Introduction
Because of the alarming disappearance of biodiversity in tropical rainforests resulting from the expansion of the agricultural frontier (Morris, 2010), understanding the processes of ecological restoration and assessing their impact on the recovery of species diversity and function is one of the most urgent tasks to buffer negative effects of land use change. Natural succession may occur relatively fast in disturbed areas close to conserved tropical rainforests, recovering biodiversity and ecological interactions without human intervention (Falcão et al., 2015; Kattan et al., 2006). However, restoration intervention may be needed in highly degraded areas, after many years of cattle grazing and in places far away from conserved forest (Myster, 2004).
Monitoring of restoration programs generally consists in evaluating vegetation recovery, assuming that animal populations eventually recover (Majer, 2009). However, it is known that the response to the recovery of plant cover depends on the life history traits of each species, such as size, dispersal and habitat preferences. In the present study we evaluated the dung beetle community and function in a restored area in a highly fragmented tropical rainforest landscape that is now dominated by pastures. Dung beetles (Scarabaeinae) are considered good indicators of habitat quality since they are taxonomically and ecologically well known, have specialization to certain habitat requirements, provide early warning of environmental change and are easily surveyed (Dale & Beyeler, 2001; Favila & Halffter, 1997). In addition, dung beetles are conspicuous components of most terrestrial ecosystems, contributing to soil fertility and aeration, nutrient recycling, secondary seed dispersal and parasite control in vertebrate populations (Nichols et al., 2008). Because of its dependence on excrement of vertebrates, in particular of mammals for feeding and reproducing, dung beetles are highly sensitive to environmental change and their conservation is considered priority (Spector, 2006).
Despite dung beetle assemblages in Central Amazonia have recovered rapidly after forest fragmentation (Quintero & Roslin, 2005), more degraded landscapes keep losing diversity, even after important local efforts of conservation or restoration (Escobar et al., 2008; Spake et al., 2015). Although dung beetle assemblages have the potential to recover with restoration in some ecosystems (Barnes et al., 2014; Bitencourt & da Silva, 2016; Davis et al., 2003; Derhé et al. 2016), sometimes they may not recover completely (Hernández et al., 2014; Medina et al., 2002) or do not recover at all (Steenkamp & Chown, 1996), even in 18 year-old restoration plantings (Audino et al., 2014), suggesting that restoration may take longer times before recovering dung beetle communities.
Here we measured dung beetle diversity and dung removal in a restored area consisting of experimental fenced plots that were excluded from cattle disturbance 10 years ago, some of which were planted with native tree species. As reference habitats, we used areas in the nearest conserved forest and neighbor pastures where cattle are still allowed to graze. We expected that restoration would help to recover dung beetle diversity and dung removal function and that diversity, number of individuals and biomass of dung beetles would decrease from forest to pastures. We propose future directions for practical conservation efforts that consider dung beetle diversity and function as indicators of restoration success at landscape scale.
Materials and methods
The study was carried out during the rainy season in July 2015 in Los Tuxtlas region, Veracruz, Mexico (18°35’51” - 18°35’36” N, 95°06’6” - 95°06’7” W) (Fig. 1). Los Tuxtlas is the northernmost remnant of tropical rainforest (Dirzo, 1992), with an altitude ranging from 0 to 1,700 m above the sea level, a mean annual temperature of 26 °C and a mean annual precipitation ranging from 1,500 to 4,500 mm (Guevara et al., 1999). The Los Tuxtlas region has been exploited for cattle grazing since the 1950´s (Guevara et al., 1999) and in 1998 was declared a Biosphere Reserve, with a protected area of 155,122 ha., of which 81% fall in the anthropic landscape where cattle pastures, crops, and forest fragments predominate, and 3 nuclear zones which occupy 19% (Guevara et al., 2004). The soils developed from volcanic ash of basaltic and basaltic-andesitic composition, and comprise alfisols and andisols in low and high altitudes respectively (Flores-Delgadillo et al., 1999).
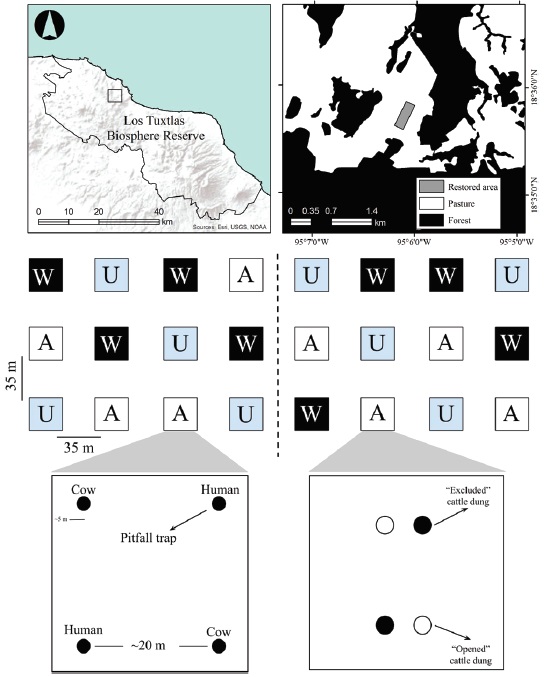
Figure 1 Restoration experiment in a Tropical rainforest in Los Tuxtlas Biosphere Reserve, Mexico. The restored area is located inside a matrix of pasture surrounded by forest (top). The restored area comprises 24 excluded plots of 3 different restoration treatments (W = planted with wind-dispersed plants, A = planted with animal-dispersed plants, and U = unplanted). Half of the restored area was used for capturing dung beetle abundance and diversity (bottom left), while the other half was used to evaluate removal of cattle dung (bottom right). Modified from (Arroyo-Rodríguez et al., 2008; De la Peña-Domene et al., 2013).
In this area, on an eroded hillside pasture located at an elevation of 180-260 m asl, a restoration experiment was established in 2006 (10 years ago) to test 3 restoration treatments. The experiment consists in 24 plots of 30 × 30 m that were excluded from cattle with living fences of Gliricidia sepium (Fabaceae); plots were arranged in a 3 × 8 grid, with a distance of 35 meters from each other (Fig. 1). The 24 plots represent a mosaic of 8 replicates of 3 different restoration treatments: 1) ‘animal’: plantings including 12 seedlings of 12 tree species dispersed by animals (birds, bats, terrestrial mammals) separated from each other by 2 m; 2) ‘wind’: plantings including 12 seedlings of 12 species dispersed by wind and 3) unplanted plots that resemble natural succession (De la Peña-Domene et al., 2013) (Fig. 1). The site was analyzed as a single restored area comprising 7.76 ha (De la Peña-Domene et al., 2014). As control habitats, we used 8 sites of the neighbor pasture where cattle are allowed to graze, located at least 100 meters away from the experimental plots and 50 m from each other to avoid interference among traps (Da Silva & Hernández, 2015; Larsen & Forsyth, 2005), and 8 forest sites in the Reserve of Los Tuxtlas Biological Station (LTBS, Universidad Nacional Autónoma de México), also separated by at least 50 from each other. The experimental plots have shown important signs of recovery in terms of tree density, canopy cover ( Martínez-Garza, Bongers et al., 2013), seed rain (Howe et al., 2010) and recruitment of tree species dispersed by birds and bats (De la Peña-Domene et al., 2014); birds and mammals have been seen (H. Howe, personal communication), suggesting that dung beetles may recognize plots as foraging sites. Plots also showed rapid signs of recovery in soil nutrients (Tobón et al., 2011), which could also improve conditions for dung beetles. Plots on the grid are within 500-1,200 m of the edge of the LTBS and > 90 m from the closest secondary forest (Howe et al., 2010).
One half of the plots were used to quantify dung beetle diversity with baited pitfall traps and the other half were used to quantify removal of cattle dung (Fig. 1). To quantify diversity, we put 4 pitfall traps (500 mL, 11 cm diameter, 8 cm depth, buried at ground level) in each plot, 2 of them baited with ca. 50 g of human dung and the other 2 baited with ca. 100 g of cattle dung which, according to the ranch owner, was free of parasiticides for at least 2 months. The traps contained ca. 250 mL of local soil as substrate in order to keep collected beetles alive and release them at the end of the experiment. These 2 dung types have been used in previous experiments to collect the whole diversity of dung beetles (Favila & Díaz, 1997). Traps inside each plot (restored area and reference habitats) were separated from each other 20 meters and had a minimum distance of 5 m from the fence (Fig. 1). Bait was replaced every 24 hours during 3 consecutive days, a sufficient time to collect most dung beetle biodiversity in the tropics (see similar procedures in Audino et al., 2014; Barnes et al., 2014; Escobar et al., 2008). The specimens were identified at species level by AD-R in the Laboratory of LTBS. Biomass of each species was calculated as the average dry mass of 20 individuals (± 1 mg) see (Díaz & Favila, 2009). Beetles were released alive at each site at the end of the fieldwork. Voucher specimens of all species (at least 1 copy) were collected and deposited in the collection of the Red de Ecoetología, Instituto de Ecologia, A.C.
In order to quantify dung removal, we placed 4 samples of 1 kg homogenized cattle dung in each plot, at ground level, being 2 of them opened to coprophagous fauna, and the other 2 excluded with 1.5 mm mesh as controls for desiccation, placed 30 cm from the open samples (Fig. 1). Dung samples were placed in a 5 L plastic bucket filled 95% with local soil and buried at ground level. At the end of the study, the soil inside the bucket was revised in order to recover the beetles responsible of dung removal. Despite the fact that cattle dung is not the most abundant resource in the forest (Amézquita & Favila, 2010) and that dung beetles can specialize in certain dung types (Louzada & Silva, 2009; Whipple & Hoback, 2012), cattle dung attracts ~1/3 of dung beetles inside the forest in Los Tuxtlas region (Bourg et al., 2016; Amézquita & Favila, 2010). Therefore, in our experiments the cattle dung provides a good approach to evaluate removal of herbivore dung (Gray et al., 2014). Dung samples were weighed after 3 days to the nearest 0.1 g to quantify dung removal, excluding the effect of desiccation.
Given that dung beetles are able to disperse long distances, we could not consider restored plots (30 × 30 m) as independent sampling units (Da Silva & Hernández, 2015) and therefore we considered the restored area as one unique habitat (~ 8 ha), immersed in a landscape composed of a mosaic of plots restored with different treatments that was compared with pastures and primary forest. Despite we discuss differences among restoration treatments, we only perform statistical analyses considering the restored area as one unique habitat.
We examined the number of individuals, biomass and diversity of dung beetles among the restored area, pastures and forest. Dung beetle diversity was estimated as effective numbers of species (qD, Jost, 2006):
where pi is the proportional abundance (number of individuals) and q is the diversity order (Jost, 2006). We used 3 diversity expressions (q - values): q = 0 is equal to species richness (0D); q ≈ 1 corresponds to typical diversity (1D), where species are included based on their proportional contribution to abundance, and is equivalent to the exponential of Shannon’s index; finally, q = 2 indicates the effective numbers of dominant species (2D) and is equivalent to the inverse of Simpson’s index (Jost, 2006; Moreno et al., 2011).
In order to accurately compare diversity among habitats we estimated sample coverage (Ĉm), which indicates the proportion of the community represented by sampled species. Therefore, Ĉm was considered as a measure of inventory completeness (Chao & Jost, 2012):
where n is the abundance of the sample and f1 and f2 represent singletons and doubletons, respectively. Ĉm ranges from 0 % (minimal completeness) to 100 % (maximum completeness). Comparisons between habitats were done under the same sample completeness (Ĉm) to satisfy the replication principle for diversity comparisons (Chao & Jost, 2012). We used 95% confidence intervals (CI) for each qD for comparisons obtained from bootstrapping (100 randomizations). We considered lack of overlapping as a statistical significant difference (Cumming et al., 2007; Chao et al., 2014). The qD ± CI 95% and Ĉm per experimental plot were obtained with iNEXT package for R (Hsieh et al., 2015).
We separated the species in functional groups (Halffter & Favila, 1993) according to body size (small: < 10 mg dry weight; medium: 10 - 100 mg and large: > 100 mg; S, M and L respectively), daily activity flight (diurnal and nocturnal; D and N respectively) and relocation behaviour of dung (tunnelers, rollers and endocoprids; T, R and E respectively). We evaluated the beta diversity in terms of the effective number of assemblages based on alpha and gamma components and regarding each diversity expression, as qDβ = qDγ / qDα (Jost, 2007). Species turnover was calculated as follows:
where N is the number of evaluated samples. qTβ ranges from 0 (minimum turnover) to 100% (maximum turnover) (Jost, 2007). Beta diversity and turnover were calculated between pairs of sampling habitats and compared to total sampling.
For comparing dung removal among the restored area, pastures and forest, we used a one-way analysis of variance (Anova) where habitat was the fixed factor. Including plot identity as a random factor in the analysis reduced model fit (ΔAIC = 2), so it was not considered in the analysis. Comparisons between habitats were done with a priori contrasts. Data were analyzed with R software version 3.2.3 (R Development Core Team, 2015), according to Crawley (2007) and Zuur et al. (2009).
Results
We collected 603 beetles belonging to 21 species and 11 genera; 88% of the individuals and 92% of total biomass were concentrated in forest (Table 1). Only 7 species (one third of total richness) were collected in the restored area (Table 1). One single species concentrated 80% of the captured individuals (Onthophagus batesi), but its total biomass (0.47 g) was 3.4 times lower than the biomass of larger species (Coprophanaeus corythus + Dichotomius satanas + Phanaeus endymion = 1.64 g), despite they were represented for only 1 or 2 individuals in the sample (Table 1).
Table 1 Dung beetles captured in a restored area and control habitats (pasture and forest) in Los Tuxtlas, Veracruz, Mexico. The functional group was defined according to the combination of 3 traits: body size (S = small < 10 mm, M = medium 10 - 100 mm, L = large > 100 mm), daily activity flight (D = diurnal, N = nocturnal) and relocation behaviour of dung (T = tunnelers, R = rollers, E = endocoprids). Numbers in parentheses represent number of individuals that were collected in traps baited with cattle dung; the others were collected in traps baited with human dung.
| Species | Funct. group | Dry mass (mg) | Pasture | Restored area | Forest | Total |
|---|---|---|---|---|---|---|
| Ateuchus illaesum | MNT | 10.9 | 1 | 1 | ||
| Canthidium centrale | MNT | 17.9 | 35 | 35 | ||
| Canthidium pseudoperceptibile | SNT | 2.9 | 4 | 4 | ||
| Canthidium pseudopunticolle | SDT | 2.3 | 2 | 2 | ||
| Canthon cyanellus cyanellus | MDR | 23.1 | 2 | 10 | 12 (1) | |
| Canthon euryscelis | SDR | 8.5 | 3 | 3 | ||
| Canthon femoralis | MDR | 15.7 | 50 | 50 (1) | ||
| Canthon indigaceus chiapas | MDR | 25.6 | 2 | 2 | ||
| Canthon vazquezae | MDR | 11.7 | 12 | 12 | ||
| Copris laeviceps | MNT | 24.7 | 27 | 27 (2) | ||
| Coprophaneus corythus | LNT | 589.5 | 1 | 1 | 7 | 9 |
| Deltochilum pseudoparile | MNR | 37.7 | 76 | 76 (4) | ||
| Dichotomius colonicus | LNT | 358.8 | 1 | 1 | ||
| Dichotomius satanas | LNT | 173.5 | 77 | 77 (2) | ||
| Eurysternus mexicanus | MDR | 22.1 | 1 | 1 (1) | ||
| Onthophagus batesi | SDT | 8.7 | 18 | 37 | 55 (1) | |
| Onthophagus landolti | SDT | 1.9 | 1 | 1 | ||
| Onthophagus rhinolophus | SDT | 9.1 | 201 | 201 (4) | ||
| Phanaeus endymion | LDT | 103.4 | 1 | 1 | ||
| Uroxys boneti | SNT | 1.3 | 2 | 22 | 24 | |
| Uroxys platypyga | SNT | 1.5 | 9 | 9 | ||
| Number of individuals | 24 | 45 | 534 | 603 (19) | ||
| Biomass (g x individuals) | 0.80 | 1.4 | 24.7 | 27.0 | ||
| Ĉm (sampling completeness) | 0.92 | 0.91 | 1.00 | 0.99 | ||
| 0 D (observed) | 5 | 7 | 14 | 21 | ||
| 0 D (~92%) | 5 | 7.1 | 14 | 21 | ||
| 1 D (~92%) | 2.4 | 2.2 | 7.2 | 8.8 | ||
| 2 D (~92%) | 1.7 | 1.5 | 5 | 6 |
In general, the total number of individuals was similar among experimental plots (12-20 individuals), and pastures (24 individuals), and 1 order of magnitude lower than in the forest (534 individuals) (Table 1). Less than 3% of total individuals were collected with traps baited with cow dung, and the remaining 97% was collected in traps baited with human dung (Table 1). Although the restored area and pastures are relatively close to the nearest primary forest (~500 m; Fig. 1), we found that 4 species (Canthon cyanellus, Coprophanaeus corythus, Phanaeus endymion, and Uroxys boneti) were occasionally captured (1 or 2 individuals) in the restored plots and only Coprophanaeus corythus was shared between forests and pastures (Table 1). Some species found in the restored area, Dichotomius colonicus, Phanaeus endymion and Eurysternus mexicanus, have also been reported in forests (Bourg et al., 2016; Favila & Díaz, 1997) but it was not the case in our sampling and other studies (Díaz et al., 2010).
Among forest, restored area and pasture, the sampling completeness was high (91 - 100%), but we made comparisons at ~92% of sampling completeness (Table 1). In this sense, the forest was more diverse (1D and 2D) than pastures and restored area. However, we did not detect differences between the 3 habitats regarding richness (0D) (Fig. 2). Note that the sampling completeness in the restored area was higher than 90% (Table 1).
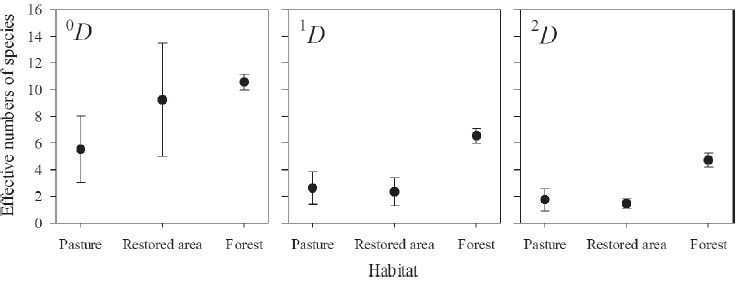
Figure 2 Comparisons of observed diversity (q D) among habitats. Vertical lines represent means ± 95% C.I.
We collected 10 functional groups of Scarabaeinae, 3 of which had the highest richness (Table 1): ‘medium diurnal rollers (5 species)’, ‘small diurnal tunnelers (4 species)’, and ‘large nocturnal tunnelers (3 species)’. Considering together the restored plots and the pasture around them, 82% of total individuals were small diurnal tunnelers (which includes the most abundant species, O. batesi). In the forest, the most abundant species was O. rhinolophus, a small diurnal tunneler that accounted for 33% of total number of individuals (Table 1).
When comparing forests with pastures and the restored area, we detected 2 effective species assemblages for all the diversity expressions, one with species from the forest and the other comprising the pastures and the restored area (Table 2). The highest values of species turnover occurred between the forest and the other 2 habitats (pastures and restored area); in particular, the maximum turnover was detected for the most abundant species (% 2Tβ~100%). On the other hand, pastures and the restored area had 1 single species assemblage, with a turnover lower than 15% (Table 2), except for species richness (0Dβ), that showed 1.7 effective assemblages and 67% turnover (Table 2). When analyzing the 3 habitats together, beta diversity and species turnover decreased from species richness (0D) to diversity of the most abundant species (2D) (Table 2). In particular, species turnover was lower than 50% for 1D and 2D (Table 2).
Table 2 Gamma, alpha and beta diversity (qDβ) and species turnover (% qTβ) between sampled habitats.
| q - value | Gamma | Alpha | qDβ | % q Tβ | |
|---|---|---|---|---|---|
| Forest - Pasture | 0 | 18.0 | 9.5 | 1.9 | 89.5 |
| 1 | 8.3 | 4.2 | 2.0 | 97.0 | |
| 2 | 5.1 | 2.6 | 2.0 | 99.7 | |
| Forest - Restored area | 0 | 18.0 | 10.5 | 1.7 | 71.4 |
| 1 | 7.4 | 3.9 | 1.9 | 88.3 | |
| 2 | 4.5 | 2.3 | 2.0 | 98.7 | |
| Pasture - Restored area | 0 | 10.0 | 6.0 | 1.7 | 66.7 |
| 1 | 2.6 | 2.3 | 1.1 | 13.7 | |
| 2 | 1.6 | 1.6 | 1.0 | 1.1 | |
| Total | 0 | 21.0 | 8.7 | 2.4 | 71.2 |
| 1 | 6.6 | 3.4 | 2.0 | 48.5 | |
| 2 | 3.3 | 2.1 | 1.6 | 31.1 |
The amount of dung removed was different among habitats (Anova F2, 37 = 16.21, p < 0.001; Fig. 3), being higher in pastures than in restored plots (t = 5.51, p < 0.001) or forest (t = 4.52, p < 0.001). Dung removal did not differ between forests and the restored area (t = 0.03, p = 0.975). In pasture and forest 2 and 6 Scarabaeinae species removed dung respectively (total abundance = 65 individuals), 2 of which were not found in plots used to quantify diversity (Table 3). However, we found that no one single dung beetle was responsible for dung removal in the restored area (Table 3).
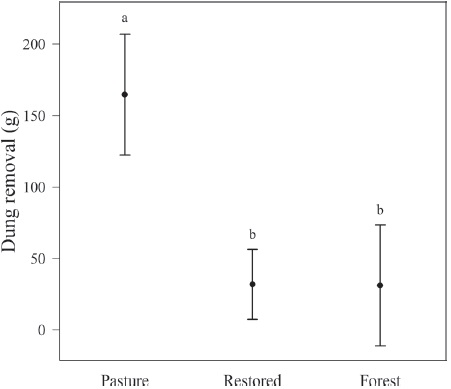
Figure 3 Cattle dung removed in restored and reference sites in Los Tuxtlas, Veracruz, Mexico. Bars represent means ± 95% C.I. Different letters indicate statistical differences.
Table 3 List of dung beetles captured in cattle dung traps used to estimate dung removal in the restored area and reference habitats (pasture and forest) in Los Tuxtlas, Veracruz, Mexico. See guild definitions in Table 1.
| Species | Guild | Dry mass (mg) | Pasture | Restored area | Forest | Total |
|---|---|---|---|---|---|---|
| Ateuchus illaesum | MNT | 10.9 | 0 | 0 | 10 | 10 |
| Copris laeviceps | MNT | 24.7 | 0 | 0 | 47 | 47 |
| Dichotomius colonicus | LNT | 358.8 | 2 | 0 | 0 | 2 |
| Eurysternus foedus* | LDE | 186.2 | 0 | 0 | 2 | 2 |
| Eurysternus mexicanus | MDE | 22.1 | 0 | 0 | 1 | 1 |
| Onthophagus batesi | SDT | 8.7 | 0 | 0 | 1 | 1 |
| Onthophagus incensus* | SDT | 8.1 | 1 | 0 | 0 | 1 |
| Onthophagus rhinolophus | SDT | 9.1 | 0 | 0 | 1 | 1 |
| Number of individuals | 3 | 0 | 62 | 65 |
*Species not found in traps used to quantify diversity
Discussion
Ecological restoration has shown to increase overall biodiversity and ecosystem service supply across countries and ecosystems (Barral et al., 2015). However, contrary to this trend, our study shows that restoration of approximately 8 ha of degraded pastures in a fragmented tropical landscape has not favored yet the recovery of taxonomic and functional diversity of dung beetles, even after 10 years of the establishment of mixed plantings of native species. Regardless of our restored area unavoidably lacks replicates, limiting the extrapolation of our findings to other tropical areas, local restoration efforts benefit from evaluating the recovery of several indicator groups, including dung beetles. The evidence of recovery of our restored plots in plant diversity, tree cover, seed rain of animal-dispersed plants and soil nutrients (see methods) have not been enough to attract dung beetles, perhaps because large and medium mammals are ephemeral and do not use the permanently restored area. Our finding contradicts in part previous suggestions that for tropical dung beetle communities the presence of tree cover is more important than dung availability (Halffter & Arellano, 2002).
Four species found in the restored area contributed with more biomass: 1) O. batesi, that is considered generalist in feeding preferences and eurytopic (registered in forests but also in forest edges and pastures) (Halffter et al., 1992); 2) C. cyanellus, a necrophagous eurytopic species (Arellano et al., 2008); 3) P. endymion, a coprophagous and frugivorous eurytopic species (Sarges et al., 2012), and 4) C. corythus, a necrophagous eurytopic species (Bourg et al., 2016). Even when these species found in restored plots are considered generalist in terms of habitat use, they differ in terms of nutritional requirements for reproduction and nidification. Future studies should evaluate whether food availability and other environmental factors such as soil properties, and natural forest physiognomy limit the use of restored habitats by certain species of dung beetles (Davis et al., 2002). Also, we cannot discard that microclimatic conditions, including temperature and humidity, are limiting the establishment of dung beetles in restoration plantings, as occurs in disturbed habitats (Larsen, 2012).
Even when local conditions are determinant of dung beetle diversity, we consider that the main reasons why dung beetle communities have not been completely recovered in the restored area are the landscape context and the potential edge effects that can be limiting the movement of beetles out of the forest, as shown in other studies with tropical dung beetles (Barnes et al., 2014; Brudvig, 2011; Holl & Aide, 2011; Peyras et al., 2013; Villada-Bedoya et al., 2017). Future studies considering variables such as forest cover, connectivity and use of damaging pesticides in surrounding farms (Alvarado et al. in press) could clarify to which extent these factors limit the recovery of beetles in some restored areas.
In spite of the high diversity of dung beetles found in forests and even in small fragments < 2 ha, where up to 14 species have been registered in our study region (F. Escobar, unpublished data), the diversity in the studied restored area was relatively low. Even when we registered one third of total species richness in the restored area, including some recognized as species from the forests (Uroxys boneti, C. corythus, P. endymion), 6 out of the 7 species captured in the restored area were represented by only 1 or 2 individuals, which probably used living fences or isolated trees in order to get to the plots (De la Peña-Domene et al., 2013; Arellano et al., 2008). Given that our sampling was limited to the rainy season, we cannot discard that other dung beetle species may be visiting the restored plots other times of the year, indicating that plots could be more suitable for such species during the dry season.
Our analysis of beta diversity detected 2 main effective species assemblages: one associated to the forest and the other considering together the pastures and the restored area. According to Bourg et al. (2016), open habitats in Los Tuxtlas region, such as pastures, harbour an own dung beetle fauna. This pattern is supported by our analysis of species turnover, that showed higher similarity of the restored area with the pastures than with the forest. This suggests that the restored area is only used occasionally by species from the forest.
Despite that the total restored area covered close to 8 ha, the experimental plots are relatively small and located inside of a pasture matrix that is surrounded by forest (Fig. 1). Therefore, mass effect of surrounding forest and pastures may be shading the impact of experimental plots in the landscape context for beetle populations (Dauber et al., 2003; Shmida & Wilson, 1985). These factors can be responsible for contrasting results found in the tropics, where 10 years without disturbance around fragments can completely recover dung beetle diversity in some forests such as the Amazonia (Quintero & Roslin, 2005), or show a partial recover in tropical forests in Australia (Derhé et al., 2016), whereas others are not recovered even after 18 years (Audino et al., 2014). Alternative restoration strategies including restoration of forest surrounding matrix or increased connectivity between fragments could be more helpful to recover dung beetle diversity and function than restoring isolated plots (Barnes et al., 2014; Spake et al., 2015).
Although our restored area apparently did not recover dung beetle diversity, we found that herbivore dung removal was as high in restoration plantings as it was in the primary forest and, not surprisingly, was higher in pastures, where cattle dung is an abundant resource. However, dung beetles were not responsible of dung removal in our studied restored site, suggesting that this important dung beetle function was also not recovered after 10 years of restoration. Probably other invertebrates, such as ants, snails and earthworms, recovered faster and contributed to dung removal in our restored area. Future studies should evaluate the presence and the function of other invertebrates that can be contributing to dung removal in our studied restored plots. Implementing functional measurements in conservation and restoration, which mostly focus on diversity measurements, is recognized as an important challenge for conservation science, as it provides information beyond what species richness and diversity give (Cadotte et al., 2011; Gray et al., 2014).
Our experiment of dung removal was carried out with cattle dung, explaining why dung removal was higher in pastures than in primary forest or restoration plots. Despite this kind of dung is not common in the forest, dung beetles are rarely specialized in dung type and ca. 1/3 of dung beetle species in the study region can be attracted to cattle dung, mainly during the rainy season (Amézquita & Favila, 2010; Bourg et al., 2016). Evidence at the same region has shown that dung beetle biomass and richness are 3 times larger in dung of native primates than in dung of exotic mammals such as cattle (Amézquita & Favila, 2010). Therefore, future studies should evaluate whether other important ecosystem services of dung beetles, such as removal of carrion or primate dung, are recovered with restoration plantings.
Our results highlight the urgent need to identify to which extent ecological restoration or alternative conservation strategies, such as conservation of remaining forest patches, can help to maintain and recover dung beetle communities (Audino et al., 2014; Barnes et al., 2014). In places such as Los Tuxtlas, where cattle farming is one of the main economic activities, restoration at a landscape level could be an effective alternative to recover biodiversity and ecosystem function (Chazdon et al., 2009; Kattan, 2008). Future studies need to consider landscape structure, the spatial ranges and nutritional requirements of key taxa, interactions with other biotic and abiotic factors and the response of several indicator groups, including dung beetles, for planning and evaluating the success or failure of restoration programs (Majer, 2009; Parker, 1997).











 nueva página del texto (beta)
nueva página del texto (beta)


