Introduction
Gastropods belonging to the family Eulimidae live in symbiotic association with members of the Echinodermata (Jangoux, 1987; Warén, 1984); however, host echinoderm species and the particular mode of symbiosis are unknown for most described eulimids (Warén, 2008). Eulimids differ from other parasitic gastropods in having the highest number of species, a high degree of sexual dimorphism and many adaptations for a parasitic life (Lorenz, 2005; Warén et al., 1984).
In Mexico, studies related to the eulimids’ symbiosis are scarce. There are only 3 papers treating species living in the Gulf of Mexico and the Caribbean Sea (Caso, 1968, 1971; González-Vallejo, 2008), and 9 other publications about symbiotic relationships in the eastern tropical Pacific (Berry, 1956, 1959; Bertsch, 1975, 1985, 1994; Brand & Muñoz-Ley, 1980; Campos et al., 2009; Salazar & Reyes-Bonilla, 1998; Warén, 1992).
In this work, eulimids associated with the slate-pencil sea urchin, Eucidaris tribuloides (Lamarck, 1816), were studied. Two species were found, which exhibit different lifestyles, Sabinella troglodytes (Thiele, 1925) is a permanent ectoparasite, whereas Nanobalcis worsfoldi Warén 1990 is a temporary ectoparasite.
Sabinella troglodytes was described by Thiele (1925) as Eulima troglodytes from the Cape Verde Islands, with a brief description of the shell and gall made in a modified spine of E. tribuloides. A similar species was collected off Palm Beach, Florida, and described by Pilsbry (1956), as Mucronalia nidorum a “gastropod domiciliary”, although he hesitated about its generic affinity. Pilsbry referred to some features like color, size, and movement of the modified spine, but did not describe mantle color patterns and added that, although he observed them alive, he “never saw one in motion.”
On the other hand, Warén (1980a), in his revision of Mucronalia, suggested that M. nidorum should be transferred to Sabinella. In 1984, he included drawings of a spine of E. tribuloides with S. nidorum (sic) inside it; he also illustrated a lateral cut of the spine in what he supposed to be the position of the male and female’s proboscis on the floor of the gall and suggested there was a perforation.
Later, Warén and Moolenbeek (1989) described Trochostilifer eucidaricola, which is also an ectoparasite of E. tribuloides, but lives attached to the peristome. They commented on some slight differences in the shape of the shells of T. eucidaricola versus S. troglodytes; the first species has a teleoconch with distinctly shouldered whorls, and the body whorl is angular with perfectly flat sides, whereas S. troglodytes has all whorls convex. Warén (1992) described a new ectoparasite, S. shaskyi, which lives in the spines of E. thouarsii from the eastern tropical Pacific, just as the Caribbean species (S. troglodytes) does. Warén made some morphological comparisons and stated that these “two species can be distinguished mainly by larval shell, which is slender with flatter whorls in S. troglodytes”. Redfern (2001: 83, fig. 353) described and illustrated S troglodytes with black and white photos in his book on the Bahamas. Later, Redfern (2013: 121, fig. 337C) again illustrated S. troglodytes in a small gall in spines of E. tribuloides.
Warén and Mifsud (1990) proposed Nanobalcis for a small group of eulimids, with Eulima nana (Monterosato, 1878) as the type species. Nanobalcis worsfoldi Warén in Warén & Mifsud 1990, was described with several shells collected in the Bahamas, Grand Cayman Island, off Fort Myers, Florida, and Aruba Island. Redfern (2001) illustrated only shells of N. worsfoldi from the Bahamas. Warén and Mifsud (1990) noted that the way of feeding, the mantle color pattern, and the interactions of this species on its host (E. tribuloides) are unknown.
Several authors have done important research on ecological aspects of eulimids, but our knowledge is still fragmentary and the complex life history of eulimids is largely unknown (Matsuda et al., 2012). Will (2009) pointed out that a study of host-parasite interactions will be doubly useful by allowing general insights into parasite systems and by increasing the knowledge of eulimid natural history.
Here we present a complete characterization of shells based on living specimens, a chresonomy list and taxonomic remarks are also provided, which might prove useful for comparison with specimens from other regions of the Caribbean Sea. At the same time, we describe biological aspects for Sabinella troglodytes and Nanobalcis worsfoldi, their means of attachment, mantle color pattern, frequency and movements on the host are recorded for the first time in the Mexican Caribbean region.
Materials and methods
One hundred and eighty sea urchins were randomly collected by snorkeling along reef crest bottoms and nearby habitats off Majahual, Quintana Roo (18°43’25.34” N, 87°42’4.30” W), on February 18, 2012. The reef lagoon is about 1.5 m deep. The other collection site was in a similar environment off Xahuayxol, Quintana Roo (18°30’15” N, 87°45’32” W), where 120 sea urchins were collected under coralline rocks at a depth of 1.5-2 m, on April 6, 2012. All sea urchins were hand-collected, placed individually in plastic bags, transferred to containers with seawater, and taken to the laboratory.
Thirty-six sea urchins and their hosts were kept alive in the laboratory. Pigmentation, movement patterns, feeding, and other aspects were observed and photographed using a stereomicroscope. Only 2 of the sea urchins had modified primary spines (with a cup-like gall) with eulimids. These 2 sea-urchins wer kept alive for 4 days; on its aboral side. This spine with internal S. troglodytes was cut off and placed in a container; when the eulimids were removed, 3 egg capsules were found on the gall’s bottom surface.
Ten adult N. worsfoldi organisms and 1 S. troglodytes were extracted from shells and reviewed for sex determination; they were then mounted on slides to observe the internal morphology of both species. A series of photographs of the shells was made and edited with HeliconFocus 5.3, and plates were assembled with Paint Shop ProCS6. For the identification of the species we used several bibliographic references (Bouchet & Warén, 1986; Pilsbry, 1956; Thiele, 1925; Warén, 1980a, b, 1984, 1992; Warén & Moolenbeek, 1989) and chresonomy lists were made for each species. Type and non-type empty shells were reviewed in the Natural History Museum of Los Angeles County (LACM), Naturalis (NCB), Leiden (previously ZMA), Museum für Naturkunde Berlin (ZMB), National Museum of Natural History, Smithsonian Institution (USNM), and the Museu Nacional, Universidade Federal do Rio de Janeiro (MNRJ). All eulimid specimens collected during this study are deposited in the ECOSUR Reference Collection, Chetumal, Quintana Roo, Mexico.
Results
From the 300 sea urchins, 139 N. worsfoldi specimens living on the spine bases were collected; 80 were from Majahual beach and 59 from Xahuayxol beach. The average frequency of sea urchins was 1-5 specimens, but only a single sea urchin with 16 eulimids moving in the body host with different shell sizes (0.5-2.5 mm) was found. In contrast, only 2 sea urchins had modified spine galls, each gall containing 2 S. troglodytes adults and 1-2 juveniles. One came from Majahual and the other from Xahuayxol.
Sabinella Monterosato, 1890.
Sabinella troglodytes (Thiele, 1925) (Fig. 1A, B)

Figure 1 (A) Sabinella troglodytes (Thiele, 1925) ECOSUR-M1385, female shell; scale bar = 0.41 mm. (B) Male shell, scale bar = 0.27 mm. (C) Eulima troglodytes syntype ZMBMoll 103264, scale bar = 0.4 mm. (D) Mucronalia nidorum (Pilsbry, 1956) holotype, ANSP 196745. scale bar = 0.49 mm. (E) Sabinella troglodytes ZMAMoll 347799, scale bar = 0.49 mm. (F) Sabinella troglodytes non-type USNM 94291 young shell, scale bar = 0.41 mm. Photo C by C. Zorn. almost half of shell length and with a round profile base, sometimes marked with a strong sinuous incremental growth scar. Protoconch multi-spiral of 3.5 slightly convex whorls, smooth, transparent; sometimes bent to the right in front view, tip rounded. Aperture is wide rounded, outer lip sinuous thin edge in lateral view and protruding along its adapical part, inner lip swollen forming a thickened columellar callus. Average size (n = 13): 3.7 mm long, 1.5 mm wide.
Type species: Eulima troglodytesThiele, 1925:146-147 (Pl. 25 Fig. 4).
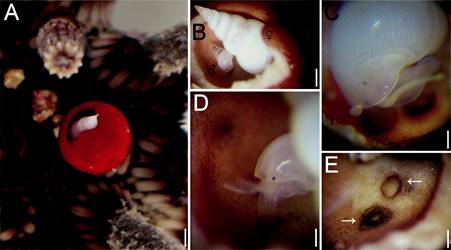
Figure 2 (A) Eucidaris tribuloides with Sabinella troglodytes host on gall; (B) gall cut showing position of male and female; (C) female attached by the proboscis; (D) male attached by the proboscis; (E) scars in gall bottom where S. troglodytes female was attached. Scale bars: A = 1.1 mm; B = 0.7 mm; C, D = 0.3 mm; E = 0.4 mm.
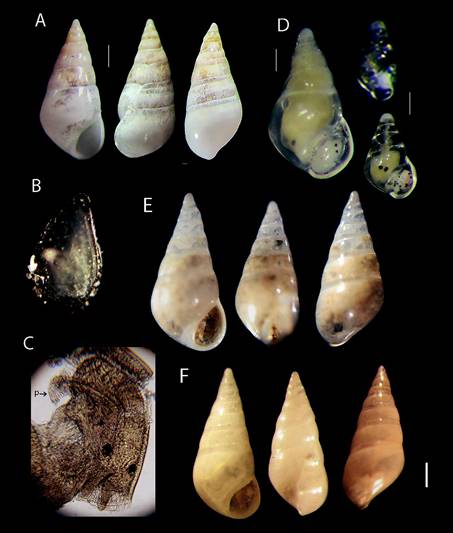
Figure 3 (A) Nanobalcis worsfoldi shell, scale bar = 0.41 mm. (B) Operculum outer view size = 500 μ. (C) Head-foot, male dorsal view, (p) penis, (40X). (D) Male specimen, scale bar = 0.37 mm and 2 young specimens where only distinguish head and eyes anatomy, scale bar = 0.1 mm. (E) Nanobalcis worsfoldi MNR-J33617 Brazil, scale bar = 0.3 mm. (F) N. worsfoldi 73-85 LACM Cuba, scale bar = 0.36 mm. be used to evaluate the affinities between specimens from the wide distribution of this species.
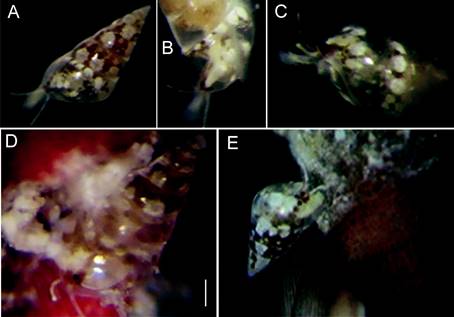
Figure 4 (A) Nanobalcis worsfoldi living color mantle pattern. (B) Translucent cephalic tentacles and black eyes in red pigment patches. (C) Lateral view showing complete translucent extended foot. (D, E) Two eulimids seemingly crawling or may be eating on the base of the spine. Scale bars: A, B = 0.4 mm; D = 0.3 mm; C, E = 0.2 mm.
Mucronalia nidorum Pilsbry, 1956:110 (Pl. 6 Figs. 4, 5, 6) junior synonym.
Rosenia nidorum Shasky, 1968:74.
Stilifer (Pelseneeria) nidorum Keen, 1971:451 (Fig. 761, Pilsbry’s figure).
Mucronalia (Pelseneeria) nidorum Abbott, 1974:130 (Fig. 1429, Pilsbry’s figure).
Mucronalia nidorum Edwards, 1977:145-146; Jong & Coomans, 1988:59; Sarasúa & Espinosa, 1977:1-4 (Figs. 1A, B; 2).
Sabinella troglodytes Lyons, 1998:23; Redfern, 2001:83 (Pl. 39 fig. 353); 2013:121 (Fig. 337C); Rosenberg et al., 2009:643; Queiroz et al., 2017 (Figs. 1A-F, 2A-G); Warén, 1980a:203; 1984:71 (Figs. 188-190 S. nidorum); 1992:193 (Figs. 47, 53-54); Warén & Moolenbeek, l989:170-172 (Figs. 2, 9-13, 15).
Taxonomic summary
Type locality: E. troglodytes north of Cape Verde Islands (original label data).
Material examined: M. nidorum holotype, ANSP 196745 off Palm Beach Florida, (from photographs: clade. ansp.org/malacology/collections). Eulima troglodytes syntype ZMB 103.264 (1 shell from photographs), north of Cape Verde Islands on E. tribuloides var. africana, no date. S. troglodytes ZMA 347799 (7 shells), Bonaire, 4.9.1948, Coll. P. Hummelinck, ZMA 162217 (label 41260), Aruba, no date. USNM 94291 (1 shell), Campeche Bank, Mexico St. 24, 200 fm (360 m). WH Rush Blake Coll., no date. ECOSUR-M-1385 (2 adults, 2 young) Majahual, QR. 2/18/2012, ECOSUR-M-1386 (2 adults, 1 young) Xahuayxhol, QR 4/06/2012.
Description: based on specimens collected for this study (ECOSUR-M-1385). Female shell, conical, spire concave, straight, white or semi-transparent. External surface smooth, glossy, with non-aligned incremental scars on each whorl. Teleoconch with 4.5 convex whorls, sutures well defined. Body whorl relatively large, constituting almost half of shell length and with a round profile base, sometimes marked with a strong sinuous incremental growth scar. Protoconch multi-spiral of 3.5 slightly convex whorls, smooth, transparent; sometimes bent to the right in front view, tip rounded. Aperture is wide rounded, outer lip sinuous thin edge in lateral view and protruding along its adapical part, inner lip swollen forming a thickened columellar callus. Average size (n = 13): 3.7 mm long, 1.5 mm wide.
Male shell spire concave, conical straight, white or semi-transparent. Teleoconch with 3.5 convex whorls, body whorl large and rounded, smooth, with fine incremental scars, sutures well defined (Fig. 1B). Protoconch transparent, with 2.5 slightly convex whorls. Aperture round, outer lip edge thin in profile view, protruding along its adapical part, inner lip swollen and forming a columellar callus. Average size: 1.5 mm long, 0.6 mm wide.
Mantle color. The female is white, semitransparent, with a series of tiny opaque spots concentrated anteriorly, and combined with another series of more opaque white spots, visible through shell. The second and third posterior whorls visceral mass is flecked with dull red stripes. Cephalic tentacles are long, slender, light yellow in color, with microscopic black dorsal dots, tips white. The male has a similar color pattern as the female in the cephalic region, visceral mass region with deep red streaks and a diffuse yellow stain between the eyes. Both specimens have tiny black spherical eyes without lens, placed basally and visible through shell.
Female and male with cylindrical snout, transparent, flexible, distal surface with rounded tip firmly attached to gall bottom. Pedal lobules are well developed and apparently functional. Operculum is oval, thin, transparent yellowish-brown in color, closing in the latter half of the aperture. No penis or other reproductive structure was found. Three transparent elliptic egg capsules were found, each with a different number of embryos of varying sizes and development stages. All capsules were attached to the gall bottom by a short stalk. The largest capsule was 1 mm long and had about 100 embryos, almost ready for release.
Distribution: Bermuda to Aruba, Bonaire and Curaçao Islands, Eastern Atlantic (Rosenberg et al., 2009).
Remarks
Thiele’s (1925) original description was based on a large specimen (3.75 mm long, 1.9 mm wide, 1.7 mm aperture height); with 8 slightly convex whorls, and flattened at the bottom from a rounded edge. The syntype ZMB103.264 of E. troglodytes is smaller (2.3 mm long, 1.5 mm wide, 0.9 mm aperture height) (Fig. 1C). The teleoconch whorls are slightly convex, gradually swelling towards body whorl, sutures are well defined. Body whorl is almost half shell length, with slightly rounded periphery and with a large sinusoid growth scar from suture to base. Aperture oval, almost 1/3 of shell length, anteriorly expanded beyond base. Inner lip flared at the base, thick columellar callus reflected, outer lip almost straight in aperture view, relatively larger, and body whorl wider with growth scar that stands out of the base. There are some differences between the syntype of E. troglodytes and our specimens, especially in the degree of swelling in whorls, the slightly less convex turns, and the aperture markedly expanded. However, there are no morphological differences between our specimens and the holotype of M. nidorum (Fig. 1D), and neither with non-type specimens from Bonaire Island (ZMA 347799) (Fig. 1E). Intraspecific variability of incremental scar position was revised; the number of whorls was variable in all empty shells and was related to adult shell size. Warén and Moolenbeek (1989:172 fig. 9) figured an unusually sized specimen (4.6 mm long) of a S. troglodytes female; the body whorls look narrower and taller, the posterior whorls have a similar size, not tapered and the periphery aperture has angulation, being very different from the male shell; all our specimens are rounded as in the M. nidorum holotype. A shell from the Gulf of Mexico (USNM 94291) is a young shell with slightly tilted apex (Fig. 1F). On the other hand, an S. troglodytes from Bahía, Brazil (Queiroz et al., 2017) has micro-sculpture (thin axial lines) along the main body whorl. Because of these and other attributes indicated above, S. troglodytes should be studied molecularly because it could be a complex of cryptic species.
All sea urchin spines showed normal movement with the exception of the modified spine because movements were slower than the others. The gall was 5.5 mm high and 4.3 mm wide and had a narrow opening. The outer spine epithelium was a uniformly dull red, without the dark banding pattern typical of other nearby spines (Fig. 2A). Inside the gall, the epithelium was only evident along the pores zone while remaining surfaces were bare. The apex of the female’s shell and subsequent body whorls protruded from the gall, whereas the rest of her shell occupied most of the space available inside the gall. The male lies under the female’s body and could be seen only after the gall outer wall top was broken (Fig. 2B). The female reacted to change in light intensity, with very slight movements, up and down inside the gall. However, they never left the gall, both males and females were permanently attached by the snout (Fig. 2C, D). Once the eulimid was detached, a circular mark formed by a rigid ring of protein was seen where the eulimid was inside the spine gall. The snout tip is firm, musculature has expanded distally into a trumpet-shaped region in the attachment site; there was no perforation (Fig. 2E).
Nanobalcis Warén & Mifsud, 1990:39.
Nanobalcis worsfoldi Warén in Warén & Mifsud, 1990. (Figs. 3A, B)
Type species: Eulima nana Monterosato, 1878:153. Nanobalcis worsfoldi Lyons 1998:23; Espinosa et al., 2005:26; Redfern, 2001:81 (Pl. 39 fig. 345); Rosenberg et al., 2009:643; Warén in Warén & Mifsud, 1990:40 (Figs. 3A-D, 4B).
Taxonomic summary
Type locality: 8 km north of Eight Mile Rock, Grand Bahama Island (Warén & Mifsud 1990).
Material examined: LACM 73-85 (2 shells, general collection). Kittery beach Guantanamo Bay, Cuba (19°53’ N, 75°07’ W) 10-15 fms (5-8 m), coll. T. Bratcher 19 November 1973. MNRJ336 (1 shell) Campos Basin 17HAB 17 (21°22’54” S, 40°19’50.56” W, Est.12, 21 July 2009, 53 m on sediment. ECOSUR-M-1387 (139 specimens), Majahual Beach and Xahuayxol Lagoon reef, Quintana Roo, Mexico.
Description: Based on specimens collected for this study (ECOSUR-M-1387). Shell solid, conical, smooth, glossy transparent, early whorls slightly tilted. Empty shells opaque white in front view (Fig. 3A). Teleoconch with 5-6 slightly convex whorls, sutures well defined, but without sub suture zone; posterior whorls of some specimens with slightly distorted axis to the left in lateral and dorsal views (Fig. 3A). Growth scars aligned on second and third whorls; body whorl occupies 50% of the total shell length. Protoconch with 2.5 slightly convex whorls, smooth, white rounded apex. Aperture round, outer lip distinctly projected at the midpoint of its height, inner lip with columellar callus located at the lower margin of the aperture. Operculum thin, oval transparent to light brown with internal reinforcement fold, narrower in the posterior part of aperture (Fig. 3B).
Mantle color. The mantle has a mottled orange-brown pigmentation, with white spots seen through the shell. Adults with large white dots situated above each suture shell whorl, color pattern was not constant in all specimens. Each cephalic tentacle slender, long, transparent with white stains and dark dots areas. Foot is short, occupying less than 30% shell length. The snout is short, smooth, with white spots. Eyes round, black, with lens (Fig. 26) placed at a variable distance between them.
Penis of male was located over left cephalic area (Fig. 3C). After preservation of the specimens, the mantle color changed to brown or pale-brown and the organism was completely retracted inside the shell (Fig. 3D). Young specimens have little mantle spots pigmentation and are mimetically transparent; only their head can be distinguished (Fig. 3D) by tiny dark stains and black eyes. All specimens with reddish pigmentation ahead of eyes, some with 1 spot, and others with a complete line composed of a series of reddish spots almost connected to each other around, or passing through their bases.
Average size of female (n = 20): 2.0-2.5 mm long; male (n = 20): 1.3-1.7 mm long.
Remarks
The type material of the species could not be reviewed, but was compared with holotype ANSP 375972 photographs (clade.ansp.org/malacology/collections photos). An intra specific difference was found between shells of young and adult individuals, young and male specimens showed slightly angular body whorls. The shape of the aperture is narrow, angulated afterwards, with obvious deposition of new material for thickening. Adults had slight differences in sinuosity of the outer lip in lateral view, and in the position of growth scars and shell thickness. A single shell from Brazil is similar to the specimens of this study, but did not show the slight inclination of early whorls (Fig. 3E) in comparison with several specimens observed and illustrated herein. The empty shell of Cuba has less convex whorls and also has a straight and higher body whorl, and the aperture is smaller than in the other shells (Fig. 3F). Morphological differences outlined above are inconclusive for the species; a molecular analysis could be used to evaluate the affinities between specimens from the wide distribution of this species.
Two individuals were placed in a container with sea water, and we observed that they were extremely motile, despite their small size (Fig. 4A-C). We could also observe them crawling along the bases of the sea urchin’s primary spines. The snail produces a mucus thread, which it attaches to a spine in order to stay in position when the spines move; this thread can be connected to other spines allowing them to displace themselves along it between spines (Fig. 4D). Sponges, bryozoans, and encrusting red algae and foraminifera covered the spines and we suppose they feed on them. There were unknown residues in the aperture of most eulimids (Fig. 4E). Three transparent globular egg-capsules were observed, attached to the base of the spines; however, it could not be confirmed if they belonged to N. worsfoldi.
Distribution: Bahamas to Lesser Antilles (Rosenberg et al., 2009).
Discussion
The present study has shown that S. troglodytes inhabits the spines and forms galls in which females, males, and egg-capsules find shelter; and that N. worsfoldi lives free, both on the same host urchin E. tribuloides. The location of the parasites is highly specific and they exhibit selective site segregation as found in similar species (Morton, 1976). The association of the second species is reported for the first time for the Mexican Caribbean Sea; other distribution localities can be found in Rosenberg et al. (2009).
Comparing the shell shape of S. troglodytes from this study and specimens examined from the Caribbean region, they appear to have a similar conchology pattern, but the syntype E. troglodytes has a smaller total size (2.3 × 1.2 mm), the profile of its teleoconch whorls is smooth, and it has fewer whorls, the aperture size is wide and there are some smaller differences on the inner lip callus. Thiele (1925) indicated a size of 3.75 × 1.9 mm and 8 convex whorls. This was likely based on another larger shell, but specimens studied here showed several intraspecific variations as mentioned in the the remarks section. According to a recent study of the Conus species in the Cape Verde Islands, 53 of the 56 recorded species on all islands are endemic, and the site constitutes an important center of endemism due to its geographic location (Peters et al., 2016). In the eulimids there are no studies covering widely distributed species that have been reported for both Atlantic coasts.
Lessios et al. (1999) studied the genetic affinities of Eucidaris species distributed in the Atlantic Ocean and concluded that, “despite the tremendous distances involved, populations from west and east Atlantic coasts are connected by recent gene flow”, and that their affinities can be best explained as the results of west to east dispersal through the Tethys Sea before its closure. The ability to cross biogeographic barriers is normally restricted to highly mobile species or species that produce propagules with high potential to disperse, at least during a particular phase of their life-cycle (Briggs, 1974), and this should be the case for these sea urchins. However, S. troglodytes should have a short planktotrophic larvae life. We observed young specimens, beginning to make a circular lateral depression on the base of the spine, which was always inhabited by another juvenile (not figured here). Further, although the long distance transport of the eggs or larvae possibly may be associated with past climatic events, or rafting, their low abundances make an effective connection between the East Atlantic and West Atlantic populations very unlikely. A molecular phylogeny is the starting point to infer biogeographic relationships within this species, and would shed light on the taxonomic status of S. troglodytes.
Members of the Eulimidae have very different attachment modes (Jangoux, 1987). Warén (1984) indicated that 5 genera form galls on the host, but only 2 species, Trochostilifer domus Warén, 1980 and S. troglodytes, use sea urchin primary spines. Pilsbry (1956) noticed that M. nidorum could move by night and leave to feed outside the gall, then return to the same spine, but strangely, he never saw them in motion. In this study, we observed that S. troglodytes secreted a viscous substance from the snout rounded tip that may allow them to firmly attach to the gall bottom or wall. This secretion is very flexible and forms a dark colored proteinaceous ring. Female and male specimens remain attached by their snout to the gall´s bottom, but do not penetrate the spine, as Warén (1984) and Warén and Moolenbeek (1989) have pointed out. The snail can probably re-adjust its position inside the gall, as shown here by the old dark ring-like mark indicating a previous attachment site (overlaying mark in Fig. 2E). After 12 hours of observation, and despite the fact that the spine was detached from the sea urchin, we saw none of them leaving the gall. Recently S. troglodytes was reported moving outside the gall (Queiroz et al., 2017), confirming the functionality of the pedal lobes observed by us. At the same time, this difference could be explained because of size limitations. As the eulimid becomes larger than the gall opening, it could not leave the gall; if smaller, leaving the gall is possible.
Warén (1984) and later Hori and Koda (1997) indicated that eulimid mantle pigmentation depends on their food. The primary spines of E. tribuloides are a good example of this; cidaroid spines have an external, polycrystalline cortex covered by epithelium; the mineral skeleton is embedded in the mesodermal stroma tissue which largely consists of fluid and different types of mesodermal cells float within this fluid (Märkel & Röser, 1983). The dark red color of the mantle that was observed in the posterior whorls of both male and female of S. troglodytes can be a result of the snail feeding on cells within the spine’s matrix or over other epithelium areas. Queiroz et al. (2017) mentioned marks around the opening and inside the gall. We also saw these marks as dark spots just only on the edge of gall’s aperture, and conclude that displacement that species makes is around and insides of gall, as was confirmed by them.
In contrast, N. worsfoldi creeps along the spine of its host and secretes a mucous thread that it uses to secure its position when the host or its spine is moving. Secretions from the pedal glands lubricate the pathway of this eulimid, and it secures its hold as it creeps over the host; this has not been reported for this species. Warén & Mifsud (1990) reported for N. cherbonnieri from New Caledonia a similar “thin thread”, remaining on sea urchin spines whenever the eulimid proboscis is retracted on. We suspect that N. wordsfoldi ingests some microorganisms living on the spines or epithelial tissue from the base’s spines, or both. However, further analyses are needed to clarify this point.
Rodríguez et al. (2001) studied N. nana, a parasite of Cidaris cidaris from the Canary Islands, and mentioned that soft parts are orange-brown with yellowish spots in the gonad-visceral zone, long black eyes, and almost transparent very long, slender cephalic tentacles. However, they did not comment on the intraspecific variation of the shells or sexual dimorphism, and by their size range N. nana is smaller than N. worsfoldi. Warén and Mifsud (1990) illustrated a marked sexual dimorphism in N. nana, but we did not find dimorphism in N. worsfoldi. We found that males have the body whorl angular on the base and smaller size. This aspect is not conclusive for this species as noted by Matsuda et al. (2012), but 1 reproductive structure like a penis was registered. In this work, contrary to what was mentioned by Rodríguez et al. (2001), who observed the same color pattern in all N. nana specimens, the mantle color pattern of N. worsfoldi is not permanent. Variable color patterns were from orange to dark green, both mixed with big white spots. These characteristic pigmentation patterns are reported here for the first time for this Caribbean zone and distribution range to Brazil.
The determination of the host is a very important aspect in the classification of the family Eulimidae, since their glossy and transparent shells offer too few characters for classification (Schiaparelli et al., 2007). The species reported here share the same host while having different ecological niches. One moves freely and is numerous in every sea urchin (N. worsfoldi), while the other remains in a gall (S. troglodytes) and its association with the host is rare or not very frequent, as was noted by McPherson (1968). Their means for attachment and behavior in the same sea urchin were different too. A better understanding of their evolutionary history is a work in progress that we hope will be resolved in the near future.











 nova página do texto(beta)
nova página do texto(beta)


