Introduction
Nematodes are ubiquitous inhabitants of soil systems due to their abundance, large number of species, adaptive strategies, functionality in multitrophic groups, and their contribution to soil biomass (Ettema & Bongers, 1993; Laakso & Setälä, 1999; Neher & Campbell, 1996; Pen-Mouratov, Rakhimbaev, & Steinberger, 2003; Yeates, Ferris, Moens, & van der Putten, 2009). Nematodes are important components of the detritus food webs (Freckman, 1988; Sohlenius, Bostrom, & Sandor, 1987), and habitat disturbances affecting the soil microenvironment and food resources can cause changes in their diversity (Freckman & Ettema, 1993). Various studies have demonstrated that nematode abundance often declines significantly when primary ecosystems are disturbed (Ferris, Bongers, & de Goede, 2001; Griffiths, Daniell, Donn, & Neilson, 2012; Hodda, Bloemers, Lawton, & Lambshead, 1997; Zhao & Neher, 2013), whereas disturbance by cultivation in agroecosystems may cause an increase in abundance, probably due to resources provided by incorporation of crop wastes (Freckman & Ettema, 1993).
Nematodes have biological characteristics and attributes that associate them with ecological processes in soil, including mineralization of plant nutrients, nutrient cycling, and decomposition of organic matter (Griffiths, 1994; Liang, Lavian, & Steinberger, 2001; Neher, 1999, 2001; Thornton & Matlack, 2002), which allow them to be used as biological indicators of disturbances that occur in terrestrial ecosystems (Baxter, Rowan, McKenzie, & Neilson, 2013; Ettema & Bongers, 1993; Freckman & Ettema, 1993; Griffiths et al., 2012; Ito, Araki, Komatsuzaki, Kanedo, & Ohta, 2015).
Few recent records exist concerning the nematodes associated with Mexican plant communities (Zullini, 1973, 1977a, 1977b) or the diversity of nematodes in Mexican Reserves (Mundo-Ocampo, Dorado, Baldwin, Morales, & Nadler, 2001), and none of these studies investigate the impact of land use changes on soil nematodes that may have occurred in those areas. The present study is not only an inventory of soil nematodes from the buffer zone of Los Tuxtlas, but it also documents the impact of land use change on soil biodiversity in one of the most important protected areas in Mexico, as measured by the composition of soil nematode communities. Accepting that nematodes play an important role in the functioning of soil and are also quite sensitive to changes in their microenvironment, this study has the following objectives: (i) to conduct an inventory of the soil nematodes from the buffer zone of the Biosphere Reserve Los Tuxtlas, Veracruz, Mexico; (ii) to calculate generic estimators and diversity indices based on soil nematodes, and (iii) to determine the effect of land use changes in the study area on soil nematodes by the measurement of common diversity indices.
Materials and methods
Field work was conducted in the Biosphere Reserve Los Tuxtlas (18°10’–18°45’ N, 94°42’–95°27’ W) located in Veracruz, Mexico. This reserve is the northernmost extension of the Neotropical rain forest and is one of the protected areas in which the processes of deforestation have been documented for prolonged periods (Dirzo & García, 1992; Martínez-Ramos, Ortiz-Rodríguez, Piñero, Dirzo, & Sarukhán, 2016).
Three sites around the reserve (López Mateos, Venustiano Carranza, and San Fernando) were chosen and 4 types of land use were selected from each site: natural forest, secondary forest, pasture, and maize fields. Natural forest in the context of this study included those areas with minimal or no recent disturbance; secondary forest included areas with previous disturbance events but in a recovery process; pasture included tracts of land supporting grass or similar vegetation eaten by domestic grazing animals; and maize fields those sowed with maize, and sometimes incorporating squash and/or beans.
Eight sampling points were established in each distinct land use area (32 soil sampling points in each locality, 96 overall), following a grid pattern with a distance between points of at least 200 m. Sampling points were positioned where grid lines crossed and 8 soil cores (of approximately 100 g each) were taken from each sampling point at a depth of 0 to 20 cm, using a 5 cm diameter soil auger tube drawn from 2 imaginary concentric circles around each point. Four cores were taken on the perimeter of the inner circle (3 m radius) and another 4 were taken on that of the outer circle (6 m radius). The 8 cores from each sampling point were combined into a single sample in a plastic bag, placed in a cool box to protect against overheating, and transported to the laboratory.
Sub-samples of 300 mL of soil were taken from each sample and the nematodes therein were extracted by sieving and then by sugar flotation (Hooper, 1986b). Once extracted, nematodes were gently killed by heating to 60 °C for 1 min and then fixed with cold 4% formalin (Hooper, 1986a). The suspension of fixed nematodes from each sample was adjusted to a volume of 10 mL and the nematodes in each of 3, 1 mL aliquots were counted. The total number of nematodes per sample was estimated from the mean of the 3 counts. After counting, samples were processed by mass dehydration in order to draw water out of the nematodes and replace it with glycerin (Franco-Navarro, 1999); 100 nematodes were picked at random and mounted in glycerine on permanent slides by the wax ring method (Hooper, 1986a) for identification under a compound microscope. If possible, 100 nematodes from each sampling site were identified to genus. All the nematodes that were collected and mounted are deposited in the Nematode Collection of the Laboratory of Nematology at the Colegio de Postgraduados, Campus Montecillo.
Accumulation curves for genera were obtained using Species Diversity & Richness software® (1998) to compare richness of genera among land uses. To determine the inventory completeness at the generic level for each land use, 3 estimates were used: Chao2, Jacknife and bootstrap (Colwell & Coddington, 1995). Comparison of abundance and generic evenness among land uses was assessed through dominance-diversity graphs, also known as abundance-range graphs (Feinsinger, 2001). Total abundance of nematodes and averages of generic richness per land use, as well as dominance (λ) that was used to assess dominance in all nematode genera in the sample with λ = Σp2i , where pi is the proportion of ith taxon (McSorley & Frederick, 1996; Pen-Mouratov & Steinberger, 2005), were also estimated. The diversity of soil nematode genera was measured with 2 indices using the Species Diversity & Richness software® (1998): (1) Simpson’s index (D), which is heavily weighted toward the most abundant species (genus) in a sample (where D = 1/C, C = Σp2i, p2i = Ni(Ni − 1)/NT (NT − 1), with Ni as the number of individuals in the ith genus and NT as the total number of individuals in the sample) (Freckman & Ettema, 1993), and (2) Shannon–Wiener index (H′), an index weighted toward rare species (genera) (where H' = −Σpi(ln pi), pi being the proportion of each taxon (genus) in the total population) (McSorley & Frederick, 1996; Pen-Mouratov et al., 2003; Pen-Mouratov & Steinberger, 2005).
Total abundance, generic richness, dominance, and generic diversity of soil nematodes by land use were compared by means of analysis of variance (Anova) and comparisons of means were tested using Tukey’s studentized range (HSD) test. All statistical procedures were performed using Statistical Analysis System software (SAS, 2005; http://www.sas.com/es_mx/software/analytics/stat.html); differences with p < 0.05 were considered significant.
Results
One hundred and twenty-four genera belonging to 13 orders and 53 families were identified; only 6 morph genera of nematodes could not be identified to genus level (Table 1). One hundred and four genera were collected in natural forest, 90 in secondary forest, 107 in pasture, and 79 in maize fields (Table 2). From the overall genera collected, 8 were detected only in natural forest (Paraphanolaimus, Desmocolex, Odontopharynx, Epidorylaimus, Bursiella, Pellioditis, Aorolaimus, Hemicycliophora), 3 only in secondary forest (Labronema, Bastiana, Microlaimus), 9 only in pasture (Dactyluroaxonchium, Oxybelondira, Takamangai, Ironus, Stenonchulus, Alloionema, Panagrocephalus, Panagrolaimus, Coslenchus) and 5 only in maize fields (Anchidiplogaster, Acephadorylaimus, Carcharolaimus, Crustorhabditis, Panagrobelus). Sixty-one genera occurred at sites characterized by all 4 land-uses (Table 1).
Table 1 Nematode genera associated with four land uses in Los Tuxtlas, Veracruz, Mexico, and their abundance in 96 soil samples (300 mL) taken and analyzed from the study area.
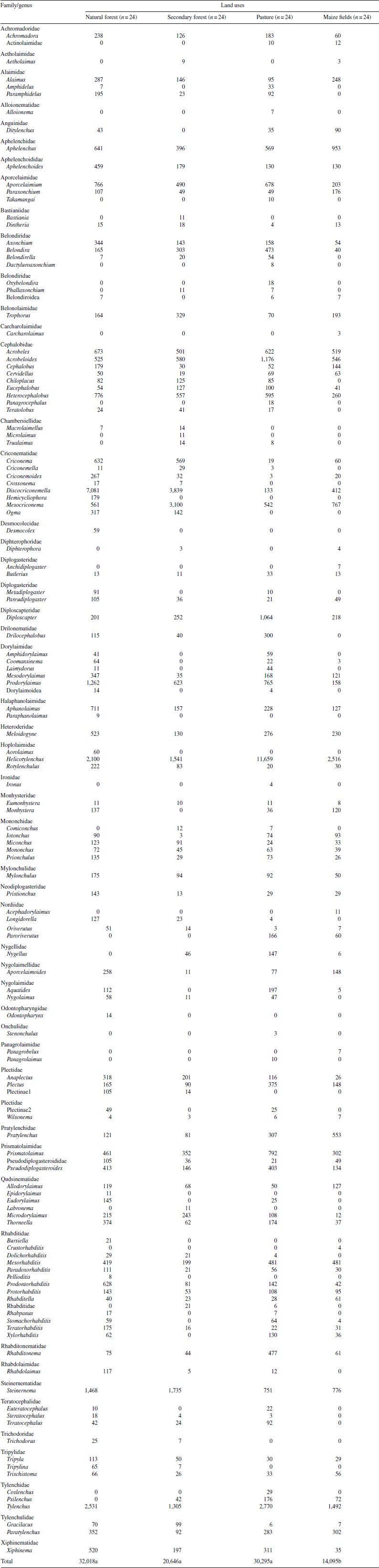
Table 2 Observed and estimated generic richness of soil nematodes associated with 4 land uses and all types of land uses in Los Tuxtlas, Veracruz, Mexico.

Completeness is a percentage of estimated richness = (minimum/maximum) × 100.
Families with the greatest abundance across all land uses were: Criconematidae > Hoplolaimidae > Cephalobidae > Tylenchidae. The most abundant genera in this study were: Helicotylenchus, the dominant genus in pasture and maize fields, Discocriconemella, which was dominant in natural and secondary forests, Tylenchus, an herbivore with high relative abundances in all land uses but particularly in pasture, and Steinernema, a bacterial feeder and entomopathogenic nematode with high values in both natural and secondary forests (Table 1). The genera with the lowest densities overall in sampled sites were Ironus, Carcharolaimus, Crustorhabditis, and Stenonchulus (Table 1).
A total of 97,054 soil nematodes were extracted from all soil samples (n = 96); 33% in natural forest, 31% in pasture, 21% in secondary forest and 15% in maize fields. Total abundance of soil nematodes by land use (n = 24) was significantly different (p < 0.05, F = 3.23) between natural forest (32,018 individuals) and maize fields (14,095 individuals), but no statistically significant differences were found between natural forest, pasture (30,295 individuals), and secondary forest (20,646 individuals) (p > 0.05, F = 2.16), despite the presence of more nematodes in the natural forest (5.3% and 35.5% more, respectively) (Table 1). Average generic richness showed significant differences (p < 0.001, F = 6.84) among land uses with the highest average in natural forest (26), followed by secondary forest and pasture (20 in both cases), and maize fields (18). Conversely, dominance of genera was significantly higher (p < 0.05, F = 3.76) in pasture (0.23 ± 0.06) than in secondary forest, maize fields, and natural forest (0.17 ± 0.04, 0.17 ± 0.03 and 0.14 ± 0.03, respectively).
Generic accumulation curves for all land uses did not reach an asymptote but all land uses may have reached a plateau with more than 100 samples (Fig. 1). Diversity estimates indicated that, in natural forest, more than 89% of the genera present were captured, in secondary forest and pasture more than 80% and 82%, respectively, and in maize fields more than 78%. The estimates predict that as many as 147 genera could be recorded for soil nematodes from all sites studied (Table 2).

Figure 1 Accumulation curve for genera of soil nematodes associated with four land uses inside the Biosphere Reserve Los Tuxtlas, Veracruz, Mexico. ⇑ indicates sampling points per land use (n = 24). Total = all samples taken in the study area (n = 96).
In dominance-diversity graphs for each land use, slopes were similar, although neither the abundance distribution pattern nor the hierarchical order of genera were similar (Fig. 2). In natural forest, 1 genus was highly dominant (Discocriconemella), 4 were moderately dominant (Tylenchus, Helicotylenchus, Steinernema and Prodorylaimus), and 3 were moderately low in abundance (Macrolaimellus, Amphidelus, and Belondira), whereas in secondary forest 2 genera were dominant (Discocriconemella and Mesocriconema), 3 were moderately high in abundance (Steinernema, Helicotylenchus, and Tylenchus), and 3 had low abundance (Stenonchulus, Iotonchus, and Wilsonema) (Fig. 2). In pasture, only 1 genus was notably abundant (Helicotylenchus) and 3 were moderately high in abundance (Tylenchus, Acrobeloides, and Diploscapter). Finally, in maize fields, 3 genera were the most abundant (Helicotylenchus, Tylenchus, and Aphelenchus), 2 genera were moderately high in abundance (Steinernema and Mesocriconema), and 3 genera had low abundance (Coomansinema, Aetolaimus, and Carcharolaimus) (Fig. 2).

Figure 2 Dominance-diversity graphs for soil nematodes associated with 4 land uses inside the Biosphere Reserve Los Tuxtlas, Veracruz, Mexico. Dis = Discocriconemella, Me = Mesocriconema, S = Steinernema, He = Helicotylenchus, Ap = Aphelenchus, Ty = Tylenchus, Ac = Acrobeloides, Dip = Diploscapter, P = Prodorylaimus, Str = Steratocephalus, Io = Iotonchus, W = Wilsonema, Mac = Macrolaimellus, Amp = Amphidelus, Be = Belondira, O = Oriverutus, Ste = Stenonchulus, Co = Coomansinema, Ae = Aetolaimus, Ca = Carcharolaimus. Ni = number of individuals of each genus; N = number of individuals of all species.
When soil nematodes in this study were assigned to trophic groups, plant parasitic nematodes and bacterial feeders were the most abundant and dominant, followed by fungal feeders, omnivores, and finally predators. Overall, the greatest number of bacterial feeders was collected from pasture and maize fields; a similar tendency was observed with fungal feeders but, in this case, the greatest number was collected from maize fields followed by pasture. Omnivores and predators showed the greatest numbers in natural and secondary forest, whereas the greatest numbers of plant parasitic nematodes were observed in secondary forest, followed by natural forest, pasture, and maize fields. A deeper analysis of the trophic structure of the soil nematode communities will be made in subsequent studies.
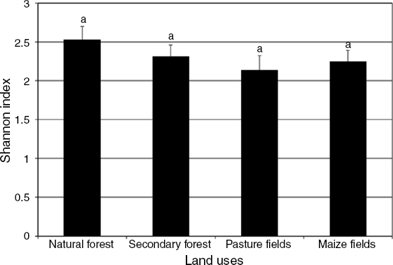
The highest Simpson’s index was observed in natural forest (9.45 ± 1.73), significantly greater (p < 0.05, F = 3.76) than in pasture (6.28 ± 1.19) but not significantly different from secondary forest and maize fields (p > 0.05, F = 2.08) (7.58 ± 1.34 and 7.42 ± 1.22, respectively) (Fig. 3). For Shannon’s index, the results followed a similar pattern to that of the Simpson’s index, with the highest value in natural forest (2.52 ± 0.18), followed by secondary forest (2.30 ± 0.16) and maize fields (2.24 ± 0.15), and with the lowest index in pasture (2.13 ± 0.19) (p < 0.05, F = 3.40) (Fig. 4).
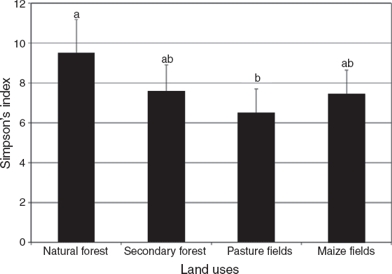
Figure 3 Simpson’s index for soil nematodes associated with 4 land uses inside the Biosphere Reserve Los Tuxtlas, Veracruz, Mexico (n = 24).
Discussion
Agricultural management practices and many other human activities disturb the soil ecosystem and affect soil nematode diversity (Freckman & Ettema, 1993; Ito et al., 2015; Neher, 2001; Zhao & Neher, 2013). The different land usage areas sampled represent the main environmental ranges of land uses in the Biosphere Reserve Los Tuxtlas that are likely to be affected in their below-ground soil biodiversity. The transition from above-ground plant heterogeneity in natural forests to extreme plant homogeneity in agriculture is reflected in the soil nematodes and their diversity.
Despite the observed and estimated number of genera being higher for pasture than natural forest, the number of nematodes and the average generic richness were higher in natural forest than in the other land uses. These 2 parameters were even higher in natural forest than values previously reported in the literature (Freckman & Ettema, 1993; Liang et al., 2001; Pen-Mouratov & Steinberger, 2005; Sohlenius et al., 1987). When the total number of genera and families from the present study are compared with those from other studies, the numbers of genera are higher than those reported from different geographical areas and environments, mostly in temperate regions (Neher & Campbell, 1996; Neher, 1999; Thornton & Matlack, 2002; Yeates, 1984).
The greatest generic richness obtained from the natural forest in Los Tuxtlas is similar to that in other studies in which richness is lower in recently disturbed soil than in soil that has not been disturbed for several decades (Thornton & Matlack, 2002), implying eradication of species during the disturbance event (Freckman & Ettema, 1993; Hodda et al., 1997). The greatest dominance of genera was found in pastures, where Helicotylenchus, a typical plant feeder, was the main genus. This is a cosmopolitan genus so its high abundance, as shown by the dominance-diversity graph, indicates the dominance of this genus over other soil nematodes is a result of the great uniformity of plants characteristic of pasture. Although the dominance of Helicotylenchus was observed in maize fields too, the difference with respect to the next most dominant genus, Tylenchus, was lower than that obtained in pasture. In contrast to the disturbed systems, in natural forest and occasionally in secondary forest, the dominant genus was Discocriconemella, a typical plant root feeder in tropical regions of the world. Dominance of this genus reflects the lack of soil disturbance, which has allowed the persistence of this native genus instead of the exotic genera found in pasture or maize fields. When the analysis of both estimates was combined, it was observed that in non-disturbed systems the number of genera (generic richness) is greater, but without an extremely dominant genus (generic dominance) above the rest; in pasture, an intermediate value of generic richness was combined with the highest generic dominance. This suggests that in this monocropping system the land use change can eliminate some genera formerly present and promote the dominance of other, mainly exotic ones, thus permanently modifying soil nematode diversity and population structure.
Nematode trophic structure in Los Tuxtlas was dominated by plant parasites and bacterial feeders (mostly represented by the Order Rhabditida), a similar situation to that observed in studies of temperate regions (Neher, 1999; Neher & Campbell, 1996; Pen-Mouratov et al., 2003; Pen-Mouratov & Steinberger, 2005; Thornton & Matlack, 2002). In pasture and maize fields, the most abundant trophic groups were bacterial feeders and fungal feeders, in similar proportions to those found in other agricultural sites (Freckman & Ettema, 1993; McSorley & Frederick, 1996; Pen-Mouratov & Steinberger, 2005). Bacterial and fungal feeders are more abundant in disturbed soils, suggesting that disturbance improves substrate quality or resource availability, presumably thereby fostering growth of bacterial and fungal prey populations (Thornton & Matlack, 2002). On the other hand, the highest percentages of omnivores and predators were observed in natural forest and secondary forest, although they were the least abundant in comparison with other trophic groups. From their position higher in the soil food web, predators and omnivores may also have a direct or indirect effect on decomposition by controlling the biomass of microbivores (Laakso & Setälä, 1999), but they are more sensitive to soil disturbance than microbivorous nematodes (Bongers, 1990). According to Thornton and Matlack (2002), higher populations of fungal and bacterial feeders are dependent on the absence of predators, so this interrelationship could explain the presence and proportion of colonizer groups (bacterial and fungal feeders) with respect to persistent groups (omnivores and predators) in both non-disturbed areas and in areas under agricultural management. The long-lived nematodes, such as omnivores and predators, which were more abundant in hardly disturbed and non-disturbed systems (natural and secondary forest), are associated with stable environments. However, when these habitats are then affected with inputs of organic matter and/or pollutants, or human activities like agriculture (in the case of pastures and crop fields), the increased bacterial activity favors colonizer nematodes that feed on bacteria and respond quickly to changes, whereas persisters nematodes (omnivores and predators) decrease (Freckman & Ettema, 1993).
The highest values of soil nematode diversity in Los Tuxtlas, calculated using both Simpson’s index and Shannon’s index, were found in natural forest. In general, the diversity indices of natural forest in this study were higher or similar to those observed in other land uses and in other reports (Freckman & Ettema, 1993; Liang, Lavian, & Steinberger, 1999; Liang et al., 2001; Pen-Mouratov et al., 2003; Yeates & King, 1997), which reflects the fact that the diversity of nematodes is favored by little or hardly disturbed soil.
In this study, the indices and generic estimators that were calculated allowed some effects of land use changes on soil nematodes and their diversity in the Mexican tropics to be detected. Nematodes were shown to be sensitive to perturbation in this study area. In little disturbed and non-disturbed areas, such as natural forest and secondary forest, richness and diversity of nematodes were significantly greater than in systems used for crop production and monocropping, such as pasture and maize fields. The simplification of above-ground plant assemblages therefore has drastic effects on below-ground nematode communities in a similar manner to that observed in other taxa.
This is the first report in Mexico on the changes brought about by human activity on soil nematode populations and diversity, both for plant-parasitic and free-living nematodes. The present study supports previous results reported in the literature and contributes to an increase in the knowledge of nematode fauna and its diversity in soils from the Mexican tropics and the changes that they undergo under the influence of different land use changes. These results may help model and predict future changes in biodiversity and species interactions with land use change.











 nova página do texto(beta)
nova página do texto(beta)