Introduction
Currently, 10% of the amphibian and reptile species known worldwide inhabit Mexico; 376 species of amphibians have been reported in this country (Parra-Olea, Flores-Villela, & Mendoza-Almeralla, 2014). Some authors consider that less than 20% of the Mexican amphibian species have been studied for parasites (Pérez-Ponce de León, García-Prieto, & Razo-Mendivil, 2002), and very few surveys have been carried out at community level (Cabrera-Guzmán, León-Règagnon, & García-Prieto, 2007; Guillén-Hernández, 1992; Mata-López, García-Prieto, & León-Règagnon, 2002; Mata-López, León-Règagnon, & García-Prieto, 2013; Paredes-Calderón, León-Règagnon, & García-Prieto, 2004; Zelmer, Paredes-Calderón, León-Règagnon, & García-Prieto, 2004). There are 36 amphibian species in the Yucatán Peninsula (Lee, 1996), but only 8 species have been studied as hosts of helminth parasites (Espínola-Novelo & Guillén-Hernández, 2008; Pearse, 1936; Terán-Juárez, 2011; Yáñez-Arenas & Guillén-Hernández, 2010). To date there are no studies on helminth communities of amphibians in the Yucatán Peninsula.
Two species out of the 36 amphibians reported for this region inhabit terrestrial environments: the marine toad Rhinella marina and the southern Gulf Coast toad Incilius valliceps. Structural differences in cover among vegetative stands (patches) create differences in thermal and moisture conditions where different amphibian species reside. Rhinella marina is distributed from southeastern Texas to Mexico, Central America, and Brazil in South America. The size of this species has been recorded up to 20 cm snout-vent length (SVL). This species presents nocturnal behavior and it can be found in open habitats, secondary vegetation, near dwellings or in water bodies (Campbell, 1998; Lee, 1996). Incilius valliceps is distributed from the southwestern United States along the Gulf of Mexico, to Costa Rica in Central America, and ranges in size from 6 to 9 cm SVL. Both species are generalists differing only in the group of arthropod they feed on and prey sizes (Gelover-Alfaro, Altamirano-Álvarez, & Soriano-Sarabia, 2001).
Aho (1990) pointed out that amphibian helminth communities are depauperate (comprising relatively few species), non-interactive (interspecific interaction among species is weak) and nematodes frequently dominate their parasite community composition. These observations were based on data obtained from temperate zones of North America, however studies have found variations to these patterns in other regions (Hamann, González, & Kehr, 2006; Luque, Martins, & Tavares, 2005). Therefore, more species of amphibians need to be examined from different latitudes and samples need to be collected from different locations in order to evaluate the spatial variability of these biological characteristics and characterize potential variation in the structure of amphibian helminth communities.
A number of authors have presented arguments for including parasites in biodiversity studies (Poulin, 2004; Poulin & Morand, 2004); in particular, studies on amphibian parasites have provided lists of parasite taxa in a host or host species, providing valuable information on parasite species richness, host habits and ecosystem interactions that can be used in monitoring and conservation programs. Other studies on parasites have been based on the relative abundance of parasites in the assemblage of host species, providing important knowledge for identifying the condition of the ecosystem. A useful approach to characterize key parasite communities is to analyze parasite diversity at two community levels: infracommunity (all parasite infrapopulations within a single individual host) and component community (all infracommunities within a given host population) (Bush, Lafferty, Lotz, & Shostak, 1997). This latter approach provides insights into understanding the role of parasites in maintaining ecosystem processes and makes it possible to describe diversity patterns quantitatively. In addition, component community level is a better approach for estimating the local pool of parasite species (Poulin and Morand, 2004). The aim of this study was to characterize the helminth communities of R. marina and I. valliceps collected from the Lagunas de Yalahau (Yucatán, Mexico) at both infracommunity and component community levels to provide diversity and species richness data, which could enhance our comprehension of the patterns in amphibian parasite communities. We used a method suggested by Chao et al. (2014) to analyze richness and diversity; the advantage of this method is that it expresses the results in units of effective numbers of species present in the community, unlike other indices where the result obtained does not directly express the number of species. These types of studies could provide an overall picture of the diversity of helminth communities in these toad species at this locality and might improve the available richness data for future comparisons.
Materials and methods
We carried out samplings on a bimonthly basis between February 2005 and November 2006 in the Lagunas de Yalahau, which are located in the central region of the Yucatán Peninsula (20°34’59.7” – 20°40’37.3” N, 89°10’49.6” – 89°15’00.5” W). We collected 54 specimens (juvenile to adult phases) of Incillus valliceps and 52 of Rhinella marina. We measured the SVL (the total length between snout and vent) of each individual and then sacrificed the specimens by cooling them and maintained them at −20 °C until examination. In order to obtain numerical data and describe the communities, we dissected each toad, collected, and identified the parasites in accordance with Espínola-Novelo and Guillén-Hernández (2008), where Chaunus marinus and Cranopsis valliceps are synonymous of R. marina and I. valliceps, respectively.
We analyzed the helminth community at 2 levels: infracommunity (all parasites of different species within the same host individual) and component community (all parasite species exploiting a host population at a given location) (Poulin, 2004). For each of the toad species, and in order to carry out the community analysis, we constructed 2 matrices, one of parasite abundances (infracommunity level) and another of presence–absence across sampling units (component community level) for each of the parasites identified (Espínola-Novelo and Guillén-Hernández, 2008). In order to evaluate whether the sample size was large enough to estimate helminth species richness within the toad populations, we performed empirical estimates of Hill numbers using the R Statistical Software. In order to characterize and compare the species diversity of parasites in both toad species, we used the framework of analysis suggested by Chao et al. (2014) with the R package iNEXT (Hsieh, Ma, & Chao, 2016). This method generalizes the sample-size-based approach of Colwell et al. (2012) and the coverage-based approach of Chao and Jost (2012) to produce and expand rarefaction–extrapolation curves of species based on Hill numbers (Hill, 1973). Hill numbers are a mathematically unified family of diversity indices, differing among themselves only by an exponent q. These indices provide a suitable framework for measuring diversity because: (1) they are expressed in units of effective numbers of species, (2) by using algebraic transformation, they are easily associated with key diversity indices such as Shannon entropy and the Gini–Simpson index, and (3) their estimations can be effectively generalized to incorporate host–parasite species assemblages (Chao et al., 2014). We calculated the first 3 Hill numbers (Hill, 1973), which are associated with estimators of species richness and species dominance, and constructed their respective rarefaction and extrapolation curves. The first Hill number (q = 0) used in the analysis estimates the expected parasite species richness (the number of species) in each toad species. The second Hill number (q = 1) is the exponential of the Shannon entropy index and estimates parasite diversity with respect to equally common species and species richness. The third Hill number (q = 2) is the inverse Simpson concentration index and measures the dominance of parasite species (abundant and prevalent species) in each toad species (for further details of the Hill numbers, see Hill, 1973).
To compare between R. marina and I. valliceps, we produced rarefaction and extrapolation sample-size-based and coverage-based curves to provide asymptotic estimators of diversity based on Hill numbers with their respective 95% confidence intervals, constructed using a bootstrap method (Chao et al., 2014). To provide an estimate of the sample size needed to achieve a fixed degree of sampling completeness [when the proportion of undetected species remains unchanged even when the sample size increases (Chao and Jost, 2012)] we constructed a sample completeness curve by combining the sample-size-based and the coverage-based estimations. We extended all extrapolations up to double the reference sample size (n = 52 and 54 for R. marina and I. valliceps, respectively) to predict parasite diversity in each toad species by doubling the sampling size.
We calculated the similarity between the 2 communities using the Jaccard Similarity Index and Percentage Similarity Index using the presence–absence and abundance of species, respectively (Krebs, 1989; Magurran, 1988). We used the Brillouin index to obtain the richness (number of species per number of hosts examined), abundance (number of parasites per number of hosts examined) and diversity and evenness at infracommunity level (Magurran, 1988). We performed these analyses using MVSP version 3.2 (Kovach, 2003). Because the distribution of the population of parasites in the host population was not normal, we used the Spearman’s rank correlation (rs) analysis (Sokal & Rohlf, 1995), in the Centurion STATGRAPHICS XV.II program (Sigma Plus, France) to evaluate the possible correlation between host sizes (SVL) and the abundance and richness of parasites.
Results
We collected samples of R. marina during 2005 (n = 39) and 2006 (n = 13) and estimated the average size (SVL) to be 15.08 cm (±2.42 std. dev.), with a range of 9–20 cm. We also collected samples of I. valliceps during 2005 (n = 36) and 2006 (n = 18), and estimated the average size (SVL) to be 5.7 cm (±1.5 std. dev.), with a range of 3.4–8.5 cm. In general, for the 3 parasite diversity estimators (species richness q = 0; exponential of Shannon entropy q = 1; and inverse Simpson concentration q = 2) all rarefaction and extrapolation sample-size-based and coverage-based graphs showed that the sample size (specimens examined) was large enough to register most of the species of parasites in both host toad species. Diversity curves showed little gain in estimates of species richness, equally common species and dominance with respect to increased sampling effort above the 50 sampling units (Fig. 1).
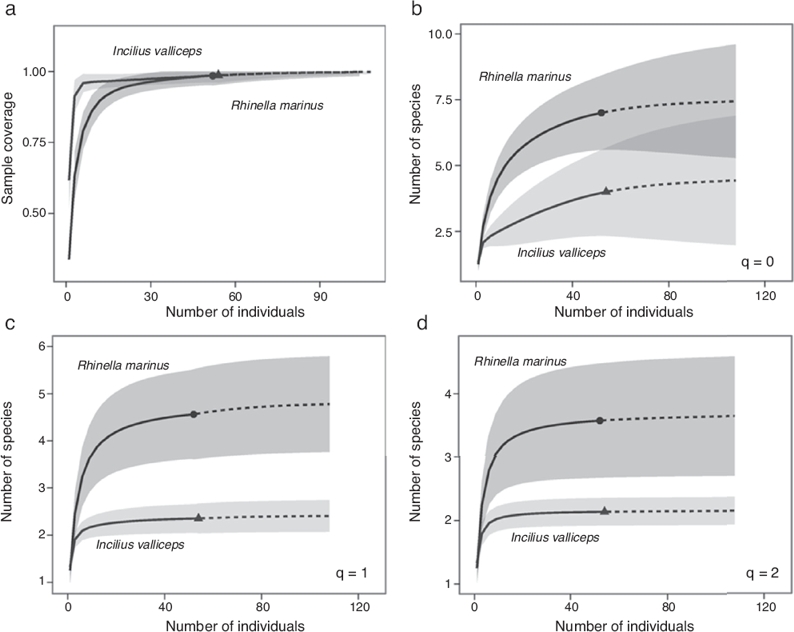
Figure 1. Estimators of diversity of parasite communities in I. valliceps and R. marina from Lagunas de Yalahau, Yucatán. (a) Sample completeness curve, sample-size-based vs. the coverage-based estimates extrapolations were extended up to double the reference sample size (50); (b) species richness q = 0; (c) the diversity of equally-common species exponential (Shannon entropy q = 1); and (d) the dominant species diversity (inverse Simpson concentration, q = 2). The 95% confidence intervals (color-shaded regions) were obtained by a bootstrap method based on 200 replications.
We recorded a richness of 4 and 7 helminth species in the 54 specimens of I. valliceps and in the 52 of R. marina examined, respectively. We estimated a maximum richness of 4.5 for I. valliceps and of 7.5 for R. marina; therefore, the sample size used in this study (sampling effort) was a good estimator of the parasite richness that may be found in both host species, at least for this sampling location (Fig. 1a). Differences in parasite species richness between the 2 host species were not statistically significant from each other when we used more than 50 individuals per sample (Fig. 1b). That is to say, above this sample size, it was likely that both hosts had a similar number of species of parasites, whereas below this sample size, the difference between the 2 toad species would be significant.
Exponential estimates of Shannon entropy (q = 0) in the hosts R. marina and I. valliceps also showed that the number of species observed and predicted were very similar, indicating that sampling included almost all equally common species that could be present in both host species. However, the number of common parasite species was significantly lower in I. valliceps than in R. marina (2 and 4 parasite species, respectively) (Fig. 1c). Furthermore, the species dominance index, estimated using the inverse Simpson concentration (q = 2) was slightly higher in R. marina than in I. valliceps (3 and 2 dominant parasite species, respectively). Although the difference between the 2 hosts was in the order of 1 species, this difference was significant (Fig. 1d). Interestingly, these results showed that in the 2 toad species, approximately half of all species of parasites were dominant species. This feature is apparently more relevant in I. valliceps where 2 out of 4 species of the parasites reported were dominant populations.
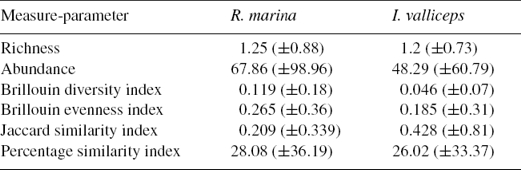
At infracommunity level, we found a maximum of 3 species in a single individual of R. marina and 2 in I. valliceps. In R. marina 11 individuals (21.1%) had no parasites, 21 (40.4%) harbored only 1 species, 16 (30.7%) harbored 2 and finally in 4 individuals (0.77%) 3 parasite species were found. On the other hand, 10 individuals (18.5%) of I. valliceps had no parasites, 23 (42.5%) had 1 species and 21 individuals (38.8%) had 2 species. The nematode Aplectana itzocanensis dominated both R. marina and I. valliceps infracommunities. This nematode species dominated in 53.8% of the infracommunities of R. marina and 66.6% of those of I. valliceps. The high dominance of A. itzocanensis produced low values of evenness and diversity in both host communities. Similarity values among hosts at infracommunity level were very low, with a mean Jaccard index value of 0.19 (±0.29 std. dev.) and similarity percentage of 29.75% (±37.1 std. dev.). Infracommunities of I. valliceps showed greater similarity to each other than those of R. marina (Table 1).
Table 1 Description of helminth communities at infracommunity level. Values are average and standard deviation are shown in parentheses.
There was a positive correlation between the size (SVL) of R. marina and parasite abundances (rs = 0.4190, p = 0.0028), however, we found no significant correlation between SVL and richness (rs = 0.1965, p = 0.1605). In I. valliceps, size was not significantly correlated with abundance (rs = 0.1843, p = 0.1796) or with richness (rs = 0.1765, p = 0.1987).
Discussion
The results showed that the sample size in this study was large enough to record the parasite species present in both toads, suggesting that the pool of parasite species present in the area is small, and that the 2 host species may eventually acquire them throughout their lives. This may explain why we found a positive correlation between host size and parasite abundance in R. marina (increased parasite abundance in larger hosts), but not between host size and the number of parasite species in either toad species. The results of rarefaction–extrapolation showed no differences in the species richness of helminths present in both host species with a sample size greater than 50 individuals. Sample sizes smaller than 50 individuals produce erroneous results. However, in relation to diversity, dominant species play a more important role in the helminth community of I. valliceps than that observed in R. marina. Consequently, in R. marina the common species are more important than the dominant ones; these differences are statistically significant. These results could be related to the movement between environments that occurs in R. marina (aquatic-terrestrial) and the preference of I. valliceps to stay longer in the terrestrial environment (Duellman & Trueb, 1994). This favors the presence of established parasites in both environments in R. marina, increasing the diversity of their community of helminths, and explains the greater dominance of a group of species in I. valliceps.
The taxonomic structure of helminth communities in R. marina and I. valliceps (see Espínola and Guillen-Hernández; 2008) fits the pattern proposed for amphibians (Aho, 1990; Barton, 1999), in which nematode species commonly dominate the community composition and where the richness is low (depauperate). However, considering records for the same host species in other locations at component community level, richness is higher than that reported for islands such as Australia (Barton, 1999) or the Caribbean (Goldberg & Bursey, 1992; Goldberg, Bursey, & Tawil, 1995; Linzey, Bursey, & Linzey, 1998; Ragoo & Oman-Maharaj, 2003) and several localities in northern latitudes, e.g. Jalisco (Galicia-Guerrero, Bursey, Goldberg, & Salgado-Maldonado, 2000), Nuevo Leon (León-Règagnon, Martínez-Salazar, Lazcano-Villareal, & Rosas-Valdez, 2005) and Texas (Mcallister, Upton, & Conn, 1989). However, richness values are lower than those reported in other areas of Mexico, such as Veracruz (Goldberg, Bursey, Salgado-Maldonado, Báez, & Cañeda, 2002; Guillén-Hernández, 1992) and Quintana Roo (Terán-Juárez, 2011). Differences in richness among the islands are probably related to the low diversity of organisms present (including both the intermediate host and helminths) on the islands in relation to those on the mainland (Biogeography theory) (MacArthur, Wilson, & MacArthur, 1967). In comparison with other studies in toads carried out in Mexico, the helminth communities of R. marina and l. valliceps from Yucatán are poorer than those previously reported from Veracruz and Quintana Roo. This may be due to the geographical position, geological history and the number of locations sampled. Veracruz is located between the Nearctic and Neotropical regions, where species from both regions can occur, whereas Yucatán is less influenced by the Nearctic zone, which decreases the probability of the presence of helminth species related to this zone (Espínola-Novelo and Guillén-Hernández, 2008). Furthermore, the Yucatán Peninsula presents a recent biota dispersal (Morrone, 2005); hence, it is likely that some helminth species or their intermediate hosts have not yet colonized this region (geological history). The lower parasite richness found in I. valliceps from Yucatán compared to that reported in Quintana Roo (Terán-Juárez, 2011) might be related to the different sampling strategies used. In the present study, we collected all hosts at a single location (Lagunas de Yalahau), while Terán-Juárez (2011) sampled 6 locations in Quintana Roo. Infracommunities are subsets of richness at the component level (Poulin, 1997), which at the same time is a subset of a large collection of species referred to as the parasite fauna of a host species (Poulin, 2004). Therefore, a greater number of samples from different locations increases the probability of finding species that are part of the richness at regional level.
An important point to discuss is that both host toad species sampled occur mainly in terrestrial environments and in general, amphibians associated with aquatic habitats have richer and more diverse communities than terrestrial or arboreal amphibians (Guillén-Hernández, 1992). The environment in which the hosts are present could explain the presence of more nematode species in both communities, compared to other taxonomic groups. In Veracruz, hosts from terrestrial habitats had more nematode species than the aquatic ones, which agrees with the previously mentioned pattern. However, in Yucatán both aquatic and terrestrial hosts had more nematodes than digeneans (Espínola-Novelo and Guillén-Hernández, 2008; Yáñez-Arenas and Guillén-Hernández, 2010). Our results suggest that biotic or abiotic conditions may be altering this pattern. A low richness or abundance of suitable invertebrate species acting as intermediate hosts (e.g. snails and dragonfly nymphs) throughout the life cycle of digeneans at this location may introduce considerable variation in parasite species richness that is not related to the characteristics of the final host species (Dobson, 1990). This may explain the low number of digenean species found. For example, the digeneans Meoscoelium monas use a snail as their first and second intermediate hosts, and Langeronia macrocirra use a dragonfly nymph as their second intermediate host. In addition, the water in this lagoon has high levels of bicarbonate (Semarnat, 2004), which may influence the presence of suitable intermediate hosts (snails) or may affect the survival of the free-living larval stages of the digeneans. Although both species are terrestrial, R. marina is often located closer to permanent water bodies than I. valliceps, which spends more time in primary vegetation and only approaches temporary water bodies for breeding (Campbell, 1998; Lee, 1996). This behavior increases the like-lihood of R. marina feeding on aquatic organisms (e.g. snails and insects) which act as intermediate hosts in the life cycles of species of the genus Mesocoelium (e.g. snail genus Lamellaxis) and Langeronia (e.g. dragonfly nymph) (Goodman, 1989; Kennedy, Killick, & Beverley-Burton, 1987) and Oncicola sp. However, our results showed that a higher number of nematode species are present in these communities and that these nematodes are abundant. This latter result might suggest that feeding habits are a minor source of parasites for terrestrial hosts, contrary to what occurs with aquatic hosts such as Lithobates spp. (Guillén-Hernández, 1992; Paredes-Calderón et al., 2004). In these cases, digeneans are an important component of their communities.
Another factor that could influence the richness and diversity of parasites is the body size of the host (SVL). According to Kuris, Blaustein, and Alió (1980), larger hosts will present a higher species richness. In amphibians, Muzzall (1991) found that the host size is correlated with parasite richness and abundance. We found a positive correlation between R. marina SVL and the number of individual worms, but not with the number of parasite species. However, the correlation value was low (rs = 0.41), which may be a consequence of several factors (host size, broad host diet, host geographical distribution, long-lived host and host population density), which are involved in the process of colonization and extinction and act in synergy (Poulin, 2004). However, similarity was higher among infracommunities of I. valliceps and it can be considered more predictive than in R. marina, since the values were low and showed that parasites probably reach each host (individual) by chance. Guillén-Hernández (1992) reported the same pattern in both species in Veracruz.
Although intermediate results have been reported between isolationist and interactive communities in Leptodactylus latinasus (Hamann et al., 2006) and unusually high richness in Rhinella icterica (Luque et al., 2005), in general amphibians harbor isolationist infracommunities (Aho, 1990; Akani, Luiselli, Amuzie, & Wokem, 2011; Bolek & Coggins, 2003; Muzzall, 1991). We found all the characteristics that define an isolationist community in the helminth communities in the present study (i.e. low species richness, high percentage of individuals harboring less than 2 species, high dominance of a single or few species). In conclusion, amphibian communities in this part of the tropics follow the same pattern as described for northern latitudes.
This study is the first to describe the amphibian helminth communities in the Yucatán Peninsula. Our results suggest that the habitat of the host, geological history and ecological conditions are important factors in the structure of helminth communities. We have paid greater attention to the results found at the component community level than at the infracommunity level since this is a better approach to estimate the local pool of parasite species (Poulin and Morand, 2004). It would be interesting to compare the communities described in this study with those of hosts from different habitats (e.g. aquatic and arboreal).











 nueva página del texto (beta)
nueva página del texto (beta)


