Introduction
Seasonality in rainfall has important effects on lizard populations (Miranda & Andrade, 2003). For example, activity levels (García, Valtierra-Azotla, & Lister, 2010; Lister & García, 1992) and growth-rates both decrease during the dry season when food availability is low (Griffiths & Christian, 1996). Habitat use also could changes between wet and dry seasons, as species adapt to seasonal fluctuations in habitat characteristics such as moisture level, temperature, insolation, food availability and predation pressure (García et al., 2010; Miranda & Andrade, 2003). Nevertheless, the extent of these changes may vary depending on local seasonal characteristics, which are a function of vegetation and soil type, geography, and topography (Kishimoto-Yamada, Itioka, Sakai, & Ichie, 2010; Suazo-Ortuño, Alvarado-Díaz, & Martínez-Ramos, 2011). For example, seasonal changes in niche relationships, activity levels, and habitat use of lizards inhabiting tropical dry forest (TDF) are different from those observed in tropical rain forest (Gienger, Beck, Sabari, & Stumbaugh, 2002; Mesquita, Colli, França, & Vitt, 2006; Watling & Donnelly, 2002), likewise these changes could be observed between the TDF and the vegetation developed along the arroyos that are immersed in it (Gienger et al., 2002; Suazo-Ortuño et al., 2011).
Elevation is an important factor affecting the environment experienced by many lizard species. Temperature is typically lower at higher elevations than that at lower elevations (Iraeta, Salvador, & Díaz, 2013). Consequently the intensity of seasonal change will differ between similar habitats located at different elevations. Relief is an important topographical factor that also affects habitat seasonality. For example, the TDF of the coast of Jalisco, Mexico, is distributed across a landscape of low hills (0-500 m asl) that facilitate water accumulation valleys and the formation of seasonal arroyos. The phenology and structure of vegetation within this arroyo is significantly modified from the surrounding TDF and is known as arroyo vegetation (AV). Here there are higher levels of moisture and productivity, greater structural complexity, reduced insolation, and lower seasonality in food availability (García & Cabrera-Reyes, 2008). In this way, the AV is unveiled as an alternative habitat in the TDF for various animal species and consequently may change their usual activity levels between seasons (García et al., 2010; Suazo-Ortuño et al., 2011). The TDF in the western of Mexico is characterized by a marked seasonality that affects the canopy cover, since almost all tree elements loss their leaves, which permits the solar radiation penetrates to the ground, affecting the thermoregulation activities of lizards (García et al., 2010). Factors as elevation and relief could change the intensity of this seasonality and consequently could affect the activities of lizards, as habitat use, and its ecological traits, as abundance.
Research related to the effects of seasonality on lizards usually focuses on comparisons of ecology and behavior between the driest and wettest months. In most of these studies, only a limited number of environmental variables have been measured during the transitions between seasons (Faria & Araujo, 2004; Irschick et al., 2005, 2006; Nogueira, Colli, & Martins, 2009). As a result, little is known about how such transitions affect lizard ecology, behavior, and population abundance. This lack of information is especially detrimental to understanding seasonal change given that the transition between wet and dry periods is a crucial time during which individuals need to prepare for stressful conditions that can last for an uncertain duration.
The objective of this study was three-fold. First, to assess the effects of the transition between the end of the wet and the beginning of the dry season on the abundance and microhabitat use of Sceloporus utiformis. Second, to determine the effect of elevation by comparing 2 TDF at different elevations. Third, to elucidate how the intensity of seasonal transitions differs between TDF and AV by measuring a number of habitat variables such as temperature, relative humidity and canopy cover.
Materials and methods
S. utiformis is a medium sized lizard with an adult average snout-vent length of 70 mm, and a large tail (more than 2 times its body length). This lizard is diurnal, terrestrial, oviparous insectivore. It is endemic to Mexico, and distributed from northern Sinaloa to Guerrero along the Pacific coast and into the central regions of Jalisco and Colima (García et al., 2010).
The study area included 2 sites. The first one was within the Chamela Biological Station, which is part of the Chamela-Cuixmala Biosphere Reserve (19°29′55.4″ N, 105°02′27.4″ W and 19°30′49.1″ N, 105°02′02.7″ W), with an elevation range of 14 and 450 m. The reserve has an area of 13,242 ha and is located in the municipality of La Huerta, Jalisco, Mexico. Mean annual temperature is 24.9 °C and mean annual rainfall is 748 mm, with 80% of the rainfall occurring between July and October (García et al., 2010). The second study site is Camp Four, a location within the Sierra Manantlán Biosphere Reserve (19°21′20.2″ N, 103°51′04.8″ W and 19°21′25.4″ N, 105°50′48.5″ W), having elevations between 998 m and 1119 m, and encompassing 139,577 ha. Mean annual temperature is 18 °C and mean annual rainfall is 1100 mm, with most precipitation occurring between June and October (Vázquez & Givnish, 1998). In the study site at Chamela, we obtained data from 2 types of vegetation: tropical dry forest (TDF) and arroyo vegetation (AV), which present similar arboreal characteristics as tropical semi-deciduous forest (Lott, Bullock, & Solís-Magallanes, 1987). In Manantlán we obtained data only from TDF. In total, we studied 3 different habitats, a tropical dry forest at low elevation (TDF of Chamela), a tropical dry forest at high elevation (TDF of Manantlán) and arroyo vegetation (AV of Chamela).
Since the objective of the study was to estimate the effects of seasonal transitions, we conducted field work from October 2005 to February 2006. This is the period when precipitation ceases and the habitat begins to dry out. To measure abundance and microhabitat use of lizards during the seasonal transition, we divided the time period into 4 shorter periods; October-November, November-December, January-February, and February-March. We established 14 transects of 350 m × 2 m each. Of those transects, 6 were in the reserve of Manantlán (MTDF), and 8 near the Chamela Station. Among the 8 transects in Chamela, 4 were established in the tropical dry forest (ChTDF) and 4 in the arroyo vegetation (ChAV). Each transect was surveyed for S. utiformis 6 times throughout the day: 08:00 h-09:20 h, 09:30 h-10:50 h, 11:00 h-12:20 h, 12:30 h-13:50 h, 14:00 h-15:20 h, and 15:30 h-16:50 h. For each transect we recorded the date, habitat type (MTDF, ChAV and ChTDF), environmental temperature, and relative humidity. These last 2 variables were recorded with a thermo-hygrometer Digital Max and Min Forestry Suppliers® every time a lizard was observed. Sex was determined only for adults using coloration patterns that differ between males and females (juveniles do not have sexually dimorphic color variation). The microhabitats where each individual was observed were also recorded. These included leaf litter, trees, herbs and rocks. Canopy cover reflects seasonality in rainfall in the deciduous TDF, and was therefore an important variable for our study. To obtain canopy cover data, we used a rectangle mirror divided in 40 squares of 1 cm × 1 cm per square, simulating a spherical densiometer. We conducted 4 measures, 1 at each cardinal point, counting the number of squares covered by canopy cover. Coverage measurements were obtained every 20 m in all transects (17 points per transect), 1 for each time period (October-November, November-December, January-February, and February-March), and hence captured changes in the canopy during the transition from the wet to the dry season. Total canopy coverage at each point was obtained by transforming the number of squares occupied in the mirror to percentages and averaging the 4 cardinal measurements for each counting point.
The data of the sums of males, females, and juveniles observed in the transect was processed by dividing the sums by the area of the transect (2100 m2) and multiplying the result by 10,000 m to convert from meters to hectares. In this way, lizard relative abundance was obtained as the number of individuals per hectare (ind/ha). Since S. utiformis abundance, environmental temperature, relative humidity and canopy cover data were not normally distributed, and were not improved by log transformation, we used non-parametrical statistics. We analyzed the data of habitat conditions and species abundance in 2 ways. To compare each variable between habitats, we used Kruskal-Wallis test (Suazo-Ortuño et al., 2011). To examine the effect of seasonal transition (temporary effect) for each variable in each habitat, we used Anova Friedman's test (non-parametric Anova), which is frequently used to measure differences in variables across different time periods (Kishimoto-Yamada et al., 2010).
A Kruskal-Wallis test to contrast the 3 habitat variables among ChTDF, ChAV and MTDF was used. Furthermore, we estimated the differences in environmental temperature, relative humidity, and canopy cover across the seasonal transition using Friedman's test for each habitat. We also used a Kruskal-Wallis test to contrast the abundance of individuals, males, females, adults, and juveniles between ChTDF, ChAV and MTDF. For each habitat we used Friedman's test to identify possible differences regarding the abundance in each aforementioned group throughout the seasonal transition. Furthermore, the differences in microhabitat used by S. utiformis lizards were contrasted between ChTDF, ChAV and MTDF using a Kruskal-Wallis test. Friedman's test was again employed to examine the effects of seasonal transition on the microhabitat use of S. utiformis in each habitat. Lastly, we used Spearman rank correlations coefficients to assess the relationship between the environmental conditions and lizard abundance with respect to study site and microhabitat.
Results
A total of 292 observations on climatic conditions and canopy cover: 83 were collected at ChTDF, 42 at ChAV and 167 at MTDF was recorded. Kruskal-Wallis analyses showed that the highest environmental temperature was registered in ChTDF, followed by MTDF, and the lowest temperature in ChAV (Table 1); whereas the highest relative humidity was registered in ChAV, followed by ChTDF, and the lowest humidity in MTDF (Table 1); lastly the highest canopy cover was registered in ChAV, followed by MTDF, and the lowest cover in ChTDF (Table 1).
Table 1 Comparison between 3 habitats using the Kruskal-Wallis tests, including habitat variables, abundance of Sceloporus utiformis lizards at different ages and sexes, and abundance of lizards in different microhabitats. Chamela tropical dry forest (ChTDF), Chamela arroyo vegetation (ChAV), and Manantlán tropical dry forest (MTDF). For each variable and habitat is presented the abundance average value and the standard error in parenthesis.
| χ 2 | p | Sites that differs p < 0.05 | ChTDF | ChAV | MTDF | |
|---|---|---|---|---|---|---|
| Temperature (°C) | 7.3 | 0.02 | ChAV vs. ChTDF & MTDF | 29.8 (0.4) | 27.3 (0.6) | 28.9 (0.3) |
| Humidity (%) | 50.8 | 0.001 | MTDF vs. ChTDF & ChAV | 61.9 (2.1) | 68 (2.6) | 44.1 (1.2) |
| Canopy cover (%) | 184 | <0.001 | ChTDF vs. ChAV & MTDF, ChAV vs. MTDF | 45.9 (1.8) | 72.9 (1.1) | 60.0 (1.4) |
| Individuals (ind/ha) | 10.2 | 0.0062 | ChAV vs. MTDF | 29.5 (6.4) | 13.0 (3.0) | 63.5 (17) |
| Males (ind/ha) | 4.8 | 0.0147 | ChAV vs. MTDF | 2.2 (1.2) | 0.6 (0.4) | 9.9 (3.8) |
| Females (ind/ha) | 5.6 | 0.681 | x | 2.2 (0.9) | 1.3 (1.0) | 7.9 (3.0) |
| Adults (ind/ha) | 8.0 | 0.018 | x | 4.4 (1.9) | 1.9 (1.3) | 17.9 (6.3) |
| Juveniles (ind/ha) | 2.9 | 0.236 | x | 25.1 (5.9) | 11.1 (3.0) | 45.2 (19) |
| Leaf litter (ind/ha) | 3.4 | 0.185 | x | 14.9 (4.0) | 7.0 (1.9) | 21.4 (7.2) |
| Herb (ind/ha) | 0.5 | 0.769 | x | 0.6 (0.4) | 0.6 (0.6) | 0.8 (0.5) |
| Tree (ind/ha) | 3.9 | 0.138 | x | 14.0 (3.9) | 5.1 (1.3) | 16.7 (6.8) |
| Rock (ind/ha) | 28.7 | <0.001 | ChTDF & ChAV vs. MTDF | 0.0 (0.0) | 0.3 (0.3) | 24.6 (6.4) |
The Friedman analyses showed that behavior of the seasonal transition was different among the 3 habitats. In ChTDF, relative humidity (Friedman test: χ 2 = 20.81, df = 3, p < 0.001) and canopy cover (Friedman test: χ 2 = 160.56, df = 3, p < 0.001) decreased significantly, while environmental temperature increased but not significantly (Friedman test: χ 2 = 1.89, df = 3, p = 0.59) (Fig. 1). In ChAV, environmental temperature (Friedman test: χ 2 = 1.08, df = 3, p = 0.78), relative humidity (Friedman test: χ 2 = 1.8, df = 3, p = 0.61) and canopy cover decreased significantly (Friedman test: χ 2 = 111.81, df = 3, p < 0.001) (Fig. 1). In MTDF, environmental temperature increased significantly (Friedman test: χ 2 = 29.95, df = 3, p < 0.001), but relative humidity (Friedman test: χ 2 = 52.38, df = 3, p < 0.001) and canopy cover (Friedman test: χ 2 = 188.22, df = 3, p < 0.001) decreased significantly (Fig. 1).
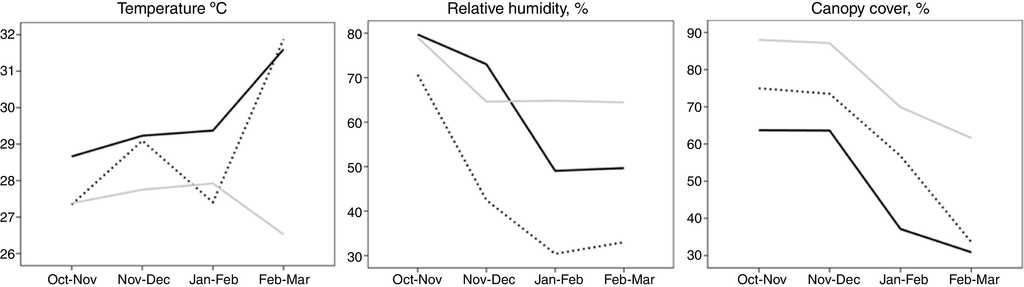
Figure 1 Average of habitat variables during the seasonal transition. Continuous black line: Chamela tropical dry forest; pointed black line: Manantlán tropical dry forest; continuous gray line: Chamela arroyo vegetation.
Aa total of 298 lizards, 163 at MTDF, 93 at ChTDF and 42 at ChAV was recorded. In the MTDF the abundance of S. utiformis was higher than in ChTDF and ChAV for the total of individuals registered, and for males and adults; whereas changes in abundance of females and juveniles were not significant (Table 1). Abundance of lizards in microhabitats was significantly higher in MTDF than ChTDF and ChAV only in the rock microhabitat; whereas the abundance in the other 3 microhabitats was also higher in MTDF than in ChTDF and ChAV but not significantly (Table 1).
In ChTDF, the relative abundance of total individuals (Friedman test: χ 2 = 10.54, df = 3, p = 0.01) and juveniles (Friedman test: χ 2 = 9.77, df = 3, p = 0.02) increased in response to the seasonal transition, while females (Friedman test: χ 2 = 5, df = 3, p = 0.17) and males (Friedman test: χ 2 = 4.8, df = 3, p = 0.19) and therefore adults decreased but not significantly (Fig. 2). Similar results were observed in ChAV where total of individuals (Friedman test: χ 2 = 6.89, df = 3, p = 0.07) and juveniles (Friedman test: χ 2 = 10.42, df = 3, p = 0.01) increased during the seasonal transition, while females (Friedman test: χ 2 = 6, df = 3, p = 0.11) and males (Friedman test: χ 2 = 4.71, df = 3, p = 0.19) and therefore adults decreased but not significantly (Fig. 2). In MTDF, the pattern was similar for the total of individuals (Friedman test: χ 2 = 6.517, df = 3, p = 0.089), juveniles (Friedman test: χ 2 = 7.615, df = 3, p = 0.055), females (Friedman test: χ 2 = 6.12, df = 3, p = 0.11), and males (Friedman test: χ 2 = 5.44, df = 3, p = 0.14) and therefore adults, however these changes were not significant (Fig. 2).

Figure 2 Average of relative abundance (individuals/hectare) of Sceloporus utiformis lizard during the seasonal transition by reproductive condition. Continuous black line: Chamela tropical dry forest; pointed black line: Manantlán tropical dry forest; continuous gray line: Chamela arroyo vegetation.
Microhabitat used by S. utiformis changed with seasonal transition in the 3 habitats. In ChTDF the use of tree microhabitat decreased during seasonal transition (Friedman test: χ 2 = 8.16, df = 3, p = 0.04), the same occurred with the use of leaf-litter microhabitat but the increase was not significant (Friedman test: χ 2 = 4.56, df = 3, p = 0.21), herb and rock microhabitats were absent in this habitat (Fig. 3). In ChAV the use of herb microhabitat decreased during seasonal transition (Friedman test: χ 2 = 9, df = 3, p = 0.02), the use of leaf-litter (Friedman test: χ 2 = 6.89, df = 3, p = 0.07) and tree (Friedman test: χ 2 = 5.06, df = 3, p = 0.17) microhabitats increased but not significantly, whereas rock microhabitat was absent in this habitat (Fig. 3). In MTDF the rock microhabitat increased during seasonal transition (Friedman test: χ 2 = 7.33, df = 3, p = 0.06), leaf-litter (Friedman test: χ 2 = 1.15, df = 3, p = 0.76) and tree (Friedman test: χ 2 = 4.56, df = 3, p = 0.21) microhabitats increased but not significantly, whereas herb microhabitat decreased (Friedman test: χ 2 = 6, df = 3, p = 0.11) but this change was not significant (Fig. 3).

Figure 3 Relative abundance (individuals/hectare) of Sceloporus utiformis lizards in several microhabitats during seasonal transition. Continuous black line: Chamela tropical dry forest; pointed black line: Manantlán tropical dry forest; continuous gray line: Chamela arroyo vegetation.
In ChTDF, environmental conditions were associated with the relative abundance of S. utiformis. However, this correlation was not observed in either ChAV or MTDF. Relative abundance of individuals of S. utiformis showed a correlation with habitat use in the 3 habitats, especially with leaf-litter and tree microhabitats (Table 2).
Table 2 Spearman correlation between relative abundance of Sceloporus utiformis and microhabitat and environmental conditions in Chamela tropical dry forest (ChTDF), Chamela arroyo vegetation (ChAV), and Manantlán tropical dry forest (MTDF).
| Leaf-litter | Herb | Tree | Rock | Temperature | Humidity | Canopy cover | |
|---|---|---|---|---|---|---|---|
| ChTDF | |||||||
| Individuals | 0.77** | −0.27 | 0.64** | - | 0.17 | −0.36 | −0.14 |
| Males | 0.27 | 0.26 | 0.10 | - | −0.52* | 0.44 | 0.56* |
| Females | 0.22 | 0.08 | 0.41 | - | −0.43 | 0.55* | 0.40 |
| Juveniles | 0.67** | −0.31 | 0.63* | - | 0.30 | −0.53* | −0.38 |
| Adults | 0.24 | 0.16 | 0.33 | - | −0.51* | 0.56* | −0.48 |
| ChAV | |||||||
| Individuals | 0.89** | 0.00 | 0.84** | 0.21 | 0.2 | −0.19 | −0.61* |
| Males | 0.09 | 0.68** | −0.19 | −0.10 | 0.00 | 0.31 | 0.40 |
| Females | 0.12 | −0.10 | 0.09 | −0.10 | −0.15 | 0.35 | 0.38 |
| Juveniles | 0.81** | −0.09 | 0.81** | 0.25 | 0.25 | −0.36 | −0.78** |
| Adults | −0.04 | 0.48 | −0.11 | −0.13 | −0.09 | 0.48 | 0.41 |
| MTDF | |||||||
| Individuals | 0.92** | −0.06 | 0.88** | 0.83** | 0.43 | −0.13 | −0.22 |
| Males | −0.09 | 0.40 | −0.12 | −0.04 | −0.30 | 0.55 | 0.82*** |
| Females | 0.21 | 0.48 | −0.01 | 0.23 | −0.29 | 0.62* | 0.80** |
| Juveniles | 0.74** | −0.29 | 0.70* | 0.63* | 0.52 | −0.45 | −0.54 |
| Adults | 0.10 | 0.46 | −0.01 | 0.12 | −0.42 | 0.51 | 0.82*** |
***p < 0.001.
**p < 0.01.
*p < 0.05.
Discussion
The results indicate that seasonal transition within the same vegetation type differed with respect to elevation (ChTDF vs. MTDF), and between the more mesic ChAV and the adjacent but drier ChTDF. These differences between habitats are reflected in the abundance and habitat use of S. utiformis. During the transition between the wet and dry seasons, the abundance of lizards in the ChTDF first displayed a rapid increase followed by a fast decrease and then a final rapid increase. The abundance of lizards in the ChAV had a continuous and slower rate of increase, and in the MTDF, lizards exhibited a slow decrease followed by 2 fast increases (Fig. 2). The elevation difference between the TDF of Chamela and Manantlán results in lower temperatures and humidity at the MTDF. The loss of relative humidity at MTDF is certainly due to the low temperature that frequently occurs at high elevations, however this low temperatures could permit water condensation that benefices the permanence of tree leaves at high TDF (Xin-Ping, Yan-Xia, Rui, Ya-Feng, & Hao, 2014). On the other hand, the AV has characteristics of a semi-deciduous forest, which maintain its canopy cover throughout the year (Suazo-Ortuño et al., 2011). We suggest that these differences in environmental conditions result in a more abrupt change in the ChTDF, followed by the MTDF and the ChAV.
S. utiformis abundance responded to both, changes in habitat type and seasonal transition. Based on our results, this lizard has a higher abundance in the MTDF than in the ChTDF and ChAV, with the ChTDF having intermediate values. It is possible that the MTDF offers better conditions to lizards that the ChTDF where conditions change more abruptly. The maintenance of vegetation conditions for longer time at MTDF, due to humidity characteristics previously discussed, could be reflected in availability of food resource that is a key factor supporting high population abundances. In the ChTDF, the abrupt seasonal transitions could have an adverse effect on the arthropods community (Suazo-Ortuño et al., 2011) and hence lower food availability. In ChAV, whose relief characteristics were discussed above, S. utiformis has low abundances, and the reasons could involve 2 factors. The first involves the thermal characteristics of this habitat. We recorded the lowest environmental temperatures in ChAV, and we hypothesized that the lower temperatures affect thermoregulation in this species (Ramírez-Bautista & Gutiérrez-Mayén, 2003), so the lizards inhabit mostly the surrounding TDF where temperatures are higher than the AV. The second factor may be an increase in interspecific competition and predation in the ChAV, since this habitat houses higher diversity of reptiles and mammals than the TDF (Gienger et al., 2002; Suazo-Ortuño et al., 2011), despite that the ChAV also could provide higher food levels than the TDF (Suazo-Ortuño et al., 2011).
A high number of correlations between abundance of lizards and habitat conditions in ChTDF was found, followed by MTDF and finally by ChAV, and the many of these correlations were negative (Table 2). The 3 habitats presented an increase of temperature, but a decrease of humidity and canopy cover during seasonal transition (Fig. 1). So, abundance of adults, males and females that decrease with seasonal transition (Fig. 2) could be positive correlated with humidity and canopy cover that also decreased, but negative correlated with temperature that increased in this lapse of time (Table 2). However, abundance of total individuals and juveniles that increased with the seasonal transition (Fig. 2) are positively correlated with temperature, but negatively correlated with canopy cover and humidity (Table 2). In ChTDF the humidity was the habitat variable more highly correlated with abundance and in ChAV and MTDF the canopy cover was the variable more strongly correlated with the abundance of lizards (Table 2).
The increase in abundance of individuals in the 3 habitats was accompanied by change in population composition. While adult male and female abundance decreased, juvenile abundance increased (Fig. 2). This is probably because many species of lizards in tropical dry forest reproduce during the rainy season, and therefore new individuals are recruited at the beginning of the dry season (Ramírez-Bautista & Gutiérrez-Mayén, 2003). It is also possible that adults hide during these months, although Ramírez-Bautista and Gutiérrez-Mayén (2003) reported that adults are active throughout the year. Nevertheless, a decrease of adult S. utiformis could lead to competition avoidance between adults and juveniles and consequently enhance juveniles survival rates, as has been reported for Lacerta vivipara (Le Galliard, Ferrière, & Clobert, 2003).
Microhabitat use of S. utiformis differed between the ChTDF and ChAV and MTDF, primarily because the last site contained a large amount of rocks, which were used by lizards as perching sites. This microhabitat was absent in both ChTDF and ChAV. In all 3 habitats, lizards were usually found on the leaf-litter and perching on trees, which could allow for wider surveillance of their territory, as in the case of several arboreal species (García et al., 2010; Lister & García, 1992; Ramírez-Bautista & Benabib, 2001).
This study aimed to investigate how seasonal transition affects habitat variables, habitat use, and relative abundance of S. utiformis. Data obtained on habitat variables (environmental temperature, relative humidity and canopy cover) and relative abundance from October 2005 to February that comprises the final part of the wet season and the beginning of the dry season, were analyzed statistically. The results showed that seasonal transition entails gradual changes in the abundance and habitat use of S. utiformis and, most likely, all the lizard species that inhabit the tropical dry forest and surrounding vegetation. Nevertheless, these changes could differ in intensity depending on the vegetation type and the elevation at each habitat. During seasonal transition, lizard abundance increases and there is also a change in the structure of the population age classes, since juvenile abundance increased and adult abundance decreased. This suggests minimal interaction (competition) between both age classes, thereby increasing the survival probabilities for the younger generation. Further studies concerning the behavior and survival of the various age classes would be needed to substantiate these hypotheses.











 text new page (beta)
text new page (beta)


