Introduction
The harpacticoid copepod subfamily Dactylopusiinae was established by Lang (1936) to include the genus Diarthrodes Thomson, 1883 and has long been contained in the Thalestridae (Huys, 1990). This subfamily was recently raised to the family level by Willen (2000), as Dactylopusiidae Lang, 1936, and was recognized by Wells (2007) and Huys (2016). Boxshall & Halsey (2004) rejected it without providing further arguments (Gómez et al., 2008).
The genus Diarthrodes was created by Thomson (1883) to allocate D. novaezealandiaeThomson, 1883 from New Zealand; the date of publication of this name is May, 1883, not 1882 as assumed for some time. Subsequently, Lang (1936) incorporated the genera Arpacticus Baird, 1845, Westwoodia Dana, 1852, Pseudothalestris Brady, 1883, Pseudowestwoodia T. Scott, 1894, and Parawestwoodia Sharpe, 1910 to it. Members of this genus are marine forms and comprise about 43 valid species worldwide (Walter & Boxshall, 2017; Wells, 2007), 16 of which have been reported from the Americas: D. nobilis (Baird, 1845), D. pygmaeus (T. Scott & A. Scott, 1895), D. tumidus (Brady, 1910), D. intermedius (Scott T., 1912), D. nanus (Scott T., 1914), D. campbelliensisLang, 1948, D. cystoecus Fahrenbach, 1954, D. falcipes Marinoni, 1964, D. dissimilisLang, 1965, D. unisetosusLang, 1965, D. tetrastachyus Yeatman, 1976, D. parvulus Pallares, 1977, D. lilacinus Pallares, 1977, D. imitator Gómez, Chertoprud & Morales-Serna, 2008 , D. tripartitus Gómez, Chertoprud & Morales-Serna, 2008 , D. hexasetosus Gómez, Chertoprud & Morales-Serna, 2008 (Gómez et al., 2008; Lang, 1965; Pallares, 1977; Reid, 1998; Suárez-Morales et al., 2006). Only a single species (D. tetrastachyus) has been hitherto recorded from the Caribbean Sea (Suárez-Morales et al., 2006). In Colombia, the knowledge on the composition of this harpacticoid family is still lagging and there are no pre vious records of species of Diarthrodes (Fuentes-Reinés & Suárez-Morales, 2014).
A recent biological survey of the aquatic fauna of a bay on the northern Colombia coast yielded male and female specimens of 2 undescribed species of Diarthrodes. They are described in full and compared with its closest congeners following the morphologic criteria and species grouping proposed by Gómez et al. (2008) for this genus.
Materials and methods
Biological samples of shallow littoral habitats were obtained from Rodadero Bay, northern Colombia, western Caribbean Sea (11°14’10” N, 74°12’06” W) (August - October 2015), mainly at the inshore areas covered by vegetation (mangrove) and with an adjacent bank of oysters. Water salinity, pH, temperature were measured with a multiparameter WTW 350i equipment. Water samples were collected manually using a 25-l bucket at both littoral and limnetic habitats. Samples were filtered with a zooplankton net (mesh = 45 μm) and preserved in 70% ethanol. Copepods were processed for taxonomical identification including the examination of the whole specimen and dissection of selected appendages. Dissected appendages were mounted in slides with glycerine and sealed with Canada balsam. The specimens were measured in ventral position, from the anterior end of the rostral area to the posterior margin of the caudal rami. Drawings were made with the aid of a camera lucida mounted on an Olympus BX51 compound microscope equipped with Nomarski DIC. Some specimens were prepared for SEM examination with a JEOL LV 5900 microscope at the Universidad Autónoma de Aguascalientes (UAA), Mexico by following standard methods: dehydration in progressively higher ethanol solutions (60-100%), critical point drying, and gold-coating. The type specimens were deposited at the Centro de Colecciones Biológicas de Meiofauna, of Magdalena University, Colombia (CBUMAGMEI). Morphological terminology follows Huys and Boxshall (1991). The following abbreviations are used in the description: P1-P6 = first to sixth legs, EXP = exopod, ENP = endopod.
Descriptions
Order Harpacticoida G.O. Sars, 1903
Family Dactylopusiidae Lang, 1936
DiarthrodesThomson, 1883
Diarthrodes bodini sp. nov. (Figs. 1-5)
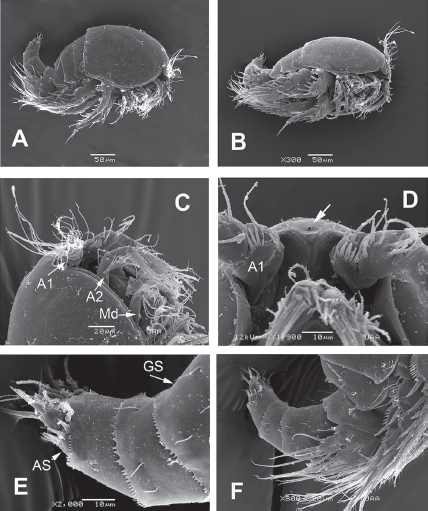
Figure 1 Diarthrodes bodini sp. nov. from Colombia. SEM-prepared female: A, habitus, lateral view; B, same, semi-lateral view; C, cephalic area, lateral view showing antennules (A1), antennae (A2) and mandibles (Md); D, cephalic area ventral view showing rostral area; E, urosome, lateral view, showing anal somite (AS) and crenulate margins; F, same, showing position and armature of leg 5.
Female. Habitus robust, globose in lateral (Fig. 1A) and ventral views (Fig. 1B), with short urosome representing ca. 25% of total length; body widest at posterior margin of cephalothorax. Total body length of holotype measured from anteriormost end of rostrum to posterior margin of caudal rami in dorsal view, 322 μm; length range: 322-357 μm, average length: 340 μm (n = 5). Rostrum wide-based, rounded, not conspicuous in lateral view (Fig. 1C, D), with single large medial pore (arrow in Fig. 1D).
Prosomites with smooth posterior margins, urosomites with crenulate margins (Fig. 1E, F). Genital double-somite representing 50% of urosome, ornamented with lateral and dorsal sensillae (Fig. 1E). Anal somite with set of ventral spinules along insertion of caudal rami (Figs. 1E, 2E). Genital field as in Figure 3E; P6 represented by short seta arising from small, oblong plate (Fig. 3E).
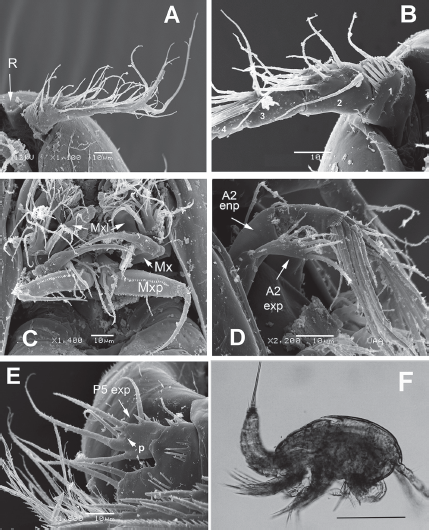
Figure 2 Diarthrodes bodini sp. nov. from Colombia. SEM-prepared female: A, left antennule, ventral view; B, same, showing armature of proximal segments; C, mouthparts, ventral view showing maxillules (Mxl), maxillae (Mx), and maxillipeds (Mxp); D, antenna showing exopod and endopod; E, fifth leg, ventral view; F, male specimen, habitus, lateral view. Scale bar: F = 100 μm.
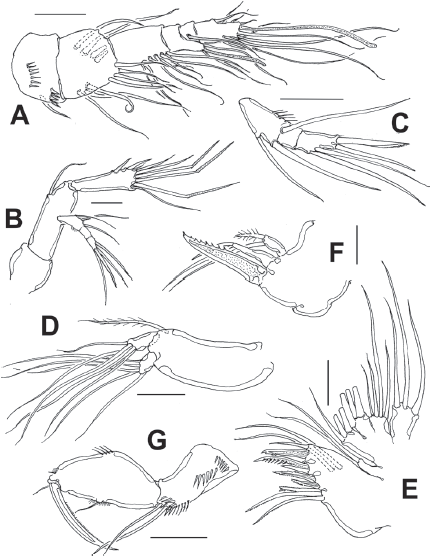
Figure 3 Diarthrodes bodini sp. nov. from Colombia, adult female: A, antennule; B, antenna; C, maxilla; D, maxilliped; E, urosome, ventral view; F, fifth leg. Scale bars: A-F = 10 μm.
Caudal rami short, about 3 times as wide as long (Fig. 1E), armed with 7 elements; elements I, II and III situated at outer distal corner; seta I slender, relatively long, ventral to stout, spiniform element II; seta III slender, about as long as element II; setae IV and V normal in width and length; seta VI shorter than seta III; dorsal seta VII shortest, situated near insertion site of seta VI (Fig. 3E).
Antennule (Figs. 2A, B, 3A) 7-segmented, with aesthetascs on fourth and distal segments; surface of first segment with set of 5-6 spinules on inner surface, succeeding segments lacking spinular ornamentation. Armature formula as: 1-(1), 2-(11), 3-(8), 4-(2+ae), 5-(1), 6(3), 7-(9+ acrothek). Second segment barrel-shaped, longest of antennule.
Antenna (Figs. 2D, 3B) with elongate allobasis elongate, lacking trace of division between basis and first endopodal segment, armed with single abexopodal seta almost reaching distal margin of free endopodal segment. EXP 1-segmented (arrow in Fig. 2D), with 5 lateral setae, and 1 seta and 1 spine on apical position. Free endopodal segment with 2 short lateral spines plus slender seta, apically 3 geniculate setae, plus 4 shorter slender setae, distal margin ornamented with row of spines.
Maxillule (Fig. 2C (Mxl)) with praecoxal arthrite armed with 2 relatively thick, long surface setae. Coxa armed with 2 setae, basis with 6. ENP and EXP 1-segmented, small, with 3 setae each.
Maxilla (Figs. 2C, 3C) with syncoxa represented by 3 endites, each armed with single seta; distalmost endite longest. Basis produced into long, relatively slender serrate claw.
Maxilliped (Figs. 2C, 3D) with basis ornamented with distal row of spinules at insertion of 2 equally long apical setae. ENP1 with row of small spinules along inner margin and additional row along medial surface and with single seta halfway of inner margin. ENP2 with long slender claw.
Leg 1 (Fig. 4A) with wide coxa, ornamented with inner row of long spinules. Basis with pair of pores on medial position and distal row of 6-7 strong spinules at insertion of endopod. Inner spine stout, relatively long, outer spine short. EXP 2-segmented, barely reaching proximal half of ENP1; first segment with single outer serrate spine, second segment with outer serrate spine plus long apical serrate spine with 3 unequally long setae. ENP 3-segmented; first segment about 4.3 times as long as wide, with plumose seta inserted halfway of inner margin, second segment as long as third segment, the latter with 2 uniserially serrate spines and single short, slender apical seta.

Figure 4 Diarthrodes bodini sp. nov. from Colombia, adult female: A, leg 1; B, leg 2; C, leg 3; D, leg 4. Scale bars: A-D = 25 μm.
Leg 2 (Fig. 4B) with inner distal corner of basis with acute process, distal margin unornamented. Rami 3-segmented. EXP1 and EXP2 each with single inner seta; EXP3 with 7 elements (4 serrate spines, 3 setae). ENP not reaching distal margin of EXP3; inner margin of ENP1 with short stout spiniform element; ENP2 with 2 inner setae; ENP3 with 5 setal elements.
Leg 3 (Fig. 4C) with smooth coxa, basis with outer basipodal seta, distal margin unornamented. EXP and ENP 3-segmented. EXP1 and EXP2 each with single inner seta, EXP3 armed with 8 setal elements. ENP reaching about halflength of EXP3. ENP1 with single inner seta, ENP2 with 2 subequally long inner setae; ENP3 with 6 setal elements.
Leg 4 (Fig. 4D) with 3-segmented EXP and ENP. EXP as in P3. Endopod reaching proximal 1/3 of EXP3. ENP1 and ENP2 each with single inner seta; ENP3 with 5 setal elements.
Setal formula of legs 1-4 as:
| leg 1 | leg 2 | leg 3 | leg 4 | |
| EXP | I-0;I,1,I,2 | I-1;I-1;III,I1,2 | I-1;I-1;III,I1,3 | I-1;I-1;II,II1,3 |
| ENP | 0-1;0-0;II,1 | 0-1;0-2;I,2,2 | 0-1:0-2;I,2,3 | 0-1;0-1;I,2,2 |
Leg 5 (Figs. 1F, 2E, 3F) with baseoendopodal lobe ornamented with 2 rows of spinules on medial surface; lobe reaching about halflength of exopodal lobe, armed with 5 elements, 2 medial elements longest, with 2 small spines between insertion points (arrowed in Fig. 3F). EXP oblong, armed with 5 setal elements; spines at insertion of 2 outermost elements and large subdistal pore (arrowed (p) in Fig. 2E).
Male. General shape of habitus slenderer than in female (Fig. 2F); total body length range: 308-336 μm, average: 322 μm (n = 7), allotype length: 322 μm. Urosome as in female dorsally, with crenulate posterior margins except for urosomites with smooth margins and rows of spinules on ventral surface; fifth pedigerous somite with preanal somite with single short row of 6-7 spinules on ventral surface; anal somite with large spinules on ventral surface at insertion of caudal rami (Fig. 5E). Caudal rami and setae as in female.
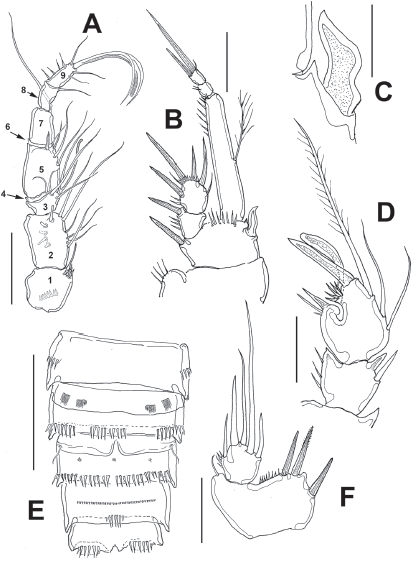
Figure 5 Diarthrodes bodini sp. nov. from Colombia, adult male: A, antennule; B, leg 1; C, detail of inner basal spine; D, modified leg 2 ENP2; E, urosome, ventral view, fifth legs omitted; F, fifth leg. Scale bars: A, B, D = 20 μm, C = 10 μm, E = 50 μm.
Antennule (Fig. 6A) 9-segmented, haplocer, with aesthetasc on third segment, acrothek on ninth segment; first segment with distal and medial rows of spinules, succeeding segments lacking spinular ornamentation. Observed armature formula as: 1(1)-2(11)-3(4+ae)-4(2)-5(4)- 6(1)-7(1)-8(1)-9(7+acrothek). Antennae and mouthparts as in female.
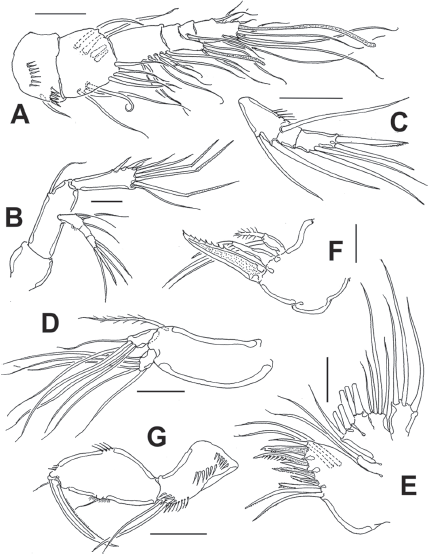
Figure 6 Diarthrodes gomezi sp. nov. from Colombia, adult female holotype: A, antennule; B, antenna; C, antennary exopod; D, mandible palp; E, maxillule; F, maxilla; G, maxilliped. Scale bars: A-G = 20 μm.
Leg 1 (Fig. 5B) as in female, except for dimorphic, leaf-like inner spine (Fig. 5C) and ENP1 length/width which is about 5.6 as long as wide.
Leg 2 (Fig. 5D) basis with inner distal corner with acute process. EXP 3-segmented, EXP1 and EXP2 each armed with inner seta; EXP3 with 7 setal elements. ENP barely reaching beyond distal margin of EXP2; ENP1 with inner spine. ENP2 sexually dimorphic, with 3 inner setae, proximal one short, subdistal longest (Fig. 5D). Proximal outer process represented by large spine with smaller adjacent spiniform elements; medial process outer subdistal process represented by cluster of 5 short spiniform elements; apical processes including strong spiniform element plus tongue-like modified setae (Fig. 5D).
Legs 3 and 4 as in female.
Leg 5 (Figs 5D, 6D) baseoendopodal lobe with row of small spinules on distal margin; lobe reaching about 1/3 length of exopodal lobe, armed with 3 subequally long spiniform elements. EXP subquadrate, armed with 5 setal elements (Fig. 5F).
Taxonomic summary
Type material. Adult female holotype (CBUMAGMEI: 0008), male allotype (CBUMAGMEI: 0009), Rodadero Bay, Magdalena, Colombia, coll. J. Fuentes-Reinés, August-October 2015. Paratypes: 3 males (CBUMAGMEI: 0010) and 3 females (CBUMAGMEI: 0011) same locality and collector. Two adult females, 2 adult males from same locality, date, and collector, specimens dissected, semi-permanent slides (ECO-CHZ 00000).
Type locality. Rodadero Bay, northern Colombia (11°14’10” N, 74°12’06” W).
Etymology. The species is named after Dr. Philippe Bodin (CNRS, Brest, France) for his many contributions to the knowledge of the marine Harpacticoida, particularly in the Atlantic Ocean.
Remarks
Gómez et al. (2008) divided this genus into 7 groups based on the number of segments in the antennary exopod and the ramal segmentation of leg 1. Diarthrodes bodini sp. nov. belongs to group II (1-2-3) by the combination of the 1-segmented antennary exopod (Fig. 3B), a 2- segmented P1EXP (Fig. 4A), and 3-segmented P1ENP (Fig. 4A). This group now contains 10 species: D. novaezealandiae, D. nanus, D. aegideus (Brian, 1927), D. gurneyiLang, 1948, D. unisetosus, D. drachi Bodiou, 1974, D. parvulus, D. latisetosus Chislenko, 1978, D. imitator, and D. bodini sp. nov. Within this group, the new species D. bodini, D. imitator from the Mexican Pacific, D. unisetosus from California, D. latisetosus from Japan, and D. aegideus from Italy diverge from the other congeners in this group by sharing 2 inner setae on the P2ENP2 (Fig. 4B). Of these species, D. bodini most closely resembles D. latisetosus in the armature formula of P1-P4, antennule segmentation and the armature and structure of the antenna. These 2 species diverge in the following characters: 1) the inner spine of P1 ENP1goes well beyond the distal margin of the P2 ENP3 in D. latisetosus (Chislenko, 1978: fig. 4.6) whereas in D. bodini it is shorter, barely reaching beyond the distal margin of bearing first ramal segment (Fig. 4A); 2) in D. latisetosus the P1 ENP1 length/width ratio is about 3.4 (Chislenko, 1978: fig. 4.6) vs. 4.3 in D. bodini (Fig. 4A); 3) in D. latisetosus the third and fourth urosomites have ventral rows of spinules with smooth margins (Chislenko, 1978: fig. 9), whereas the corresponding somites lack ventral spinules and have crenulate margins in D. bodini (Figs. 1E, F, 3E); 4) in the new species D. bodini the P3ENP reaches the insertion of the inner proximal seta of P2 EXP3 (Fig. 4C) whereas in D. latisetosus P3ENP this ramus is shorter, barely reaching the distal margin of P2 EXP2 (Chislenko, 1978: fig. 5.2 ); 5) likewise, in D. bodini the P4ENP is clearly longer than in D. latisetosus; it reaches about the proximal1/3 of P4EXP3 (Fig. 4D) whereas in the latter species, the P4ENP is clearly shorter, reaching midlength of P4EXP2 (Chislenko, 1978: fig. 5.3 ), 6) in the new species the female P5ENP is ornamented with strong spines at insertion of 2 outer setae (Figs 2E, 3F), whereas in D. latisetosus these accessory spines are absent (Chislenko, 1978: fig. 4.7); 7) the female P5 ENP has a pair of spiniform apical elements (arrowed in Fig. 3F) which are absent in D. latisetosus (Chislenko, 1978: fig. 4.7); 8) in the new species the setal elements on the female P5 EXP are uniformly wide, whereas D. latisetosus has a remarkably thicker element on P5 ENP (Chislenko, 1978: fig. 4.7); 9) the seminal receptacle is curved, with parallel lateral bean-like sacs at each side in D. latisetosus (Chislenko, 1978: fig. 4.9) whereas in D. bodini lateral sacs have a rounded shape in a different position (Fig. 3E); 10) the male modified basipodal spine of P1 is curved, apically acute in D. bodini and straight, apically rounded in D. latisetosus (Chislenko, 1978: fig. 5.7); 11) in D. latisetosus the male P2ENP2 lack a medial notch and its ornamentation is reduced, lacking additional spiniform elements (Chislenko, 1978: fig. 5.6) whereas a strong notch and modified setal elements including a cluster of 5 spines are present in D. bodini (Fig. 5D), 12) the male antennule is 7-segmented in D. latisetosus (Chislenko, 1978: fig. 5.10) vs. 9-segmented in D. bodini (Fig. 5A).
Diarthrodes gomezi sp. nov. (Figs. 6-9)
Female. Habitus resembling that of D. bodini, robust, globose in lateral and ventral views (Fig. 8D), with urosome representing ca. 30% of total length; body widest at posterior margin of cephalothorax. Total body length of holotype measured from anteriormost end of rostrum to posterior margin of caudal rami in dorsal view, 560 μm. Rostrum wide-based, rounded, with large medial pore (Fig. 7F).
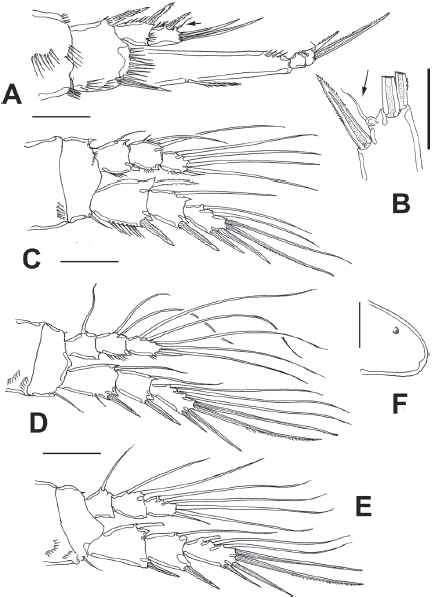
Figure 7 Diarthrodes gomezi sp. nov. from Colombia, adult female holotype: A, leg 1; B, detail of EXP3, leg 1, small apical seta arrowed; C, leg 2; D, leg 3; E, leg 4; F, rostrum, semi-lateral view. Scale bars: A, C-E = 25 μm, B, F = 10 μm.
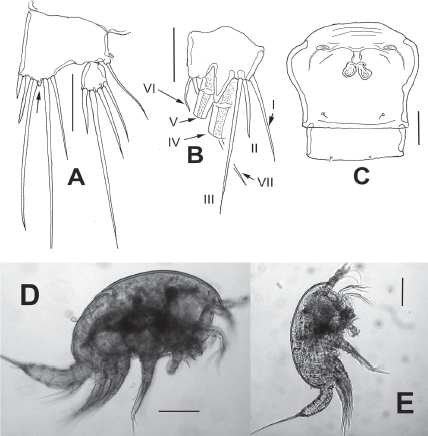
Figure 8 Diarthrodes gomezi sp. nov. from Colombia, adult female holotype: A, fifth leg, medial protuberance arrowed; B, caudal ramus, ventral, showing caudal setae I-VII; C, genital double and postgenital somites, ventral view; D, habitus, lateral view; E, adult male allotype, lateral view. Scale bars: A-C = 20 μm, D, E = 100 μm.
Prosomites and urosomites with smooth posterior margins. Genital double-somite representing 50% of urosome, ornamented with pair of ventral sensillae near posterior margin; genital field as in Fig. 8C, with sixth leg represented by oblong fused plate armed with single short seta. Anal somite with set of ventral spinules along insertion of caudal rami.
Caudal rami short, about 3 times as wide as long (Fig. 8B), with 7 elements; elements I, II and III situated at outer distal corner; seta I slender, longer than seta II; seta III slender, long about twice as long as element II; setae IV and V normal in width and length; seta VI short, slender. Dorsal seta VII long, situated near insertion site of seta VI (Fig. 8B).
Antennule (Fig. 6A) 6-segmented, with aesthetascs on fourth and sixth segments; surface of first segment with 2 rows of spinules; succeeding segments lacking spinular ornamentation. Armature formula as: 1-(1), 2-(11), 3-(8), 4-(2+ae), 5-(1), 6-(9+ ae). Second segment longest of antennule.
Antenna (Fig. 6B, C) with elongate allobasis, armed with single abexopodal seta almost reaching midlength of succeeding free endopodal segment. EXP 3-segmented, armed with 2-1-4 setal elements on each segment; distal elements represented by stout seta and serrate spine. Free endopodal segment elongate, with 2 short lateral spines and slender seta, distally 3 geniculate setae, plus 3 shorter slender non-geniculate setal elements.
Mandible (Fig. 6D) with strong gnathobase, gnathal blade with irregular edge. Coxa-basis elongate, armed with single seta; palp with 1-segmented EXP and ENP, armed with 5 and 4 long setae, respectively.
Maxillule (Fig. 6E) with praecoxal arthrite armed with 2 relatively long proximal setae, 7 spiniform, distal elements plus 2 long medial setae. Coxa reduced, with 2 slender setae, basis with 6 setal elements. ENP and EXP 1-segmented, subrectangular, armed with 4 and 3 setae, respectively.
Maxilla (Fig. 6F) with syncoxa bearing 2 endites armed with 2 setae each; distalmost endite longer than proximal. Basis produced into strong, serrate claw.
Maxilliped (Fig. 6G) basis with rows of long spinules, 1 proximal, 1 medial and 1 at insertion of 2 equally long apical naked setae. ENP1 with row of small spinules along inner margin near insertion of short inner seta; additional row on opposite position. ENP2 represented by long slender claw with small seta at insertion point and serrate inner margin.
Leg 1 (Fig. 7A) with wide coxa, ornamented with inner and outer rows of spinules plus 2 rows on medial margin. Basis with rows of long spinules on inner margin; distal row of 8-9 long, slender spinules at insertion of endopod. Inner and outer basipodal spines stout, relatively long. EXP 2-segmented, barely reaching proximal 1/3 of ENP1; first segment with single outer serrate spine, second segment with outer serrate spine plus short slender subapical seta (arrowed in Fig. 7A, B) and 2 apical unequally long spines. ENP 3-segmented; ENP1 about 4.2 times as long as wide, with short plumose seta inserted at proximal 1/3 of inner margin, ENP2 as long as ENP3, the latter with 2 uniserially serrate spines, outer one about halflength than inner one, plus short, slender apical seta.
Leg 2 (Fig. 7C) coxa with row of 5 spinules on outer corner. Inner distal corner of basis with short acute process, distal margin with row of small spinules at insertion of ENP. Rami 3-segmented. EXP1 and EXP2 each with single inner seta; EXP3 with 6 elements (4 spines, 2 setae). Endopod short, barely reaching distal margin of EXP2; inner margin of ENP1 with short seta; ENP2 with 2 inner setae; ENP3 with 5 setal elements.
Leg 3 (Fig. 7D) coxa with 2 rows of spinules on outer corner, basis with single outer seta, distal margin unornamented except for minute spinule on distal inner margin near insertion of ENP. EXP and ENP 3-segmented. EXP1 and EXP2 each with single inner seta, EXP3 armed with 7 setal elements. ENP barely reaching distal margin of EXP2. ENP1 with single inner seta, ENP2 with 2 unequally long inner setae; ENP3 with 6 setal elements.
Leg 4 (Fig. 8E) with 3-segmented EXP and ENP. Coxa and basis and EXP as in leg 3 except for shorter basipodal seta. ENP not reaching distal margin of EXP2. ENP1 and ENP2 with 1 and 2 inner setae, respectively; ENP3 with 5 setal elements. EXP3 with 7 setal elements.
Setal formula of legs 1-4 as:
| leg 1 | leg 2 | leg 3 | leg 4 | |
| EXP | I-0;I,1,II | I-1;I-1;II,II,2 | I-1;I-1;II,II,3 | I-1;I-1;II,II,3 |
| ENP | 0-1;0-0;II,1 | 1-1;0-2;I,2,2 | 0-1:0-2;I,2,3 | 0-1;0-2;I,2,2 |
Leg 5 (Fig. 8A) baseoendopodal lobe with outer row of small spinules at insertion of basal seta; lobe reaching beyond halflength of exopodal lobe, armed with 5 elements, 2 medial elements longest, with small protuberance between insertion points (arrowed in Fig. 8A). EXP subquadrate, armed with 4 setal elements, innermost thickest, medial one longest.
Male. General shape of habitus as in female, although slightly slenderer (Fig. 8E); total body length of allotype: 462 μm (n = 1). Urosome longer than in female, genital somite with ventral pair of plates each armed with short, inconspicuous single seta (Fig. 9H). Caudal rami and setae as in female.
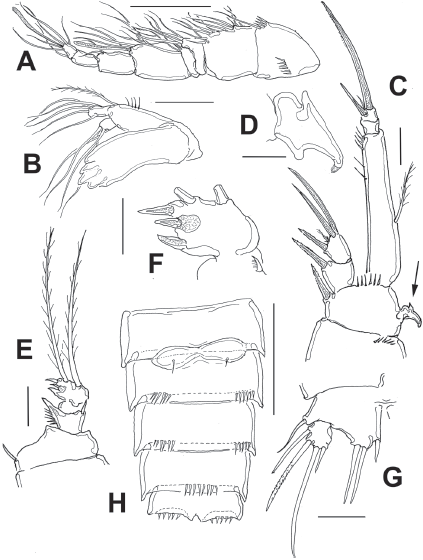
Figure 9 Diarthrodes gomezi sp. nov. from Colombia, adult male allotype: A, antennule; B, mandible and palp; C, leg 1; D, detail of leg 1 basipodal modified seta; E, modified leg 2 ENP; F, detail of leg 2 ENP 2; G, leg 5; H, urosome, ventral view, fifth legs omitted. Scale bars: A = 50, B, C, E, G = 20 μm, F, D = 10 μm.
Antennule (Fig. 9A) 8-segmented, haplocer, with aesthetasc on fifth segment; first segment with 2 rows of spinules, succeeding segments lacking spinular ornamentation. Armature formula not fully discernible: 1-(1), 2-(12), 3-(4), 4-(3), 5-(5+ae), 6-(2), 7-(0), 8(6+ae). Antennae and mouthparts as in female except mandible with 1 seta less on exopodal lobe (Fig. 9B).
Leg 1 as in female, except for dimorphic, modified inner spine (arrow in Fig. 9C, D).
Leg 2 (Fig. 9E, F) modified. ENP 2-segmented, ENP1 with inner spine, outer margin produced into acute process. ENP2 sexually dimorphic, with tripartite medial outer process; each lobe armed with short apical spiniform element; distal margin with 2 long setae.
Legs 3 and 4 as in female.
Leg 5 (Fig. 9G) baseoendopodal lobe smooth; lobe reaching about halflength of exopodal lobe, armed with 3 spiniform elements, innermost shortest. EXP subquadrate, armed with 4 setal elements.
Taxonomic summary
Type material. Female holotype (CBUMAGMEI: 0012-1-8), male allotype (CBUMAGMEI: 0013-1-8), zooplankton sample, August-October 2015, Rodadero Bay, northern Colombia (11°14’10” N, 74°12’06” W), collector J.M. Fuentes-Reinés. Specimens partially dissected, semi-permanent slides.
Type locality. Rodadero Bay, northern Colombia (11°14’10” N, 74°12’06” W).
Etymology. The species is warmly dedicated to Dr. Samuel Gómez (Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México (UNAM), Mexico) for his contributions to the taxonomic knowledge of this genus of harpacticoid copepods.
Remarks
Diarthrodes gomezi can be placed in group VII (3-2-3) (sensuGómez et al. 2008) by its 3-segmented antennary exopod, 2-segmented P1EXP and 3-segmented P1ENP. This group comprises 13 species: D. minutus (Claus, 1863), D. ponticus (Kriczagin, 1877), D. major (Scott & Scott, 1895), D. assimilis (Sars, 1906), D. roscoffensis (Monard, 1935), D. feldmanni Bocquet, 1953, D. cystoecus, D. orientalis Apostolov, 1975, D. tetrastachyus, D. septentrionalis, D. lilacinus, D. brevipes Wells & Rao, 1987, and D. gomezi sp. nov. Of these, D. ponticus and D. cystoecus have been reported to display a high morphologic variability in different geographic areas (Apostolov, 1973; Brian, 1921, 1928; Farran, 1913; Monard, 1928; Sewell, 1940; Thompson & Scott, 1903; Wells & Rao, 1987); thus, they could represent a species complex.
Diarthrodes gomezi is unique among related congeners in possessing P3-P4EXP3 with 7 elements (Fig. 7D, E). Among species in Diarthrodes group VII (sensu Gómez et al., 2008), D. gomezi most closely resembles D. minutus in the following characters: 1) antennule segmentation, 2) number of setae and antennary segmentation, 3) setal formula of P1 and P2. However, the spine/seta formula of P1EXP2 of D. minutus is ambiguous because Claus (1863) and Sars (1906) illustrated P1EXP2 with 5 elements, while Apostolov and Marinov (1988) showed the P1EXP2 having 4 setal elements only. The new species D. gomezi can be distinguished from D. minutus by: 1) the P1ENP1 inner seta is remarkably long, it reaches the distal margin of P1ENP2 (Lang, 1948: Abb. 218 6P1) whereas in D. gomezi it is clearly shorter, barely reaching beyond distal 1/3 of same segment (Fig. 7A); 2) in D. gomezi the P1EXP2 is armed with 4 elements (Fig. 7A) vs. 5 in D. minutus (Lang, 1948: Abb. 218-6P1female; Wells 2007: p. 373); 3) the small apical seta on P1ENP3 is clearly longer in D. minutus (Lang, 1948: Abb. 218, 6P1) than in D. gomezi, which shows a short, inconspicuous seta (arrowed in Fig. 7A); 4) the male P2ENP2 has 3 short, strong spines and 2 long apical setae in D. gomezi (Fig. 9E) vs. a distinct armature represented by 2 long spines and 3 setae in D. minutus (Lang, 1948: Abb. 218-6P2ENP male), 5) in D. gomezi the male P5EXP is armed with 4 setal elements (Fig. 9G) vs. 5 setae in D. minutus (Lang, 1948: Abb. 218, 6P5 male).
The new species, Diarthrodes bodini and D. gomezi are currently known exclusively from the type locality, Rodadero Bay, on the Caribbean coast of Colombia. They were found in the littoral zone in an area covered by mangrove with a small adjacent bank of oysters at a depth of 0.70 m and water temperature ranging between 30 and 32 ºC, salinity 36.1 psu, pH 8.3. Most species of Diarthrodes are strictly benthic and may be eurytopic (Bodin & Le Guellec, 1992). Several species are known to live as epi-or endophytic forms in association with algae (Shimono et al., 2007); they display a unique feeding strategy, by segregating a mucus-trap to agglutinate bacteria. The 2 described species of Diarthrodes were found in the plankton net but it is likely that more species of this genus could be recovered by sampling the invertebrate community dwelling among algal patches (Alper et al., 2010; Curvelo & Corbisier, 2000). Further research on the biology, life cycle and feeding of the Colombian species, and their possible association with algae, are clearly interesting research subjects regarding this genus in the surveyed area.











 nueva página del texto (beta)
nueva página del texto (beta)


