Introduction
The genus Copelandia Bres. was proposed by Bresadola (1913) to accommodate those Panaeolus (Fr.) Quél. species presenting metuloidal pleurocystidia. The bluing reaction of the basidiomata -a common feature in most species- is indicative of the presence of psilocin, as well other psychoactive compounds, which renders these species the status as hallucinogenic (Guzmán et al., 2000; Singer, 1986). The strong morphological similarities with Panaeolus led some authors to recognize Copelandia as synonym of the Panaeolus (Gerhardt, 1996; Ola’h, 1969), although most authors consider it as an autonomous genus (Singer, 1986; Watling & Gregory, 1987). Copelandia, Panaeolus and Panaeolina Maire compose the Panaeoloideae clade (Moncalvo et al., 2002) but the systematic position of Copelandia remains unclear. Based on molecular phylogenetic analysis performed by Padamsee et al. (2008), Nagy et al. (2013) and Örstadius et al. (2015), members of Panaeolus and allies were not considered as belonging to Psathyrellaceae. In the same way, a multigene phylogeny of the Bolbitiaceae considered Panaeolus s.l. as a sister group, not a member of this family (Tóth et al., 2013). Thus, is expected that future work will confirm that Copelandia and Panaeolus are distinct genera and to what family they belong.
The genus is comprised of about 10 species (Guzmán et al., 2000), of which Copelandia cambodginiensis (Ola'h & R. Heim) Singer & R.A. Weeks (as Panaeolus cambodginiensis Ola'h & R. Heim), C. cyanescens (Berk. & Broome) Singer and C. tropicalis (Ola'h) Singer & R.A. Weeks (as Panaeolus aff. tropicalis Ola'h), are known from Brazil (De Meijer, 2006; Wartchow et al., 2010).
During an investigation of the agaricoid fungi in seasonal semideciduous forest (Oliveira & Cortez, 2016; Silva-Filho et al., 2016) in the western region of Paraná State, south Brazil, we examined collections belonging to Copelandia, which are described, illustrated and discussed as follows.
Materials and methods
Specimens were collected in 2 localities, comprised of fragments of Seasonal Semidecidual Forest (Atlantic Forest Biome) in the western region of Paraná State: RPPN Fazenda Açu, municipality of Terra Roxa and PE de São Camilo, municipality of Palotina. Morphological analysis (both macro- and microscopical) followed standard procedures for agaricoid fungi (Singer, 1986). Colour names and codes used in the macroscopical descriptions are based on Kornerup and Wanscher (1978). In the basidiospores description, Q is the quotient between the length and width, Qm is the medium value of Q and n is the number of measured basidiospores/number of analyzed basidiomata/ number of collections. Microscopic measurements and photographs were made under an Olympus CX31 optical microscope with a Toup Cam FMA050 digital camera and measurements were taken through software Toup tek Toup View. All specimens were dried in an open air drier (±40 ºC) and are preserved at the mycological collection of the Herbarium of Universidade Federal do Paraná (HCP).
Description
Copelandia cyanescens (Berk. & Broome) Singer, Lilloa 22: 473 (1951) Figure (1, 2, 6-10)

Figures 1-5 Copelandia basidiomata: 1, 2 Copelandia cyanescens - HCP 1030. 3-5 Copelandia mexicana. 3-4 HCP 1031; 5 HCP 1032. Scale bar = 5 mm. Photos: Alexandre Silva-Filho.
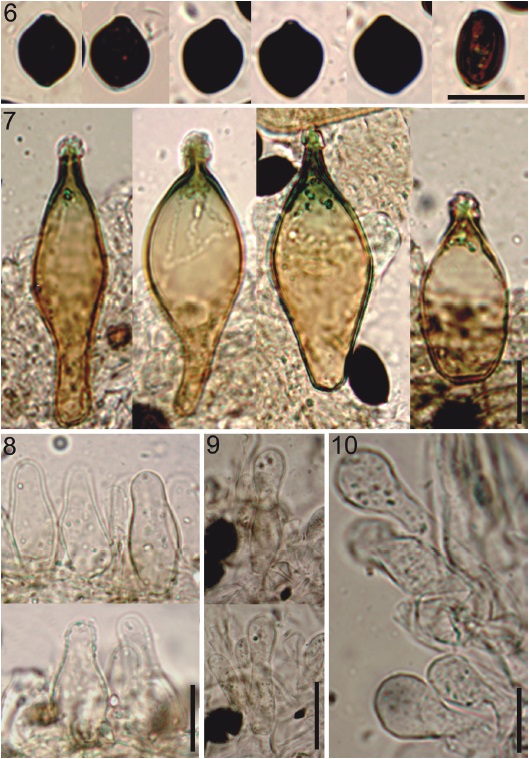
Figures 6-10 Copelandia cyanescens - HCP 1030. 6, basidiospores; 7, pleurocystidia; 8, cheilocystidia; 9, pileipellis; 10, caulocystidia. 6-10, scale bar = 10 µm. Photos: Alexandre Silva-Filho.
≡ Panaeolus cyanescens (Berk. & Broome) Sacc., Syll. Fung. 5: 1123 (1887)
Pileus 22-56 mm diam., broadly parabolic, convex, to broadly-convex, surface smooth, hygrophanous, yellowish-white (1A2) with light yellow (1A5) at the center, making bluish grey (23C3) to dark blue (25F4) when wounded and touched, margin non striated, acute to slightly reflexed (Fig 1). Lamellae adnate, crowded, with 2 lamelullae, edge smooth to slightly crenate, discolor paler with the sides, consistency fleshy, medium grey (1E1) to grey (1D1) with spots (Fig. 2). Stipe 48-110 × 2-6 mm, central, cylindrical, equal to slightly bulbous at base, fistulose, surface smooth to slightly fibrillose, slightly velutinous near the apex, consistency fleshy, with white mycelial pad in some specimens, yellowish white (1A2) to yellowish grey (2C2) at apex, brownish grey (4D2, 5C2) at base, turning bluish when touched on the pileus. Context 2 layered: the upper layer 1.5-2 mm thickness, yellowish with (1A1), and the lower layer 1.5-2 mm thickness, olive grey (1E2), fleshy. Veil absent. Spore print dark grey (1F1).
Basidiospores 11.5-14.5 × 9-11 µm (Q =1.13-1.48, Qm = 1.28, n = 30/1/3), limoniform in face-view, ellipsoidal in side-view, apically truncate by a germ-pore, thick-walled, reddish brown to black in KOH (Fig. 6), not bleaching in 95-98% sulfuric acid. Basidia 17-22.5 × 9-12.5 µm, broadly clavate to cylindric-clavate, tetrasporic, hyaline. Pleurocystidia 33.5-68 × 15-25 µm, as metuloids, abundant, pedicellate-utriform, broadly-fusiform, with acuminate and incrusted apex (muricate), thick-walled ≥ 1.5 µm, light brown with brown wall and greenish apex in KOH (Fig. 7). Cheilocystidia 21-37 × 7-14 µm, abundant, lageniform, obpyriform, some sphaeropedunculate, wall slightly thickened (< 1 µm), hyaline in KOH (Fig. 8). Lamella edge sterile. Lamellar trama irregular, with filamentous to inflated hyphae 5-20.5 µm diam., smooth, thin-walled, yellowish brown in KOH. Subhymenium a layer of subparallel hyphae, 1.5-4 µm diam., smooth, thin-walled, yellowish brown in KOH. Pileipellis an epithelium of isodiametric to pyriform elements, 11.5-29.5 µm diam., smooth, thin-walled, hyaline in KOH. Pileocystidia scarce, and in fascicles in the pileus margin 24-45 × 5.5-10 µm, lageniform to lageniform-subcapitate, thin-walled, hyaline in KOH (Fig. 9). Hypodermium with interwoven, filamentous hyphae, 2.5-8.6 µm diam., smooth, light brown in KOH. Pileus trama with irregular and inflated hyphae, 8-34.5 µm diam., yellowish brown in KOH. Stipitipellis a cutis, composed of filamentous hyphae 3-4 µm diam., smooth, yellowish brown in KOH. Stipe trama regular, 4.5-18 µm diam., smooth, wall slightly thickened, < 1 µm, yellowish brown to green grayish in KOH. Caulocystidia 22-47 (-94.5) × 7.5-14 µm, sphaeropedunculate, clavate or lageniform, non-incrusted at apex, thin-walled, hyaline in KOH (Fig. 10). Clamp connections present.
Taxonomic summary
Examined specimens: Brazil. Paraná State, Palotina, P.E. São Camilo, alt. 332 m, solitary and in small groups, in pasture, on cow dung, 25 May 2015, A.G.S. Silva-Filho 439 and 440 (HCP 1027, HCP 1028); ibid, 21 Jun 2016, A.G.S. Silva-Filho 783 (HCP 1030).
Distribution: cosmopolitan (Guzmán et al., 2000).
Copelandia mexicanaGuzmán, Bol. Soc. Mex. Micol. 12: 27 (1978) Figures (3-5, 11-15)

Figures 11-17 Copelandia mexicana - HCP 1031. 11, basidiospores; 12, section of lamellae; 13, pleurocystidia; 14, cheilocystidia; 15, caulocystidia; 16, pileipellis; 17, pileocystidia. Scale bar = 10 µm. Photos: Alexandre Silva-Filho.
Pileus 6-10 mm diam., at first convex, umbonate, convex slightly umbonate when mature, surface smooth, slightly striated to strongly translucent striated almost up to center, hygrophanous, at first olive brown (4D10), olive (3F4), margin greyish green (1D4), at maturity brownishgrey (5C2, 5D2) to light grey (5D1), margin pastel grey (5C1) to pale grey (5B1) (Figs. 3-5). Lamellae adnexed, crowded, with 2 lamelullae, edge even to slightly wavy, pale discolor with the sides, consistency fleshy, at first pastel grey (5C1), light grey (5D1) to olive grey (1D2), becoming brown (5F6) at maturity (Fig. 4). Stipe 11-56 × 1-3 mm, central, cylindrical, equal to slightly bulbous at base, fistulose, surface smooth, velutinous near the apex, consistency fleshy, with white mycelial pad in young specimens, at first greyish brown (6D5) at apex, yellowish grey (4B2) at base, in maturity specimens pastel grey (5C1) to light grey (5D1) at apex, greyish brown (5D3) to brown (5E5) at center toward the base (Fig. 5). Context thin (1-1.5 mm thick), fleshy, brownish grey (5C2), olive brown (4D1). Veil absent. Spore print not observed.
Basidiospores 6-9 × 4.5-6.5 µm (Q = 1.16-1.89, Qm = 1.44, n = 75/3/3), lenticular, limoniform in face-view, broadly ellipsoid in side-view, with truncate base and distinct germ-pore, thick-walled, reddish brown in KOH (Fig. 11), not bleaching in 95-98% sulfuric acid. Basidia 13-18 × 5-12 µm, cylindric-clavate to clavate, tetrasporic, hyaline. Pleurocystidia 24-50 × 7-16 µm, as metuloids, not abundant, pedicellate-utriform, some subcylindrical or subfusoid with obtuse apex, thick-walled, < 1 µm at the base and central portion, 1.5-3 µm in extreme apex, which also is incrusted crystals at apex (muricate), light brown in KOH (Fig. 13). Cheilocystidia 24-37 × 4-9 µm, abundant, obclavate, lageniform, wall slightly thickened, < 1 µm, without crystals incrusted at apex, hyaline in KOH, rarely thick-walled with crystals incrusted at apex (Fig. 14). Lamella edge sterile (Fig. 12). Lamellar trama irregular, with filamentous to inflated hyphae 5.2-15 µm diam., thin-walled, smooth, yellowish brown in KOH. Subhymenium with filamentous hyphae, 1.6-3.5 µm diam., smooth, thin-walled, light brown in KOH. Pileipellis an epithelium of cylindrical, isodiametric to pyriform elements, 9.5-22 µm diam., smooth, thin-walled, yellowish brown in KOH (Fig. 16). Pileocystidia scarce 22-40 × 4-8.5 µm, vesiculose-clavate to lageniform, thick-walled 0.5-1.5 µm, mostly yellowish brown in KOH, rare brownish (Fig. 17). Hypodermium a layer of filamentous hyphae, 2-8.5 µm diam., parallel, horizontally arranged, smooth, thin-walled, light brown in KOH. Pileus trama with inflated hyphae 3-5.5 µm diam., thin-walled, yellowish brown in KOH. Stipitipellis composed of a cutis of filamentous hyphae 1-3.5 µm diam., parallel, smooth, yellowish brown in KOH. Stipe trama regular, with inflated hyphae, 4.5-19.5 µm diam., smooth, yellowish brown in KOH. Caulocystidia 14-49 × 3-11 µm, pyriform, cylindrical, lageniform, wall slightly thickened (< 1 µm), non-incrusted at apex, hyaline in KOH (Fig. 15). Clamp connections absent.
Taxonomic summary
Examined specimens: Brazil. Paraná State, Terra Roxa, RPPN Fazenda Açu, alt. 332 m, solitary on decaying wood, 15 Jun 2015 A.G.S. Silva-Filho 491 (HCP 1031); ibid, 14 Oct 2015, A.G.S. Silva-Filho 632 (HCP 1032); ibid, 12 Nov 2015, A.G.S. Silva-Filho 641 (HCP 1033).
Distribution: previously known only from Mexico (Guzmán et al., 2000) and now from Brazil.
Discussion
The basidiospores not bleaching in 95-98% sulfuric acid and the metuloidal pleurocystidia are features that accommodates our species in Copelandia, subfamily Panaeoloideae Singer (Pegler, 1983; Singer, 1986). We prefer to maintain the generic name Copelandia as previosly adopted by Singer (1986).
Copelandia cyanescens compared with our C. mexicana specimens, produces more robust basidiomata, with larger pileus (22-56 mm), margin non striated, large basidiospores (11.5-14.5 × 9-11 µm), metuloidal pleurocystidia with pale yellowish brown walls, and hyphae with clamp connection.
The metuloidal pleurocystidia with deep green apex is a noteworthy feature that distinguishes Copelandia chlorocystis Singer & Weeks from other species belonging to this genus. This feature was observed in our collections of C. cyanescens (Fig. 7), but according to the original description, C. chlorocystis has smaller basidiospores (10.3-13 × 8-9.5 µm), which are produced in 2 and 3 spored basidia, and apparently do not grow on any kind of dung (Weeks et al., 1979).
Copelandia mexicana is characterized by the non-coprophilous habitat, small pileus (≤ 10 mm) and basidiospores (≤ 9 µm long), metuloidal pleurocystidia with crystals incrusted at apex with light brown content in KOH and presence of pileocystidia (Guzmán, 1978). All these features are in agreement with our south Brazilian sample collection, supporting the identification. It seems to be a rare and poorly known species of Copelandia, originally described from Mexico.
In the same manner, as mentioned by Guzmán (1978) in the protologue, our sample collection of Copelandia mexicana did not turn conspicuously blue when bruised or touched, as reported for most species of Copelandia (Gerhardt, 1996). However, the black spots noted on the dried basidiomata could be an evidence of this character, suggesting it to be a neurotropic fungus. Copelandia mexicana was described with a brownish pileus and stipe (Guzmán, 1978) and in our collection this feature was observed only in younger specimens (Fig. 3) that subsequently become grayish in maturity (Figs. 4, 5). In addition, our specimens have some cheilocystidia with a swollen base (Fig. 14), while the original description and illustration does not report this. Unfortunately, C. mexicana is only known from the original description by Guzmán (1978) and, to our knowledge, it has not been since recorded from México or other localities.
Copelandia tropica Natarajan & Raman from India and C. mexicana are closely related species; both have non-coprophilous habit, smaller basidiospores (≤ 9 µm long) and hyphae without clamp connections. However, C. tropica presents a larger pileus (ca. 35 mm), longer and white stipe (ca. 100 mm) and there is an absence of pileocystidia (Natarajan & Raman, 1983). Copelandia affinis Horak is another species that grows on soil and occasionally on decomposed wood (Horak, 1980). But, the mushroom described from Papua New Guinea has larger pileus (25 mm) with a surrounding disc conspicuously wrinkled, longer basidiospores (9-10 × 5-6.5 µm), larger cheilocystidia (9-14 µm diam.), and presence of clamp connections (Horak, 1980).
Copelandia mexicana was described by Guzmán (1978) as a species of Copelandia, but Gerhardt (1996), in a world revision of Panaeolus (including Copelandia as subgenus), considered that species as nom. excl., referring especially to the pale color of the basidiospores in KOH and the non-typical cystidia, which he reminded those of Inocybe. (Fr.) Fr. Gerhardt (1996) cannot assign C. mexicana to any known genus, but concludes that it does not belong to his concept of Panaeolus. However, Guzmán et al. (2000), in their world catalogue of neurotropic fungi, listed C. mexicana as an autonomous species. Based on the examination of Brazilian collections, the general morphology (both macro- and microscopical) recalls Panaeolus s.l. (incl. Copelandia) and we consider that Copelandia, by virtue of microscopic features, especially the presence of metuidal cystidia, is the correct genus for this taxon until further evidence arise.
Copelandia cyanescens is a common species found in pastures growing on cow dung (Weeks et al., 1979) and it is the widest distributed species of the genus, reported from all continents, especially due to general interest as neurotropic mushroom -although possibly misidentified many times. In Brazil, there are few records and the distribution is poorly known. Wartchow et al. (2010) recorded from Pernambuco and De Meijer (2006) from Paraná States as Panaeolus cyanescens (Berk. & Broome) Sacc. Copelandia mexicana up to now was only known from Mexico, according to Guzmán et al. (2000), and herewithin we expand its distribution to South America, with possible occurrence throughout the Neotropical forests from South America to Mexico.











 nueva página del texto (beta)
nueva página del texto (beta)


