Introduction
The family Saturniidae (Lepidoptera) comprises 2349 species in 169 genera and 9 subfamilies (Minet, 1994; Nieukerken et al., 2011; Regier et al., 2008). Saturniids have a worldwide distribution and their greatest richness is found in tropical regions (Lemaire, 1978, 1980, 1988). Saturniids are mostly nocturnal and can easily be captured using a source of light, and are taxonomically well known (Lemaire, 1978, 1980, 1988). They play an important role in ecosystems since they are herbivorous (Kagata & Ohgushi, 2012) and also prey for many animals and other insects (Bellows, Owens, & Huddleston, 1982; Collins, 2013; Peigler, 1994; Young, 1982). Caterpillars of some species are a vital source of proteins and fats in rural communities (Gahukar, 2011; Munthali & Mughogho, 1992; Ramos-Elorduy, Landero-Torres, Murguía-González, & Pino, 2006). Cocoons are economically important in silk industry (Peigler, 2012) and they show a high potential in medical applications (Rockwood et al., 2011; Tulachan et al., 2014).
In Mexico, saturniids are represented by 213 species in 38 genera and 5 subfamilies (Balcázar & Beutelspacher, 2000). The 4 states of the country with the greatest richness of Saturniidae are Chiapas, Veracruz, Oaxaca and Puebla (Balcázar & Beutelspacher, 2000). Veracruz has 102 species of Saturniidae, which is 53% of the known species in the country (Revueltas-Morales, 2003). The Saturniidae fauna known from Veracruz was mainly recorded from 4 municipalities: Santiago Tuxtla, San Andrés Tuxtla, Naolinco and Las Minas (Balcázar & Beutelspacher, 2000; Beutelspacher, 1978; Hernández-Baz, 1992; UNIBIO, 2015). These records provide valuable knowledge on their richness and seasonal flight activity.
Large saturniid moths are susceptible to environmental conditions, especially temperature (Heinrich & Bartholomew, 1971). Higher temperatures speed up pre-flight warm-up produced by the contractions of the flight muscles and they initiate flight only at body temperatures above 32 °C (Bartholomew & Casey, 1973). In warmer conditions, moths maintain body temperature that allow for extended periods of flight activity for seeking mates and host plants for oviposition (Bartholomew, Vleck, & Vleck, 1981; Heath & Adams, 1967). Many ectothermic insects depend on external thermal conditions such as evapotranspiration and temperature to maintain viable populations and these variables are the best predictors of species richness and abundances of some families of large moths and butterflies (Axmacher et al., 2009; Choi, 2008; Jaroensutasinee, Pheera, Ninlaeard, Jaroensutasinee, & Choldumrongkul, 2011; Kerr, Vincent, & Currie, 1998).
Mexico has 91 endemic species (47%) of Saturniidae, of which 45 species occur in Veracruz (Balcázar & Beutelspacher, 2000; Revueltas-Morales, 2003). Most of these species are at high risk or vulnerable due to the destruction and fragmentation of their habitats. Despite of the well-known taxonomy of the Saturniidae family, ecological studies on seasonality in the species composition in Mexico are scarce and inventories of saturniids are still far from complete, since some ecosystems and States have received little attention (Balcázar & Beutelspacher, 2000). Tropical semi-deciduous forests (TSDF) cover less than 4% of the national territory in isolated fragments at altitudes between 0 and 1300 m (Rzedowski, 2005). This type of vegetation shows a clear seasonal pattern, nonetheless, there are no ecological studies on Saturniidae in TSDF in Mexico. Therefore, it is necessary to collect throughout the year records of saturniid richness and seasonal flight activity. Thus, the aim of this study was to survey the richness, abundance and composition of Saturniidae throughout the year in central Veracruz, Mexico. Additionally, we explored whether or not environmental variables are a significant predictor of Saturniidae richness and abundance.
We expect to find a positive relationship between climatic variables that directly reflect conversion and fluxes of solar radiation (temperature and evapotranspiration) with richness and abundances. These variables can strongly affect ectothermic insects, especially large moths. In consequence, we expect that richness and abundance would be higher in spring when compared with summer, fall and winter.
Material and methods
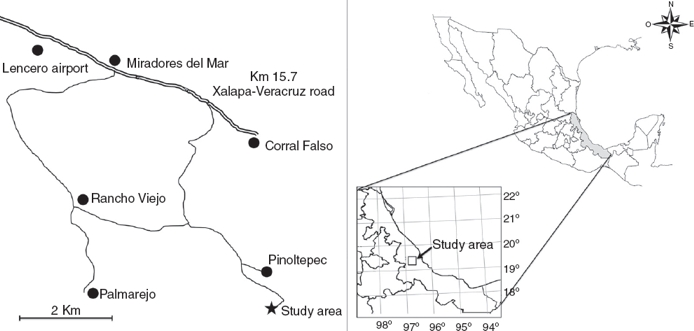
The study was conducted in a fragment of a TSDF located near Pinoltepec, municipality of Emiliano Zapata, Veracruz, Mexico, 19°26’7.01” N, 96°45’9.20” W, with an altitude of 757 m (Fig. 1). The vegetation present is a transition between tropical rain forest and tropical deciduous forest. TSDF is characterized because 50% of the trees drop their leaves for a short time during dry season (Rzedowski, 2005). The climate is warm with a clear rainy season from June to September, with an average temperature of 22.17 °C and rainfall of 216.6 mm, and a dry season from October to May with an average temperature of 19.8 °C and rainfall of 43.5 mm.
Sampling was performed on a monthly basis from September 2014 to August 2015. Captures were restricted to periods without strong moonlight, windy days and heavy rains by sampling 1 night during the new moon phase (Yela & Holyoak, 1997). We used a light trap, which consisted of a white 2 × 2 m sheet in a vertical position at a height of 1 m above the ground in front of a 250 watts mercury vapor bulb powered by a portable generator (Camargo & Becker, 1999; Padrón, 2006). Specimens were sampled throughout 1 entire night from 18:00 to 6:00 h to effectively sample the saturniid assemblage (Lamarre et al., 2015). Moths were collected manually and killed with an injection of commercial liquor in the thorax. The specimens were spread within 24 h and left to dry for 2 weeks at room temperature. The identification of the material collected was based on the illustrations of Draudt (1930) and Druce (1897), and then confirmed by dissecting genitalia and comparing with preparations made and photographed by Lemaire (1978, 1980, 1988). The specmens collected are stored at the INBIOTECA-UV for future studies.
Data analysis
The alpha diversities of our study and previous studies in Mexico on Saturniidae in Pinus-Quercus forest in Rancho Nuevo, Chiapas (Beutelspacher & Balcázar, 1998) and La Michilía, Durango (Díaz & Balcázar, 1997) were calculated using Chao1estimator of species richness and coverage-based rarefaction curves (Chao & Jost, 2012). With this approach, it is possible to compare different sample sizes extrapolating the smallest samples and compare species richness estimates at equal sample coverage. The analyses were done using iNEXT online software (Hsieh, Ma, & Chao, 2013) configured at 40 knots and bootstraps with 300 replications (Carneiro, Mielke, Casagrande, & Fiedle, 2014; Zenker et al., 2015). Richness comparisons were done at 99% coverage. Shannon-Wiener diversity index (H′) and Pielou’s Evenness index (J′) were calculated using PAST version 2.02 software (Hammer, Harper, & Ryan, 2001). Due to the lack of information to perform coverage-based rarefaction curves and diversity indices, we compared richness and abundances of Saturniidae with the species lists of Beutelspacher (1978) from Las Minas, Veracruz; Hernández-Baz (1992) from Los Tuxtlas, Veracruz; Beutelspacher (1982a) from Chamela, Jalisco; Beutelspacher (1986) from Huitzilac, Morelos; and Beutelspacher (1982b) from Cahuaré, Chiapas. Spearman correlation analysis was used to evaluate whether environmental variables (monthly mean temperature, evapotranspiration and rainfall) are significant predictors of Saturniidae richness and abundance using STATISTICA software (StatSoft Inc, Tulsa, OK, USA). p values less than 0.05 were considered statistically significant. Mean monthly temperatures were graphically and statistically compared with the monthly abundance and species richness of saturniids. Weather information was obtained from monthly averages over 30 years from Rancho Viejo Meteorological Station, which is about 3 km from the sampling site.
Results
A total of 336 saturniids moths were collected after 144 h of sampling effort. The specimens belonged to 4 subfamilies and 2 tribes, 18 genera and 30 species (Table 1). In addition to the species attracted to light, we also recorded Automeris io Lemaire nearby the study area. The species accumulation curves of predicted richness based on 144 h of samples reached an asymptote with 336 individuals and the sample coverage was 0.99%, indicating that we effectively sampled the Saturniidae assemblage that is attracted to light (Fig. 2).
Table 1 Saturniidae species collected throughout the year in tropical semi-deciduous forest in central Veracruz, Mexico, classified by subfamilies and tribes, with months in which each species was collected and numbers of individuals.

a Means endemic species of Mexico according to Balcázar and Beutelspacher (2000).
b This species was recorded nearby the study area but it was not attracted to light, therefore it was not included in the analysis.
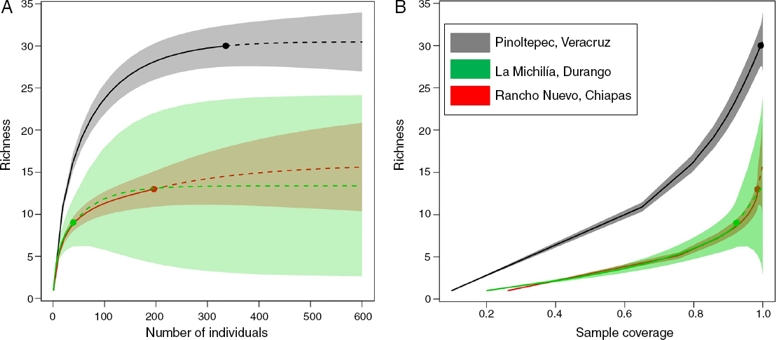
Figure 2 (A) Size-base rarefaction (solid curves) and extrapolation (dash curves) comparing Saturniidae species richness in Pinoltepec, Veracruz (present study), Rancho Nuevo, Chiapas (Beutelspacher & Balcázar, 1998) and La Michilía, Durango (Díaz & Balcázar, 1997); (B) coverage-based rarefaction and extrapolation curve. Shadow areas represent the 95% confidence intervals (bootstrap method with 300 replication).
The extrapolated curve and sample coverage suggested that Saturniidae diversity in Pinoltepec, Veracruz is 50% higher than Rancho Nuevo, Chiapas and La Michilía, Durango (Fig. 2A and B). In comparison with previous studies, the type of vegetation in Mexico that showed higher specific richness is Pinus-Quercus forest and tropical semi-deciduous forest (this study) with 31 species follow by tropical rain forest. Tropical deciduous forest, Pinus-Quercus forest showed the lowest specific richness (Table 2).
Table 2 Diversity measurements of Saturniidae assemblage in tropical semi-deciduous forest in central Veracruz, Mexico compared with faunal studies on Saturniidae in Mexico.
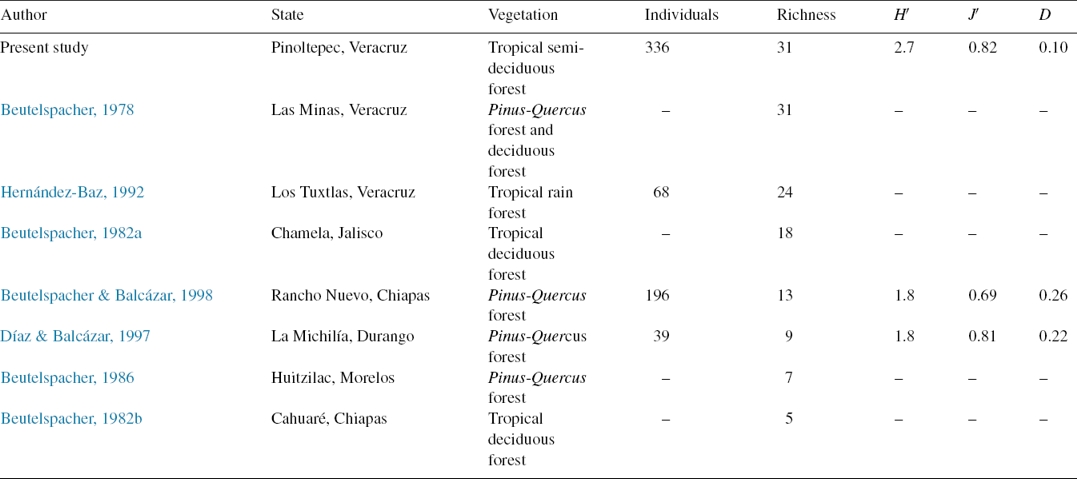
H′: Shannon-Wiener’s index of diversity; J′: Evenness; D: Simpson’s index of dominance.
The most abundant subfamily was Hemileucinae represented by 169 individuals (51%) followed by Ceratocampinae with 104 individuals (31.4%), Arsenurinae with 34 (10.5%) and Saturniinae with 28 individuals (8.45%). The subfamily with the most number of species was Hemileucinae with 13 (43%), followed by Ceratocampinae with 10 species (33%), Saturniinae with 4 (1.3%) species and Arsenurinae with 3 species (1%).
Hylesia was the most abundant genus with 96 individuals (28.57%), followed by Syssphinx with 37 (11%). The least abundant genera were Pseudodirphia and Ciao with 2 individuals (0.59%) and Citheronia (0.29%) with only 1 individual. Syssphinx was the genus with most species recorded with 5 species (16.6%), followed by Automeris (13.3%) and Hylesia (10%). Four genera (Molippa, Adeloneivaia, Rothschildia, Hylesia) were represented by 2 species and 12 genera (Ciao, Citheronia, Pseudodirphia, Hyperchiria, Dysdaemonia, Lonomia, Antheraea, Copaxa, Othorene, Periphoba, Eacles, Arsenura) were represented by only 1 species each (Table 1).
The most abundant species in this TSDF fragment was H. coinopus, with 25.8% of the total collected individuals, followed by Arsenura armida armida (Cramer) with 8.33%, and E. imperialis decoris with 7.73% (Fig. 3). Only 1 individual of C. lobesi jordani and Rothschildia lebeau aroma Schaus were captured during the study (Table 1). Additionally, the most conspicuous species throughout the year were Othorene verana (Schaus), Syssphinx quadrilineata quadrilineata (Grote & Robinson), Automeris tridens (Herrich-Schäffer) since they were captured in 6 of the 12 months of the study. Species trapped in a single month were Caio championi (Druce), Hylesia acuta Druce, Hyperchiria nausica (Cramer), Molippa nibasa (Maassen & Weyding), P. mexicana, and R. lebeau aroma (Table 1; Fig. 3).

Figure 3 Relative abundance of species and number of specimens of Saturniidae recorded in Pinoltepec, Emiliano Zapata, Veracruz, Mexico in tropical semi-deciduous forest.
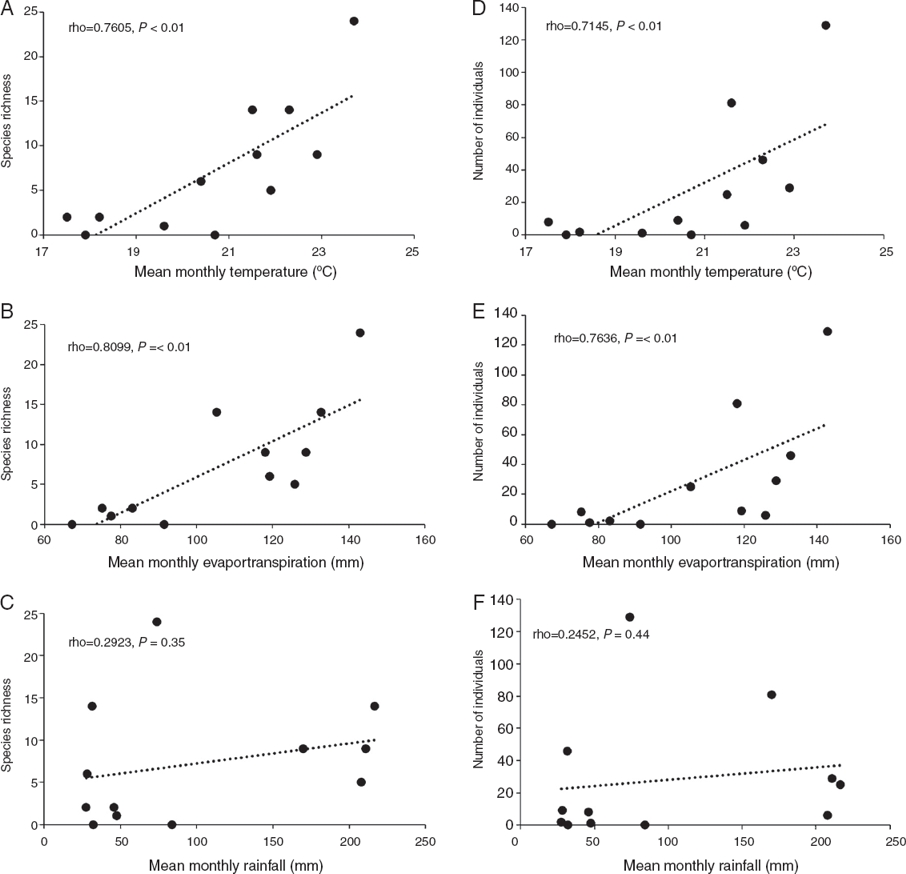
Two peaks of species richness were recorded: the first one occurring during the August-September period (19 species) and the second one during the April-May period (26 species). The hottest month of the year (May) presented the highest richness and abundance with up to 24 species (77%) and 129 individuals (37.5%) in 1 sampling night. Species richness and abundance increased as temperature increased, from February to May, and decreased when temperatures were lower, from October to January (Fig. 4).

Figure 4 (A) Specific richness and (B) numbers of individuals of Saturniidae captured per month compared with mean monthly temperature (dash line) and evapotranspiration (solid line). (C) Specific richness and (D) number of individuals of Saturniidae captured per month compared with mean monthly rainfall.
Spearman correlation analysis of temperature and evapo-transpiration indicated a positive relationship of these 2 environmental factors with monthly richness and abundance fluctuations. Temperature is significantly correlated with richness (Δ = 0.7605, p < 0.01) and number of individuals (Δ = 0.7145, p < 0.01) (Fig. 5A and D). These relationships were similar to those observed between evapotranspiration with richness (Δ = 0.8099, p = < 0.01) and numbers of individuals (Δ = 0.7636, p = < 0.01) (Fig. 5B and E). Despite of the high mean temperatures in June-August, the abundance and richness levels were considerably lower than in the former period. Rainfall increased from May to August, however, there was no relationship of this environmental factor with richness (Δ = 0.2923, p = 0.35) and number of individuals (Δ = 0.2452, p = 0.44) (Fig. 5C and F).
Discussion
The species richness recorded in this study represents 6.87% of the known Saturniidae species from Mexico (Balcázar & Beutelspacher, 2000) and 30.39% of the 102 species of the state of Veracruz (Revueltas-Morales, 2003). Most importantly, we recorded the occurrence of 6 Mexican endemic species (Antheraea polyphemus mexicana Hoffman, Automeris montezuma (Boisduval), Citheronia lobesi jordani Draudt, Hylesia coinopus (Dyar), Molippa ninfa (Schaus), and Pseudodirphia mexicana (Bouvier)) in this type of vegetation (Balcázar & Beutelspacher, 2000). According to the abundance criteria of Rabinowitz, Cairns, and Dillon (1986) C. lobesi jordani is a rare species (1–2 specimens), A. polyphemus mexicana, A. montezuma, M. ninfa, and P. mexicana are common (3–19 specimens) and H. coinopus is abundant (20–50 specimens) in TSDF. The species A. armida and M. nibasa were previously considered endemic to Mexico (Balcázar & Beutelspacher, 2000). Nonetheless, new records from Costa Rica, Honduras and Brazil of these species confirm that are widely distributed in the Neotropics (Costa, Cotzek, & Janzen, 2003; Miller et al., 2012; Miranda et al., 2015). TSDF is distributed in isolated fragments and forms a complex mosaic with tropical deciduous forest, palm forest and other type of vegetation (Rzedowski, 2005). It is probable that these complex environments would be an important refuge for many other endemic species of Lepidoptera. Habitat destruction, land change use and fragmentation may be detrimental to endemic species, especially those species occurring in low population densities as in the case of C. lobesi jordani, A. polyphemus mexicana, A. montezuma, M. ninfa, and P. mexicana. However, it is necessary to carry out ecological studies at a broader geographical scale to increase our knowledge regarding the biology and the immature forms of these species to define priorities for conservation.
The Shannon-Wiener’s index observed in Pinoltepec is higher than those observed in previous reports of Saturniidae in Mexico (Beutelspacher, 1982a, 1982b, 1986; Beutelspacher & Balcázar, 1998; Díaz & Balcázar, 1997). Nonetheless, the species richness is similar to the work of Beutelspacher (1978) who recorded 31 species, the highest richness in Mexico for a locality. These 2 sites contribute to 50% of the 102 species of Veracruz and they shared 11 species (Beutelspacher, 1978; Revueltas-Morales, 2003). The high diversity in these 2 study areas may be due to the fact that they are located in ecotone zones that may include species of adjacent communities.
On the other hand, differences in richness between our findings and previous works are probably due to differences in experimental procedure, type of vegetation and sampling effort. Many species lists of Saturniidae in Mexico have been recorded with the use of a black light instead of a mercury vapor light, and the vegetation corresponds to Pinus-Quercus forest, tropical deciduous forest and deciduous forest (Beutelspacher, 1978, 1982a, 1982b, 1986; Beutelspacher & Balcázar, 1998; Díaz & Balcázar, 1997). In the work of Hernández-Baz (1992) the study of Saturniidae richness in the tropical rain forest was carried out during 7 months, restricted from 8:00 pm to 12:00 am. Future works with major sampling effort could increase the Saturniidae species richness for tropical rain forest. Additionally, results from flight activity of saturniids strongly suggested the need to monitor lights all night in order to maximize catch size and effectively sample the Saturniidae assemblage (Lamarre et al., 2015).
Our results showed that most of the species (93.3%; 28 species) occurred in May and September in this area. We collected 77% of the species and 37.5% of the individuals in May, the hottest moth of the year. According to our results, 86.6% of the total Saturniidae species were recorded during the spring season. There is a positive relationship between temperature and evapotranspiration with richness and abundance of Saturniidae. This result concurs with previous 1 year studies on Saturniidae in Mexico where around 74% of the species were collected during the spring and during the hottest month of the year in Pinus-Quercus and tropical deciduous forest (Beutelspacher, 1978, 1982a, 1986; Beutelspacher & Balcázar, 1998; IBUNAM, 2015; SMN, 2000). Since a similar richness pattern has previously been reported for Sphingidae in tropical rain forest with 87.6% of the number of species caught in spring suggesting a correlation with dry season rather than rainy season (León-Cortés & Pescador, 1998). Conversely, in Pinus-Quercus forest in the Biosphere Reserve of “La Michilía”, Durango, the diversity of Saturniidae seems to have a positive relationship with the wet season when the availability of host plants increases (Díaz & Balcázar, 1997). The food of caterpillars of the Saturniidae consists of mature leaves and consequently their richness may be independent of the rainy season in tropical ecosystems. According to Rzedowski (2005), TSDF remains green during the driest season of the year, thus possibly food is abundant throughout the year.
One other explanation is that large saturniid moths need to thermoregulate their bodies before initiating flight (Heinrich & Bartholomew, 1971). The cost of this thermoregulation is high at colder environmental temperatures as they need more time to heat their bodies (Bartholomew et al., 1981) and many biochemical processes slow, such as metabolism and assimilation of energy (Hemmingsen, 1960). Diversity of ectothermal insects should increase with increases in environmental temperature (Allen, Brown, & Gillooly, 2002). Saturniidae do not feed as adults due to vestigial or the absence of mouthparts and warmer conditions would help to extended periods of flight activity to copulate and for finding host plants (Bartholomew et al., 1981; Heath & Adams, 1967). In the larval stage, higher temperatures may speed up physiological processes, and is most likely a reproductive strategy to secure more offspring and increase their fitness avoiding adverse abiotic factors (Choi, 2008).
Thus, since many environmental factors influence species richness and abundance, further studies are needed to unravel the stimuli and factors that regulate the insect-plant interactions and oscillation of richness and abundance. Our results highlight the importance of conserving this type of vegetation since it represents an important refuge for endemic species that exhibit some of the highest richness of Saturniidae in Mexico. Additional studies at landscape-level including habitat changes, are necessary to estimate the possible loss of species of ecological importance.











 nueva página del texto (beta)
nueva página del texto (beta)