Halacaridae are prostigmatid mites with more than 1,100 described species around the world (Bartsch, 2009), and as a group they are mainly known as the marine mites. Eight species of halacarids have been reported from Mexico: Agaue variabilis MacQuitty, Agauopsis filirostris MacQuitty, Copidognathus ilsebartschi MacQuitty, Halacarus newelli MacQuitty, Simognathus cramerae Rivas, Actacarus giganteus Krantz, Copidognathus yucatanensis Chatterjee & De Troch, and C. unicustatus Bartsch; the former 5 are distributed along the Pacific (MacQuitty, 1983, 1984; Rivas, 2006) and the latter 3 in the Caribbean Sea (Chatterjee & De Troch, 2001; Krantz, 1971).
This family is mainly marine, occurring at depths ranging from the littoral zone to about 7,000 m (Bartsch, 1988); however, some 60 species can be found in continental and coastal fresh and brackish waters (Bartsch, 1996, 2008). In particular, the subfamily Limnohalacarinae is composed of 5 genera: Hamohalacarus Walter, Himejacarus Imamura, Parasoldanellonyx Viets, Soldanellonyx Walter and Limnohalacarus Walter; all of them with species that inhabit continental waters.
Particularly, the 13 species of Limnohalacarus have a worldwide distribution, living in fresh to slightly brackish waters (Bartsch, 2013; Pepato & Costa, 2015): Limnohalacarus africanus Walter, L. australis Bartsch, L. capernaumi Petrova, L. cultellatus Viets, L. dentatus Bartsch, L. fontinalis Walter & Bader, L. inopinatus Fain & Lambrechts, L. lanae Green, L. major Bader, L. mamillatus Fain & Lambrechts, L. novus Bartsch, L. portmanni Bader and L. wackeri (Walter).
Only 2 species of Limnohalacarinae have been reported from the Neotropics. The first one, L. cultellatus, originally described from the Antilles, has also published records from the northern USA and El Salvador (Bartsch, 2011), the Caribbean region (Bartsch, 1984; Viets, 1940) and Brazil (Bartsch, 2011; Pepato & Costa, 2015). The second species, L. mamillatus, was described from an aquarium in Belgium and has been recorded in Brazil (Pepato & Costa, 2015).
Regarding anchialine systems, only a few records of aquatic mites exist from caves in Bermuda (Bartsch & Iliffe, 1985). No previous records of halacarids exist for the Yucatán Peninsula in Mexico, where large anchialine systems with hundreds of kilometers of flooded passages have developed. The typical taxa occurring in the anchialine systems of Yucatán are crustaceans: remipedes, amphipods, isopods, ostracods, thermosbaenaceans, and caridean shrimps (Álvarez, Iliffe, Benitez, Brankovits, & Villalobos, 2015).
We herein present a new record of Limnohalacarus cultellatus from Cenote Bang which is part of the Ox Bel Ha system, Tulum, Quintana Roo, Mexico. The samples of L. cultellatus, obtained in 2013 are the first for Mexico and also the first from an anchialine habitat. The morphological variations observed relative to the original description are presented (Table 1), and an identification key for females of all the species of Limnohalacarus is included.
Table 1 Records and variation of the total body size and some of the plates of Limnohalacarus cultellatus.
| Distribution | Habitat | Length/width ratio | References | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Idiosoma | Gnathosoma | AD | OC | PD | AE | GP | |||
| Margarita, Curacao, Aruba and Bonaire Islands | Piped water, rivers and water in fissures | 1.41 | 1.35 | 1.00 | 1.12 | 1.87 | 0.80 | 1.20 | Viets (1940) |
| Andhara Pradesh, India | Brackish water, bay near confluence of a river | 1.37 | 1.42 | 1.13 | 1.10 | 1.92 | 0.58 | 1.12 | Chatterjee and Chang (2005) |
| Georgia and Wisconsin, USA | River | 1.19 | 1.65 | 1.16 | 1.00 | 1.78 | 0.62 | 0.80 | Bartsch (2011) |
| Northern Madagascar | River, riffle | 1.19 | NA | 1.10 | 1.10 | 1.70 | NA | NA | Bartsch (2013) |
| Rio Grande do Sul, Brazil | Lake with salinity gradient (brackish to freshwater) | 1.47 | 1.28 | 1.01 | 1.06 | 1.53 | 0.60 | 0.71 | Pepato and Costa (2015) |
| Yucatán Peninsula, Mexico | Anchialine cave | 1.34 | 1.36 | 1.13 | 1.53 | 1.83 | 0.57 | 1.17 | Present study |
AD, anterior dorsal plate; AE, anterior epimeral plate; GP, genital plate; NA, not available; OC, ocular plate; PD, posterior dorsal plate.
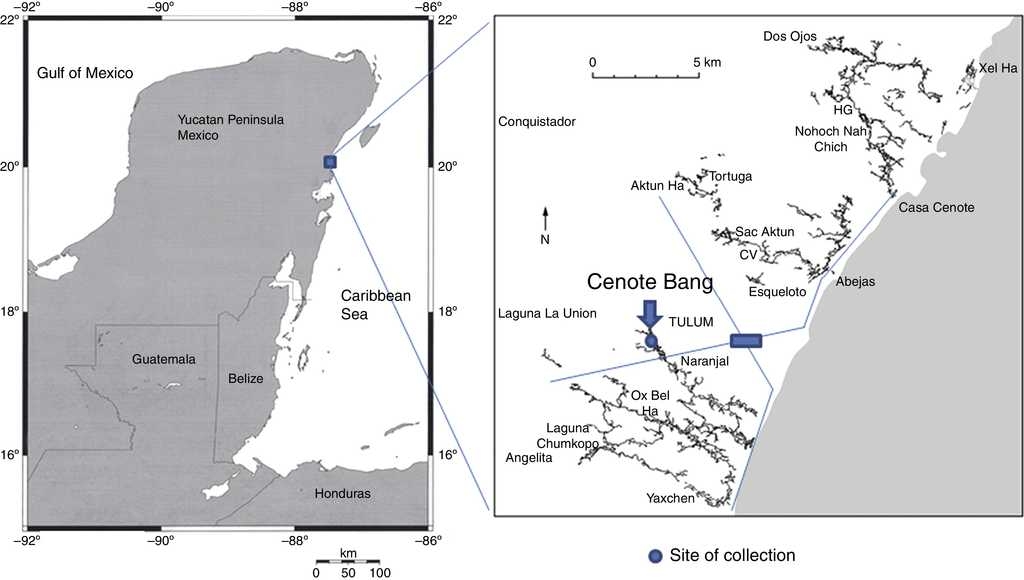
The Ox Bel Ha system develops just to the south of the town of Tulum, Quintana Roo, Mexico (Fig. 1). Cenote Bang is located in the innermost section of the system about 12 km from the shore. Around Cenote Bang the submerged galleries develop at an average depth of 18 m and the halocline is present at a depth ranging from 18 to 21 m.
Samples were obtained with a plankton net (300 μm) from 0 to 8 m in the cenote. The contents were preserved in 70% ethanol. At the laboratory, mites were sorted under a stereoscopic microscope, cleared in lactic acid and mounted in Hoyer's medium. The illustrations and measurements in micrometers (μm), were made using a Nikon Optiphot-2 phase contrast microscope. Microphotographs were obtained with an AxioCam MRC5 camera using a Carl Zeiss AxioZoom V16 microscope.
The specimen was collected under the scientific collector's license issued to F. Álvarez (FAUT 0104) by the Mexican environmental authority (Semarnat). The organism was deposited in the National Acarological Collection (CNAC) of the Institute of Biology, UNAM, México City, with the catalog number CNAC009211.
The abbreviations used in the diagnosis and redescription are: AD (anterior dorsal plate), AE (anterior epimeral plate), AP (anal plate), ds (dorsal setae, from anterior to posterior: ds-1 to ds-5), P-2 to P-4 (second to fourth palpal segment), GA (genitoanal plate), ¿ (famulus), gac (genital acetabula), glp (dorsal or lateral gland pores, from anterior to posterior: glp-1 to glp-5), GP (genital plate), GO (genital opening), OC (ocular plate), pas (parambulacral seta), PD (posterior dorsal plate), PE (posterior epimeral plate), pgs (perigenital setae), sgs (subgenital setae), ω (solenidium), legs (numbered I to IV). Segments, from distal to proximal region are: tarsus, tibia, genu, telofemur, basifemur, and trochanter. The chaetotaxy formulas exclude the solenidia, famuli and parambulacral setae from the trochanter to the tarsus. The number of bipectinate spines is given in parentheses. Lengths of leg segments are measured along their dorsal margin. Measurements are expressed in micrometers (μm).
Complementary description
Classification follows Krantz & Walter, 2009: 98-100.
Subclass Acari Leach, 1817
Superorder Acariformes Zakhvatkin, 1952
Order Trombidiformes Reuter, 1909
Superfamily Halacaroidea Cunliffe, 1955
Family Halacaridae Murray, 1877
Subfamily Limnohalacarinae Viets, 1927
Genus Limnohalacarus Walter, 1917
Limnohalacarus cultellatusViets, 1940
Material examined. One female (CNAC 009211), Cenote Bang, Tulum, Quintana Roo, Mexico (29°57′30.63″N, 50°09′14.50″W), 4 December 2013, colls. O. Cortes and F. Álvarez.
Diagnosis [according with Viets (1940), Bartsch (2011, 2013) and Pepato and Costa (2015)]. Length 275-325 μm. Dorsal plates reticulated, except for smooth anteriormost part of AD. Medial and lateral eye pigment absent. Length: width ratio of AD 1.0-1.2. OC divided and reticulated, with narrow triangular sclerite bearing gland pore. PD 1.5-2.1 times longer than wide and 2.5 times longer than AD. Second pair of dorsal setae present. Ventral plates separated. GP with 4-9 pairs of gac, 3, rarely 4, pairs of pgs and 2 pairs of sgs. Gnathosoma 1.3-1.5 times longer than wide; rostrum slender. Both pairs of maxillary setae slender. Pharyngeal plate removed from margin of gnathosomal base by more than half its length. P-2 basally abruptly widened, in lateral view rectangular with straight dorsal margin. Telofemur I, 1.6-1.8 times longer than wide. Tarsi with spiniform lamellae near bases. Trochanters I-IV with 1, 1, 1, 1 setae, basifemora with 4, 3, 2, 1 setae, genua III with 4 setae, and tibiae I-IV with 7, 6, 7, 6 setae. On tibiae I-III, 1, 1, 2 pectinate setae, on tibia IV all setae slender. Tibiae III and IV with 4 and 3 ventral setae, respectively. All tarsi with pairs of pas singlets. Claws on tarsus I slender, claws with lamellar basal process and delicate apical tines. Claws of tarsi II-IV with series of tines from apical accessory process to basal process. Male: not known.
Redescription. Female. Idiosoma 329 μm long, 245 μm wide (Fig. 2A). Dorsal plates reticulated (Fig. 2B). AD hexagonal, posterior border truncate, 1 small anterior projection in the middle toward the front (Fig. 3A), length 67 μm, width 76 μm, length:width ratio 0.9. Medial eye pigment absent, ds-1 on an anterior area of AD and ds-2 on membranous cuticle between AD and OC. OC between slightly rounded and sub-pentagonal, 43 μm long, 23 μm wide, length:width ratio 1.5, separated from a triangular sclerite, 26 μm long, 14 μm wide, bearing gland pore.
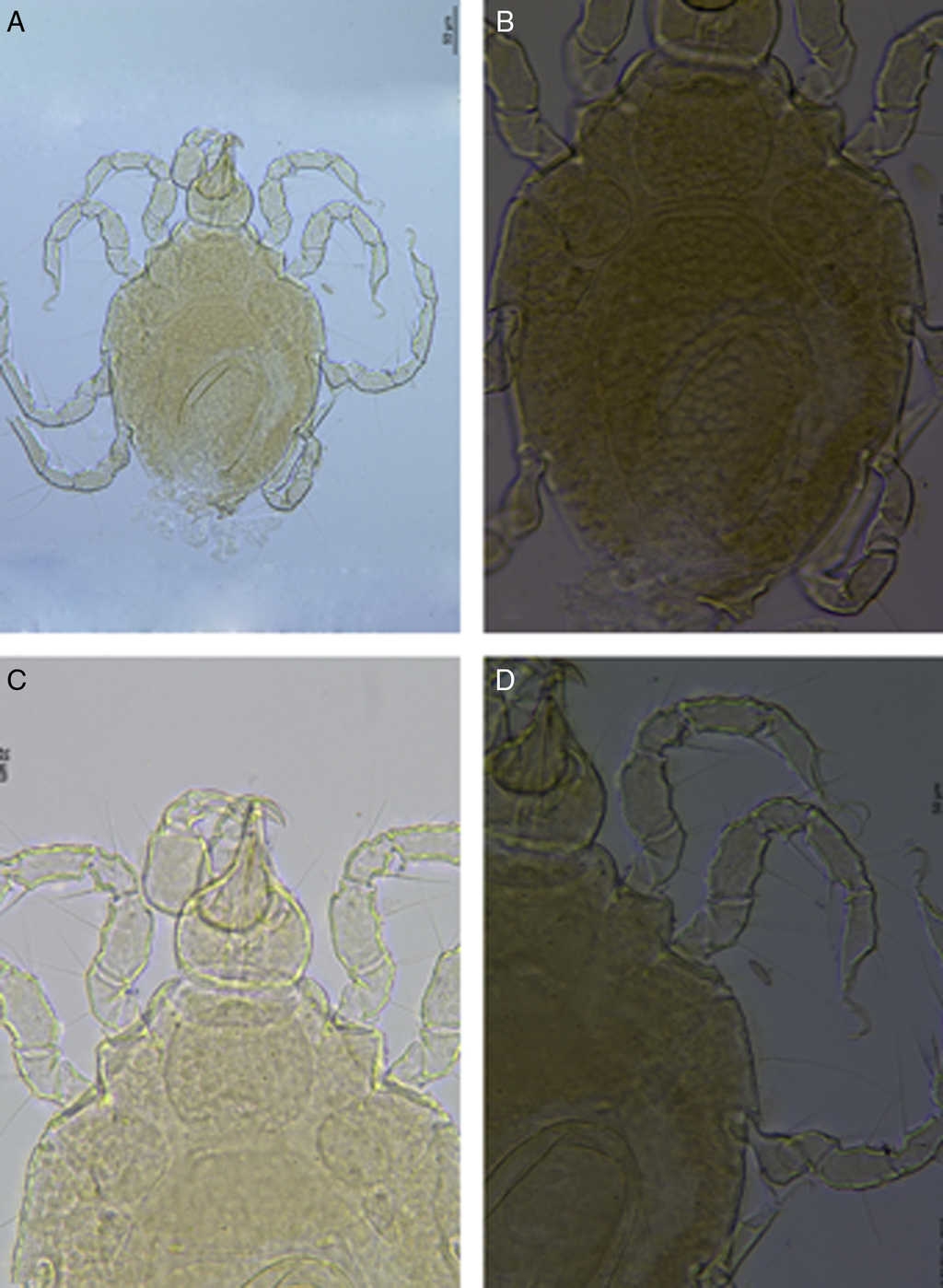
Figure 2 Limnohalacarus cultellatusViets, 1940, female: A, habitus; B, idiosoma, dorsal plates; C, gnathosoma; D, legs I and II.
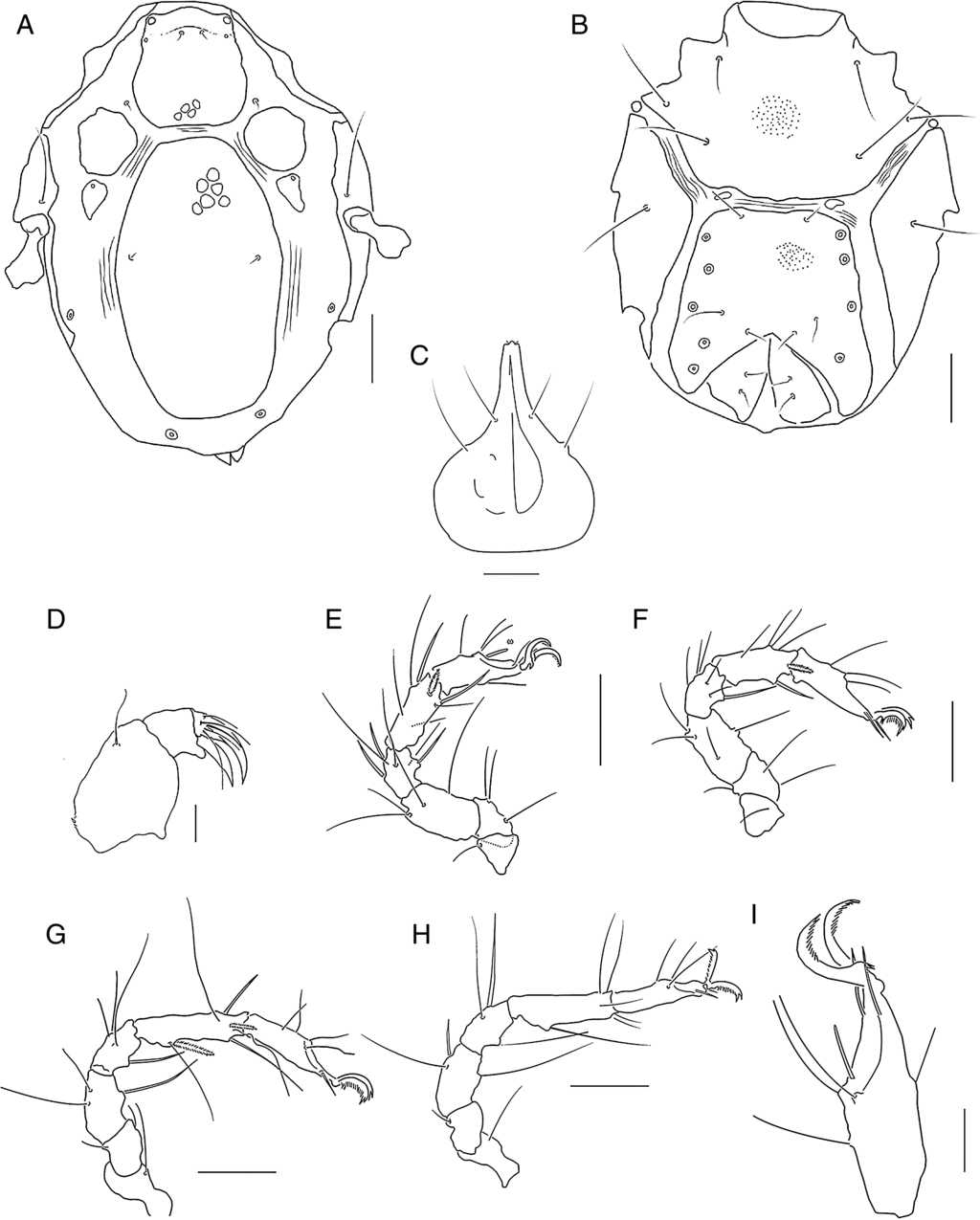
Figure 3 Limnohalacarus cultellatus Viets, 1940, female: A, idiosoma, dorsal view; B, idiosoma, ventral view; C, gnathosoma, ventral view; D, pedipalp, lateral view (scale bar=20μm); E, leg I; F, leg II; G, leg III; H, leg IV; I, tarsus I. Scale bars=50μm.
Corneas and eye pigment not evident. PD elongated, 218 μm long, 119 μm wide, 3.2 times longer than AD. PD length:width ratio 1.8. Pairs of glp-4 and glp-5 on striated cuticle lateral to PD. Ventral plates AE, pairs of PE and GP separated by membranous cuticle. AE 116 μm long, 215 μm wide, with 3 pairs of ventral setae, PE with 3 setae. GP 162 μm long, 138 μm wide, with 3 pairs of pgs and 11-14 gac, GO in posterior part of ventral shield (Fig. 3B). Genital sclerites large, 68 μm long, 31 μm wide, with 2 pairs of sgs. Length of gnathosoma 97 μm, width 71 μm, gnathosoma length:width ratio 1.36; rostrum (Fig. 2C), 40 μm long, equal to 0.41 of gnathosoma length. Dorsal and basal pairs of maxillary setae slender (Fig. 3C). Dorsal margin of P-2 straight (Fig. 3D). Legs slender (Figs. 2D, 3E-H). Length/height of telofemora I-IV: 2.6, 1.8, 1.7, 1.7 times, respectively. Telofemur/tibia length ratio I-IV: 0.99, 0.94, 0.66, 0.60. Leg chaetotaxy from trochanter to tarsus (solenidia, famuli and parambulacral setae excluded): I: 1, 4, 4, 6, 7(I), 4; II: 1, 3, 4, 6, 6(I), 3; III: 1, 2, 3, 4, 7(II), 4; IV: 1, 1, 3, 3, 6, 3. Tibia III with 2 bipectinate ventromedial and 2 slender and smooth ventrolateral setae. All tarsi with pairs of pas singlets. Claw I with long tines arranged along inner flank and distal outer portion of claw (Fig. 3I). Claws on tarsi II-IV with tines and a developed basal lamellar process.
The habitat of Halacaridae has been mainly in the marine environment, which is interpreted as a successful secondary adaptation from their terrestrial prostigmads ancestors (Bartsch, 1989). The fact that around 5% of halacarid mites live in continental waters raise interesting questions of how they were able to invade freshwater habitats and how the ways of life played a role in this process (Bartsch, 1996; Delamare-Deboutteville, 1960). The evolutionary pathways of marine groups to freshwaters through subterranean systems implies interactions with hydrogeological processes at different scales (Ward & Palmer, 1994), aspects to consider for the invertebrate fauna of anchialine systems.
The halacarid recently collected from Cenote Bang fit well the diagnostic characteristics established by Bartsch (2011, 2013), Pepato and Costa (2015), and Viets (1940), for Limnohalacarus cultellatus; the main measurements of the specimen are within the recorded range based on records given for the species around the world. In addition to the original description by Viets (1940) for L. cultellatus, 9 records, including the one presented here from the Yucatán Peninsula are known (Table 1). All of these are almost always based on 1 or 2 individuals, and because of this limited number an assessment of the morphological variation is difficult to obtain, it has yet to be established if it is a wide ranging species with a significant degree of morphological variation or a species complex, that in the future could be resolved incorporating molecular information about these mites. According to Bartsch (2013), L. cultellatus, L. australis (Australia), and L. inopinatus (Aquarium in Belgium) are members of the same clade. The differences observed between these species in the PD length: width ratio, the number of apical tines on claws I, and presence of rather thick, barbed setae on tibia IV could be interpreted as intraspecific variation and not as a variation between 2 species.
The species of Limnohalacarus have a cosmopolitan distribution in spite of not having efficient dispersal mechanisms. However, it is thought that the combination of an ancient radiation to freshwater since the Mesozoic and the possible parthenogenetic mode of reproduction of some of the species could contribute to their wide geographical distribution (Bartsch, 1996, 2008). The efforts to study halacarid mites in environments such as the anchialine caves are necessary to answer questions about the delimitation of the species as well as to understand the diversification process.
The third author gratefully acknowledges the funding awarded though a Conacyt grant 155644(546) “Processes that create and maintain the biodiversity in an extreme environment: the anchialine systems of Yucatán”. We appreciate the assistance provided by Drs. Ilse Bartsch and Almir Pepato with part of the literature cited. We would also like to thank Olinka Cortes for obtaining the samples and Susana Guzmán from IBUNAM for the technical assistance in taking the microphotographs. We also thank the two anonymous reviewers for their contributions to the manuscript.
| Key for females of all the known species of Limnohalacarus | |
|---|---|
| 1a. Gland pores (glp) within OC, ventral plate fused | 2 |
| 1b. Gland pores (glp) outside OC, ventral plates separated | 8 |
| 2a. Gnathosoma length:width ratio 1.0 to 1.2 | 3 |
| 2b. Gnathosoma length:width ratio 1.3 to 1.7 | L. major |
| 3a. 0-1 subgenital setae | 4 |
| 3b. 2 or more subgenital setae | 5 |
| 4a. Apical maxillary setae wide | L. lanae |
| 4b. Apical maxillary setae slender | L. mamillatus |
| 5a. 4 or more small tines on claws III and IV | 6 |
| 5b. 2 strong tines on claws III and IV | L. dentatus |
| 6a. Length of idiosoma less than 300 μm | 7 |
| 6b. Length of idiosoma higher than 300 μm | L. fontinalis |
| 7a. AD lenght/width 0.9 and OC lenght/widht 1.4 or more | L. novus |
| 7b. AD length/width 0.7 and OC less than 1.4 | L. portmanni |
| 8a. Parambulacral setae of leg I doublets | 9 |
| 8b. Parambulacral setae of leg I singlets | 11 |
| 9a. 5 or more perigenital setae | 10 |
| 9b. 3-4 perigenital setae | L. africanus |
| 10a. Eye pigment and / or cornea absent | L. capernaumi |
| 10b. Eye pigment and / or cornea present | L. wackeri |
| 11a. Tibia IV with 1 pectinate spiniform setae | L. inopinatus |
| 11b. Tibia IV without pectinate spiniform setae | 12 |
| 12a. AD completely reticulated | L. australis |
| 12b. AD reticulated, except anterior most part | L. cultellatus |











 nueva página del texto (beta)
nueva página del texto (beta)