Introduction
The genus and species Potosa dybasiGoodnight & Goodnight, 1947 were originally described in the family Phalangodidae, subfamily Phalangodinae, based on 2 males collected in Huichihuayán, San Luís Potosí, Mexico. This genus was poorly diagnosed as those phalangodines with 5 distinct areas, a median spine on mesotergal areas II and III, first area of dorsal scutum without a median line, ocularium separated from the anterior margin, and bearing median spine, and tarsal count 4(2):6(3):6:6 (Goodnight & Goodnight, 1947). These authors considered Potosa related to Spinolatum Goodnight & Goodnight, 1942 ENT[Grassatores: incertae sedis, according to Kury (2003)ENT] from Guyana, mentioned a brief comparison between these 2 genera. Later, Goodnight and Goodnight (1953) synonymized Potosa with Karos Goodnight & Goodnight, 1944 without any justification. Posteriorly, Kury (2003) based on examination of male genitalia proposed some new familial assignment, transferring Karos from Phalandodidae to Stygnopsidae.
Cruz-López and Francke (2015) performed a cladistic analysis of Karos based on morphological data, reverted Goodnight and Goodnight (1953) synonyms in Karos, including Potosa. Also, they rediagnosed the genus, redescribed P. dybasi and described a new species: Potosa reddelli Cruz-López & Francke, 2015. Cruz-López and Francke (2015) detected a peculiar synapomorphy for Potosa: a swollen, sexually dimorphic metatarsus I of the males, with a ventral glandular opening (Cruz-López & Francke, 2015: figs. 60A, B). Surprisingly, this feature was illustrated by Goodnight and Goodnight (1947), but it was not mentioned in the original description until an additional record for P. dybasi published by Goodnight and Goodnight (1973). This glandular opening plus morphology of the male genitalia makes of Potosa easily recognizable from other genera among Stygnopsidae (Cruz-López & Francke, 2015).
There is a poor knowledge on the biological data of the genus Potosa thus far. The genus is distributed in the central portion of Huasteca region, known only from 3 localities in the South of San Luís Potosí and the North of Hidalgo states (Cruz-López & Francke, 2015).
The relationships of Potosa are still unknown, morphology-based phylogeny indicates that Karos is the sister group of Potosa, while molecular and total evidence analyses indicate that Potosa is the sister group of the clade (((‘Hoplobunus’ planus + Mictlana), ‘Hoplobunus’ aff. planus) + ((‘Karos’ depressus + Crettaros), Karos))), in Stygnopsidae, Karosinae (Cruz-López & Francke, 2015, 2017). In addition, Cruz-López and Francke (2017) included molecular data of a male voucher Potosa CNANOp-0089, which in the present study is described as a new species Potosa elsanto sp. nov. This new species described herein is based on 2 males, the glandular opening is illustrated in detail. Also, a dichotomic key for the recognition of the 3 known species of the genus is included.
Materials and methods
The type material was deposited in the Colección Nacional de Arácnidos (CNAN), Universidad Autónoma de México (UNAM). Additional material of Potosa spp., from CNAN and Texas Tech University (TTU), USA, was examined (see other material examined section). Color images were obtained using a Nikon Coolpix S10 camera attached to Nikon SMZ645 microscope. SEM’s photos were taken using a Hitachi S-2460N Scanning Electronic Microscope following the procedure suggested by Acosta et al. (2007). Plates of figures were made and edited through Photoshop CS5. Morphological nomenclature follows Cruz-López & Francke (2015, 2017), dorsal scutum nomenclature according to Kury and Medrano (2016), pedipalpal armature follows Acosta et al. (2007), and macrosetal nomenclature of male genitalia (A to E groups) according to Kury and Villarreal (2015), with modifications proposed by Cruz-López and Francke (2017). Distribution map was generated through ArcGIS Online basemaps using ArcGIS online content (available at: www.arcgis.com). Measurements are given in mm and µm. Abbreviation: MS = macrosetae.
Description
Potosa elsanto sp. nov. (Figs. 1-8, 11-25)

Figures 1-2 Potosa elsanto sp. nov. Male holotype. Habitus, dorsal and lateral view, respectively. Scale: 0.5 mm.
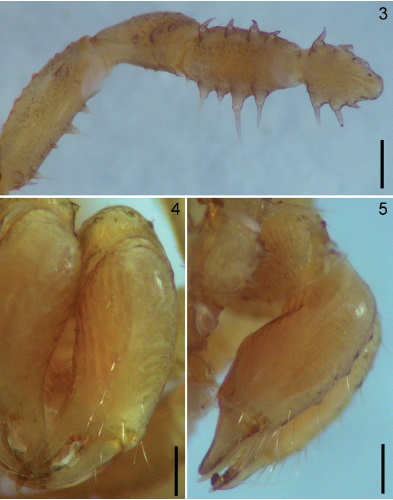
Figures 3-5 Potosa elsanto sp. nov. Male holotype. 3, pedipalp, ventral view. 4-5, chelicera, frontal and ectal views respectively. Scale bar: 0.35 mm (Fig. 3), 0.25 mm (Figs. 4-5).

Figures 6-10 Potosa elsanto sp. nov. Male holotype: 6-8, lateral view of legs I, II and IV. Potosa. dybasi male (TTU): 9-10, lateral view of legs II and IV. Abbreviations: met I= metatarsus I; dis I, II and III = distitarsus I, II and III; met IV = metatarsus IV.

Figures 11-16 Potosa elsanto sp. nov. (DNA voucher CNANOp0089). Male. 11-12, metatarsus I, ventral and lateral views respectively. 13, detail of limit between astragalus and calcaneus of leg I. 14-16, detail of ventral glandular opening on metatarsus I. Abbreviations: gl op = glandular opening, met I = metatarsus I; ast = astragalus; cal = calcaneus. Scale: 250 µm (Figs. 11 and 12, 100 µm (Fig. 13), 150 µm (Fig. 14), 100 µm (Fig. 15), 50 µm (Fig. 16).
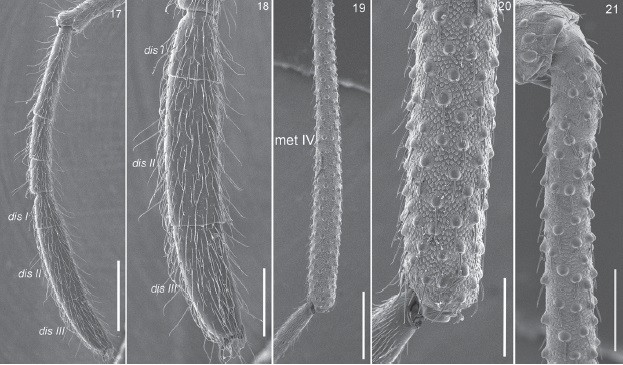
Figures 17-21 Potosa elsanto sp. nov. (DNA voucher CNANOp0089). Male. 17, lateral view of complete tarsus II. 18, detail of the distitarsus of leg II. 19, entire metatarsus IV. 20, apical portion of metatarsus IV. 21, basal portion of metatarsus IV. Abbreviations: dis I, II, III = distitarsus I, II and III, met IV = metatarsus IV. Scale bars: 500µm (Figs. 17, 19), 250 µm (Figs. 18, 21), 200 µm (Fig. 20).

Figures 22-24 Potosa elsanto sp. nov. (DNA voucher CNANOp0089). Male genitalia. 22-24, dorsal, lateral and ventral views respectively. Abbreviations indicate setal groups, using the same color as Kury and Villarreal (2015), for easy recognition. Scale: 50 µm.

Figure 25 Detail of apical follis of Potosa elsanto sp. nov. (DNA voucher CNANOp0089). Abbreviations: f = follis; lat pr = lateral projections; sp pr = spiniform projections; St = stylus. Scale: 25 µm.
Potosa sp. CNANOp-0089: Cruz-López & Francke (2017)
Diagnosis
Potosa elsanto sp. nov., can be easily recognized from the troglomorphic P. reddelli by the following combination of characters: presence of middle spine on the ocularium (Fig. 2), mesotergal areas II and III with median tubercles developed (Figs. 1, 2), metatarsus I in males very swollen with glandular opening in the middle of metatarsus (Fig. 11), and by the presence of an additional pair of MS E on the penis (Fig. 24), instead 2 pairs as in P. reddelli. Also the new species described here can be recognized from P. dybasi by the following combination of characters: spine of the ocularium not acute, contiguous with the ocularium (Fig. 2), distitarsus II of leg II very large, longer than distitarsus I, instead of similar size on P. dybasi (Figs. 7, 9, 18); penis with sockets of macrosetae D rounded, well-marked (Fig. 22); metatarsus IV of males swollen apically and slightly curved in the middle instead of femur straight and not swollen apically on P. dybasi (Figs. 8, 10, 19).
Description (male holotype). Dorsum: scutum gamma type (γ), with median bulge rounded and well-marked from sulcus I to sulcus III; constriction I deep and rounded, constriction II shallow, continuous with the coda; coda straight, posterior apices of scutum softly rounded (Fig. 1). Prosoma hexagonal shape, wider at the level of ozopores. Ocularium in the middle of prosoma, base elliptical, triangular in lateral and frontal view, with small apical spine, contiguous with the ocularium (Fig. 2). Mesotergum with 5 well-marked areas, area I larger than the remaining, area II slightly smaller than area I; areas III and IV similar in shape; sulci II and III retrocurved, IV and V straight. Area I with few and central small tubercles, areas II and III with similar ornamentation, but with the central tubercle developed in a backward spine, larger in area III; areas IV and V with transversal row of spiniform tubercles. Free tergites with transversal row of tubercles, similar to those on mesotergal areas IV and V, but smaller. Lateral channels projected as clear areas at level of mesotergal area I, just on the wider portion of scutum, these clear areas are softly rounded; corners of area V and free tergites I and II with similar projections (Figs. 1, 2). Venter: coxae IV covering almost all area in ventral view, all coxae covered with scattered small tubercles and few setae. Stigmatic area in triangular shaped, genital operculum rounded, small. Spiracles visible. Chelicera: small, bulla weak, cheliceral fingers with at least 5 small and contiguous teeth (Figs. 4, 5). Pedipalp: femur sub-rectangular, with dorsal surface slightly convex; ventrally with a row of 5 spiniform setiferous tubercles, the basalmost longer, on the meso-apical surface, with a setiferous tubercle. Patella cylindrical, with 3 setiferous tubercles on mesal side. Tibia with 4 setiferous tubercles on each side as follow: IiII, (3 > 1 = 4 > 2). Tarsus with 3 setiferous tubercles on each side as follow: III, (1 > 2 > 3). Claw shorter than tarsus (Fig. 3). Legs: Measurements in the Table 1. All legs are slender, covered by small tubercles on trochanters to metatarsi, tarsi covered by small setae. Metatarsus I very swollen, with the glandular opening in the middle, dome-shaped, calcaneus on the apical portion of metatarsus (Figs. 6, 11-16). Distitarsi II and III of the leg II bigger and swollen, distitarsus II noticeably larger than distitarsus I (Figs. 7, 17, 18). Femur IV covered by scattered tubercles, slightly curved in the middle and swollen apically (Figs. 8, 19-21). Tarsal count 4(2):6(3):6:6. Male genitalia: pars distalis flattened dorso-ventrally, apical margin of flimsy lamina slightly curved ventrally; apical margin irregular shape, with 2 lateral pointed lateral lobes (Figs. 22-25). The follis originate in the middle of pars distalis, completely exposed, with the latero-apical projections ventrally projected; the spiniform projections covering all interno-apical surface of follis; stylus originated from the inner follis, with apical bristles (Figs. 22-25). Macrosetal pattern as follow: MS C forming a longitudinal row on lateral margins of pars distalis, these setae are long and curved, widely separated each other; a pair of long and curved MS A, just below of row of MS C, on the basalmost part of pars distalis; 2 pairs of long MS D forming a high trapeze, below to the base of follis, sockets of these setae well-marked, rounded; ventrally with 3 pairs of small MS E, E1 and E2 subapically, forming a rectangle, an additional pair of small MS E on the basal pars distalis, between the lowest C and A pairs (Figs. 22-24).
Table 1 Measurements (mm) of Potosa elsanto sp. nov., male holotype.
| Trochanter | Femur | Patella | Tibia | Metatarsus | Tarsus | |
|---|---|---|---|---|---|---|
| Pedipalp | 0.40 | 1.03 | 0.66 | 0.76 | - | 0.63 |
| Leg I | 0.46 | 1.63 | 0.76 | 1.23 | 1.66 | 1.03 |
| Leg II | 0.56 | 2.66 | 1.06 | 2.43 | 2.40 | 2.16 |
| Leg III | 0.50 | 1.73 | 0.73 | 1.60 | 1.90 | 1.16 |
| Leg IV | 0.70 | 3.10 | 1.26 | 2.60 | 3.10 | 1.30 |
Female. Unknown.
Identification key to the species of Potosa based on males.
| 1. Ocularium without medium spine | ……………………………………….P. reddelli Cruz-López & Francke, 2015 |
| 1'. Ocularium with medium spine | …………………………………………………………………………………….2 |
| 2. Distitarsus II of leg II similar length as distitarsus I (Fig. 9). Sockets of genital setae D softly marked. Metatarsus IV apically not swollen (Fig. 10) | ……………………………………………..P. dybasi Goodnight & Goodnight, 1947 |
| Distitarsus II of leg II noticeably longer than distitarsus I (Fig. 7). Sockets of genital setae D well marked. Metatarsus IV apically swollen (Fig. 8) | ………………………………………………………….Potosa elsanto sp. nov. |
Taxonomic summary
Distribution. This species is known only from the type locality (Fig. 26).
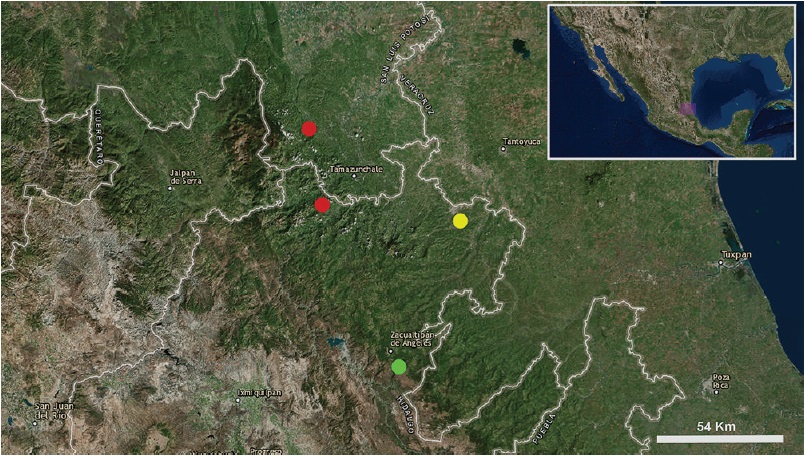
Figure 26 Known records of the 3 species of the genus Potosa: P. dybasi (red circles), P. reddelli (yellow circle), and P. elsanto sp. nov. (green circle).
Type data: male holotype (CNAN-T0037) from Ejido Atopixco (20º35’27.96” N, 98º36’51.48” W, 2,000 m asl) Zacualtipán Municipality, Hidalgo, Mexico. July 20, 2015 (O. Francke, G. Contreras, D. Barrales, L. Olguín, D. Guerrero colls.).
Other material examined: male of Potosa elsanto sp. nov. (DNA voucher CNANOp0089) from 2.5 Km N from intersection Zacualtipán-Santiago Tianguistengo (20º40’29.24” N, 98º40’07.93” W, 2,050 m asl) Zacualtipán Municipality, Hidalgo, Mexico. October 8, 2011 (J. Cruz, G. Contreras, J. Mendoza, D. Barrales colls.). Male of P. dybasi (TTU) from Cueva de El Jobo Municipality (21º25’22.94” N, 98º57’06.58” W) Xilitla Municipality, San Luís Potosí, Mexico. November 18, 1972 (J. Reddell, T. Raines colls.). Male holotype of P. reddelli (CNAN-T0797) and male and 5 females paratypes (CNAN- T0798) from Cueva de San José, San José, Ahuantempa, (21º05’55.96” N, 98º22’58.98” W) Huejutla Municipality, Hidalgo. March 18, 1981 (J. Reddell, D. McKenzie, T. Archey, F. Endres colls.).
Etymology. The specific epithet is a noun in apposition in honor to Rodolfo Guzmán Huerta, a.k.a “El Santo”, an emblematic Mexican wrestler and actor who was born in Hidalgo, Mexico, where the type locality is located.
Natural history. The specimens of P. elsanto sp. nov. were found living in sympatry with Chapulobunus unispinosus Goodnight & Goodnight, 1946, Crettaros sp., Hoplobunus sp., Karos sp., (Stygnopsidae), 2 undetermined species of Cosmetidae, Martensolasma catrina Cruz-López, 2017, and Trilasma sp. (Nemastomatidae).
Discussion
We have made considerable efforts to collect more specimens of this species and additional material of P. dybasi. We visited the localities recorded for the 2 previous described species at least once a year since 2011; however, we have not found any additional specimens of P. dybasi, and we only collected the 2 males reported here of P. elsanto sp. nov. For these 2 species, the females are still unknown. So far, Potosa has been the most difficult genus to collect in the field, despite the fact that we have used different collecting methods recommended for the study of tropical harvestmen (Curtis, 2007; Tourinho et al., 2014), it has been a problematic task to obtain additional specimens.
The presence of sexually dimorphic glandular openings among Laniatores is generally related to chemical intraspecific communication, may be for sexual purposes, however, the use of these glands are still unknown (Willemar et al., 2010). According to Willemart et al. (2010), glandular openings on metatarsus I in males have been reported in the literature for the families: Assamidae, Cosmetidae, Cranaidae, Stygnopsidae (for P. dybasi) and Triaenonychidae. But with exception of P. dybasi, these structures have never been studied using a SEM, therefore this is the first study showing detailed those glandular openings. On the other hand, Willemart et al. (2010) described glandular areas according by their similarities, following their criteria, “pores grouped inside a large (and generally deep) opening” are found on metatarsus and tarsus I of Cranainae, Gonyleptidae or Manaosbiidae. Willemart et al. (2010) showed different types of these type of glands from different species, but for the specific case of Isocranaus strinatii Šilhavý,1979 ENT[Cranaidae or Manaosbiidae? compare table 1 and 2 of Willemart et al. (2010) and Kury (2003)ENT], the authors illustrated an enlarged glandular opening on the ventral side of second tarsomere of leg I (Willemart et al., 2010: fig. 2B). At the moment the latter is the most similar opening to those on Potosa, but on the metatarsus instead first tarsomeres.
Thus far, similar glandular openings on legs are widespread among different lineages of Laniatores, but for Stygnopsidae, Potosa is the only genus that exhibits such structures. For this reason, this makes the genus an interesting group for future behavioral and evolutionary research.











 nueva página del texto (beta)
nueva página del texto (beta)


