Bopyrid isopods, except the endoparasite subfamily Entophilinae, are obligate crustacean ectoparasites, mainly on decapods (Poulin, 1995; Williams & Boyko, 2012). The effects of the presence of these parasites on their hosts includes the destruction or alteration of gonadal tissue, changes in reproductive behaviour, reduction in size or delay of gonad development, the latter defined as parasitic castration (Baudoin, 1975; Reinhard, 1956). Although these effects are usually more conspicuous in female hosts, the severity of gonadal alterations is variable and the host may not always be fully castrated (Calado, Vitorino, & Dinis, 2006).
Boyko and Williams (2009) pointed out that although there are several reports on the taxonomy and biology of bopyrid isopods, the information on the geographic range and host specificity of this group is scarce for most species. The regions that appear to lack or contain low numbers of bopyrids may be due to an artefact of limited sampling (Shields, Boyko, & Williams, 2015). Thus, even when numerous studies have reported on the taxonomic and ecological information for hosts, reports about their parasite species are often overlooked or omitted.
The western Atlantic between North Carolina, USA, through the Gulf of Mexico and the Caribbean is the region with the third highest bopyrid diversity with a total of 79 species, only behind the North West Pacific (NWP, 123 species) and East Asia Sea (116 species) (Shields et al., 2015; Williams & Boyko, 2012). Bopyrids that infest brachyurans also show the highest diversity in the NWP (44 species), but the Caribbean is the fourth most diverse region for these parasites with 9 species (Shields et al., 2015). For Mexico, there are 43 bopyrid isopod species reported, 19 on the Pacific coast and 24 on the Atlantic coast (Román-Contreras, 2008; Román-Contreras & Martínez-Mayén, 2011; Romero-Rodríguez & Martínez-Mayén, 2017). Nevertheless, the detailed information on the geographical distribution of each species within the country is incomplete.
According to Markham (1985) the first species of bopyrid isopod described in America was Leidya distorta (referred as Cepon distortus) (Leidy, 1855) associated with the host Uca pugilator (Bosch, 1802) sampled from the coasts of New Jersey, USA. Although this bopyrid has been reported from other locations on the western Atlantic coasts (Fig. 1), the present study represents the first report of the presence of L. distorta from the Atlantic coast of Mexico. Likewise, the association of this bopyrid with Uca spinicarpa (Rathbun, 1900) represents a new host record for the superfamily Ocypodoidea, since this parasite was previously reported infecting to Ucides cordatus (Linnaeus, 1763) and several Uca species including U. pugilator, U. minax (LeConte, 1855), U. pugnax (Smith, 1870), U. vocator (Herbst, 1804), and U. uruguayensis (Nobili, 1901) (Bourdon & Bowman, 1970; Lemos-de Castro, 1973; Roccatagliata & Torres-Jordá, 2002) (Fig. 1). The distribution of U. spinicarpa spans from the western coast of Florida, USA, to the Laguna de Términos, Campeche (Arruda-Becerra & Alves-Coelho, 2010) and in the coastal lagoon of Progreso Yucatán, Mexico (Guillén-Hernández, García-Varela, & Pérez-Ponce de León, 2008) (Fig. 1).
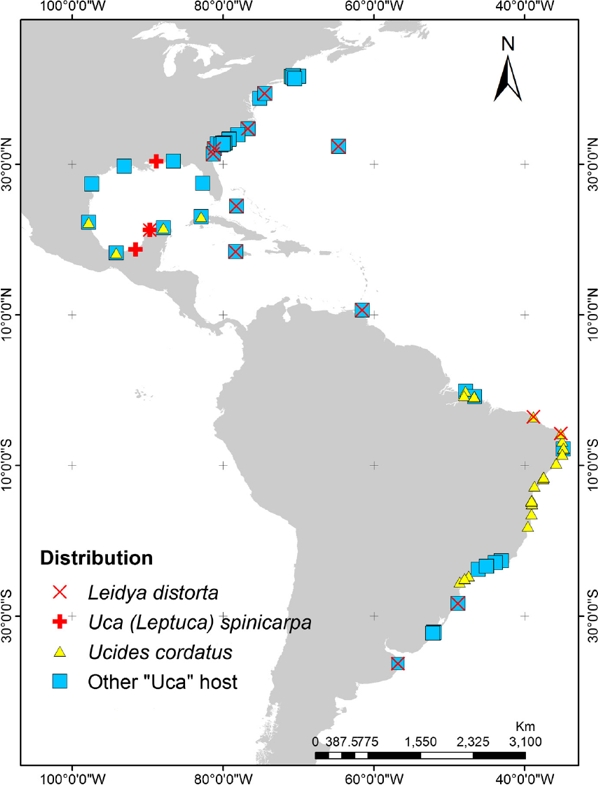
Figure 1. Distribution of Leidya distorta and its hosts throughout the western Atlantic region. Uca species included in the “other Uca hosts” refer to U. pugilator, U. minax, U. pugnax, U. vocator, and U. uruguayensis. Information taken from: Bourdon and Bowman (1970), Lemos-de Castro (1973), Raz-Guzmán and Sánchez (1992), Roccatagliata and Torres-Jordá (2002), Guillén-Hernández et al. (2008), and http://www.iobis.org/es, http://www.GBIF.org.
A total of 2,467 individuals of U. spinicarpa were collected by hand from March 2002 to July 2007 in the lagoon system of Chelem, situated in northern Yucatán (21°17’ N, 89°40’ W), as part of a project to document fish parasites and invertebrates from the coast of Yucatán, Mexico. Based upon this sample of fiddler crabs, 93 individuals were parasitized by the bopyrid isopod L. distorta, which represents a prevalence of 3.77%. This value is lower than the prevalence reported by Torres-Jordá and Roccatagliata (2002) for the same bopyrid species infecting U. uruguayensis (9.3%) in the Río de la Plata estuary, Argentina.
The males of U. spinicarpa (n = 82; prevalence = 4.07%) were more parasitized than females (n = 11; prevalence = 3.06%) although the mean length and width of the carapace was similar for both sexes (Table 1). This contrasts with results reported for L. distorta infesting to U. uruguayensis where the prevalence was similar for both crab sexes of the same size class, although was biased to males as a function of the crab size (Roccatagliata & Torres-Jordá, 2002). Rather, the greatest levels of infestation observed for males of U. spinicarpa may correspond to differences in the relative abundance of each sex, as was suggested by Beck (1979). Accordingly, the sex ratio of the studied sample of U. spinicarpa differs significantly from the ratio 1:1 and was strongly skewed towards males for both parasitized (0.88:0.12 M:F; χ2 = 31.7, p < 0.05) and unparasitized individuals (0.85:0.15 M:F; χ2 = 657.6, p < 0.05).
Table 1 Carapace size of fiddler crab Uca spinicarpa specimens sampled in the lagoon system of Chelem, Yucatán, Mexico.
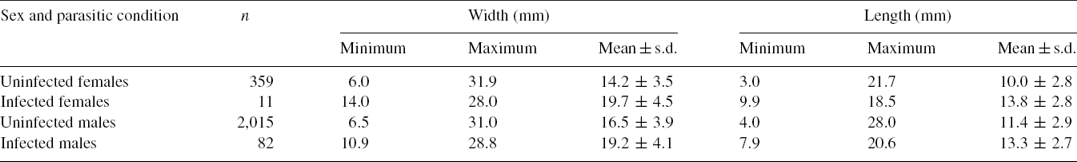
Although there is no consensus on how the infestation by bopyrids influences host growth, it is generally accepted that these parasites have a negative effect on the growth of their hosts because the maximum size of parasitized individuals is usually lower than that of their non-parasitized counterparts (O’Brien & van Wyk, 1985). However, this differs from our results since U. spinicarpa specimens infested with L. distorta had a greater mean size than unparasitized specimens (Table 1). Further work is needed in order to properly evaluate the influence of L. distorta on U. spinicarpa growth.
We recorded a total of 137 bopyrid isopods from 93 infested fiddler crabs in our sample (90 females, 45 males and 2 cryptoniscus larvae), which represents a mean intensity (number of parasites per infected host) of 1.47 and mean abundance (average number of parasites in the host sample) of 0.06. All the bopyrid isopods examined matched the morphological characteristics described by Richardson (1908) and Bourdon and Bowman (1970) for L. distorta. Based on the criteria established by these authors, females were classified as: juvenile –the development of the oostegites ranged from incipient to a length that reached half way dawn the ventral side of the female, but without meeting at midline–; subadult –females with slight or completely overlapping oostegites but without a fully closed brood pouch (Fig. 2B)–; non-ovigerous adult –females overlapping oostegites, brood pouch fully developed but with no embryos; and ovigerous females– same as to the previous category but with embryos in the brood pouch.
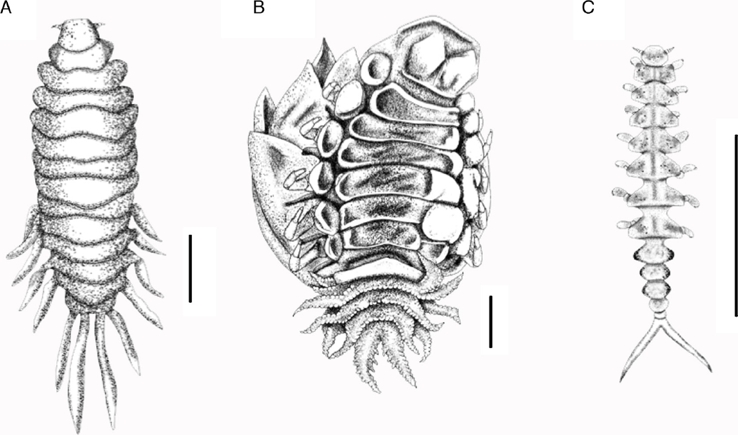
Figure 2. Developmental stages of Leidya distorta specimens infecting the gill chamber of the fiddler crab Uca spinicarpa in Laguna de Chelem, Yucatán, Mexico. A, immature female; B, mature female; C, male. Scale bar = 1.0 mm.
The size of the juvenile females (n = 36, 39.5%) ranged from 1.0 to 6.9 mm of total length (TL), with a mean of 4.1 ± 1.4 mm TL. For 7 of these females, the lateral plates of the 5 pleomeres were long and slender, but with smooth margins and were not tuberculate (Fig. 2A). Thirteen juvenile females had foliaceous processes on the dorsal side of the pleomeres, mainly on the first and second ones, and these were of a variable length, e.g., the shorter foliaceous processes situated on the first pleomere reached the distal edge of the third pleomere, whereas the longer one exceeded the pleon distal edge. For one juvenile female, a foliaceuos processes were observed on the first 3 pleomeres, the longest pair was situated on the first pleomere and surpassed the distal edge of pleon, whereas the pair on the third pleomere was only slightly development. The development of the frontal lamina and the lobes of the cephalon in juvenile females appear to be associated with body size, as the smallest females frequently lacked these features.
The 9 subadult females (10%) had a size interval between 4.0 and 7.0 mm, and a mean size of 5.6 ± 1.0 mm TL. Foliaceous processes on the pleomeres were not observed on any of these specimens. Six specimens had slight bulges on the dorsal side of pereomeres 1–6 which were more evident in larger females, since they possessed incipient rounded dorsal bosses.
In general, the dorsal bosses of adult females were conspicuous and subquadrangular; however, 6 of these specimens lacked developed dorsal bosses and only exhibited the lifting of a sharp defined edge in the mid-dorsal portion of pleomeres 1–6, which formed a strong carina. For 3 of the specimens, we observed 2 dorsal bosses close together for pereomere 5 which produced a bilobate appearance. The size interval of 20 non-ovigerous females (22%) ranged from 4.0 to 7.2 with a mean size of 5.8 ± 0.8 mm TL. The 26 ovigerous females (28.5%) ranged from 4.5 to 8.0 with a mean size of 6.4 ± 0.8 mm TL.
The body of L. distorta males infesting U. spinicarpa ranged from narrow and elongated with well-defined and separated pereomeres (Fig. 2C), as illustrated by Richardson (1908), to a robust and compact body because the pereomeres were very close together, this is similar to the description by Bourdon and Bowman (1970). Likewise, 36 out of the 45 males (80%) had pigmentation on the side of the pleomeres, mainly on pleomeres 1–3. This feature was not reported in previous studies of this species and might be retained from their cryptoniscus larval stage since larvae recorded in this study had the side edges of all pleomeres pigmented. The size intervals of males ranged from 0.8 to 4.8 mm TL with a mean size of 1.5 ± 0.6 mm TL.
Bourdon and Bowman (1970) noted morphological differences in females of L. distorta infesting different hosts that are consistent with some of the patterns recorded in the present study. Such morphological variation could be associated with early development of some females stimulated by the presences of male specimens. Males could promote accelerated development of the ovaries and influence the morphology of the female (e.g., development of the oostegites), as proposed for the bopyrid Bopyrina abbreviata Richardson, 1904 (Romero-Rodríguez, Román-Contreras, & Bortolini-Rosales, 2017). It remains unknown whether the population characteristics of L. distorta are influenced by the host species they infect or if there are morphological differences for this parasite among populations of the same host species inhabiting different geographical areas. Likewise, it would be interesting to survey other potential brachyuran hosts distributed along the Atlantic coast of Mexico in order to better describe the relationships between this parasite and its hosts, including impacts on morphological, as well as demographic aspects.











 nueva página del texto (beta)
nueva página del texto (beta)


