the species richness indicated that the families Serranidae (16 species; 10.7%), Haemulidae (12 species; 8.1%), and Pomacentridae (10 species; 6.3%) were the most speciose. The remaining families had lower levels of species richness (< 9). Haemulon and Lutjanus, with 7 species each, were the most species-rich genera, followed by Chaetodon, Stegastes, and Sparisoma with 5 species. The genera Calamus, Scarus, and Gymnothorax, were represented by 4 species (Fig. 2), while the rest (78 genera) were represented by not more than 3 species in the area (Fig. 3).

Figure 2 Some fish species belonging to species-rich genera in the SMASE: A) the Smallmouth grunt Haemulon chrysargyreum; B) the Princess parrotfish Scarus taeniopterus; C) the Foureye butterflyfish Chaetodon capistratus; D) the Green moray Gymnothorax funebris; E) the Threespot damselfish Stegastes planifrons, and F) the initial phase of the Redband parrotfish Sparisoma aurofrenatum. Photos: J. Calle-Triviño (C), B. Jiménez (A, D, E) and C. Cortés-Useche (B, F).

Figure 3 Some fish species in the SMASE: A) the Scrawled filefish Aluterus scriptus; B) the Longlure frogfish Antennarius multiocellatus; C) the Atlantic trumpetfish Aulostomus maculatus; D) the Longspine squirrelfish Holocentrus rufus; E) the Smooth trunkfish Lactophrys triqueter, and F) the Yellow stingray Urobatis jamaicensis. Photos: J. Calle-Triviño (A), B. Jiménez (B, D, E) and C. Cortés-Useche (C, F).
We documented 14 new records for the area of influence of the SMASE (9.4% of the total): Cosmocampus elucens Poey, 1868, Emblemariopsis sp. Longley, 1927, Haemulon parra Desmarest, 1823, Hemiramphus balao Lesueur, 1821, Hemiramphus brasiliensis Linnaeus, 1758, Hippocampus reidi Ginsburg, 1933, Lutjanus cyanopterus Cuvier, 1828, Halicampus crinitus Jenyns, 1842, Mycteroperca bonaci Poey, 1860, Ophioblennius macclurei Silvester, 1915, Seriola dumerili Risso, 1810, Stegastes adustus Troschel, 1865, Balistes capriscus Gmelin, 1789 and Pterois volitans Linnaeus, 1758 (Fig. 4).

Figure 4 Some newly recorded fish species in the SMASE: A) the Short pipefish Cosmocampus elucens; B) the Banded pipefish Halicampus crinitus; C) the Blackhead blenny Emblemariopsis sp.; D) the Dusky damselfish Stegastes adustus; E) the Longsnout seahorse Hippocampus reidi, and F) the Red Lionfish Pterois volitans. Photos: J. Calle-Triviño (C), B. Jiménez (B, F), A. Luis-Báez (E) and C. Cortés-Useche (D).
Discussion
The list of species registered in this work constitutes the first record for the reefs La Pared, Coralina, Atlantic Princess, Magallán, Punta Cacón, and Pepito I and II, all in the SE region of the Dominican Republic.
The species richness (150) is consistent with other studies, including the most represented families being Serranidae, Scaridae, and Haemulidae (Bustamante et al., 1998; Schmitt et al., 2002; Williams et al., 1983). Additionally, most of the species observed in this study (93.3%) are widely distributed in coral reefs of the tropical western Atlantic (Joyeux et al., 2001), including the lionfish, which originated in the Indo-Pacific Ocean and has widely invaded the Atlantic coast and Caribbean region (Schofield, 2009). Only 6 species present in the SMASE are on the Red List of the International Union for Conservation of Nature (IUCN), 5 species are assessed as vulnerable (Coryphopterus personatus Jordan & Thompson, 1905, Elacatinus prochilos Böhlke & Robins, 1968, Lachnolaimus maximus Walbaum, 1792, Lutjanus cyanopterus Cuvier, 1828, and Balistes capriscus Gmelin, 1789) and 1 as endangered (Epinephelus striatus Bloch, 1792).
This study recorded 150 species, which represents a 17% increase in the number of species compared with the most recent previous study, which identified 125 species (Schmitt et al., 2002). However, it is worth noting that the use of other sampling techniques, such as a detailed cryptic survey or rotenone collections, would increase the number of species recorded (Del Moral-Flores et al., 2013). Nonetheless, the RDT is useful for species detection, and Schmitt et al. (2002) concluded that it is a good sampling method for achieving a better representation of the fish community (more species) (Francisco-Ramos & Arias - González, 2013).
Local species richness may be related to environmental conditions and ecological relationships (e.g., topographic complexity), with different reefs providing different amounts of habitat availability and diversity in which fishes can feed and reproduce (Gratwicke & Speight, 2005). These reefs present rough surfaces with holes that serve as refuges, and they have substrates with complex structures. Furthermore, their proximity to mangroves and sea grasses may increase species richness (Rogers et al., 2014).
This study adds 14 new records of fishes present in coral reefs of the SE region of the coast of the Dominican Republic. A comprehensive inventory of fish species is critical for an assessment of the biodiversity and ecological relationships on reefs and will play a significant role in the conservation of endangered coral reef habitats.











 nova página do texto(beta)
nova página do texto(beta)


