Introduction
Characithecium Mendoza-Franco, Reina, & Torchin, 2009 was proposed to include Urocleidoides costaricensis (Price & Bussing, 1967) Kristky & Leiby, 1972, a species considered as incertae sedis by Kritsky, Thatcher, and Boeger (1986) due to the presence of overlapping gonads, mid-ventral vagina, ventral anchor larger than the dorsal, and ventral bar showing a median posterior projection (Mendoza-Franco et al., 2009).
Rossin and Timi (2014) revised the diagnosis of Characithecium to include 4 new species collected in Argentina and 1 new combination. The genus currently comprises 6 species: Characithecium chascomusensis (Suriano, 1981) Rossin & Timi, 2014, Characithecium costaricensis (Price & Bussing, 1967) Mendoza-Franco, Reina, & Torchin, 2009, Characithecium longianchoratum Rossin & Timi, 2014, Characithecium robustum Rossin & Timi, 2014, Characithecium quadratum Rossin & Timi, 2014 and Characithecium chelatum Rossin & Timi, 2014.
Urocleidoides astyanacis Gioia, Silva & Artigas, 1988 was described from specimens parasitizing the gills of Astyanax fasciatus (Cuvier, 1819) and Astyanax scabripinnis (Jenyns, 1842) in Brazil (Gioia, Cordeiro, & Artigas, 1988). This species was also recorded in Astyanax altiparanae Garutti & Britski, 2000 by Azevedo, Madi, and Ueta (2007). However, Mendoza-Franco et al. (2009) considered U. astyanacis as a junior synonym of C. costaricensis. This study describes a new species of Characithecium found on the body surface and gills of A. aff. fasciatus and Astyanax jacuhiensis (Cope, 1894) from Lake Guaíba, State of Rio Grande do Sul, Brazil.
Material and methods
A total of 70 specimens of A. aff. fasciatus and 60 of A. jacuhiensis were collected with seine nets between March 2012 and November 2013 in Lake Guaíba, municipalities of Guaíba (30°08′28″S, 51°18′53″W) and Barra do Ribeiro (30°17′11″S, 51°18′01″W), State of Rio Grande do Sul, Brazil. Individual fishes were stored in separate plastic bags and kept under refrigeration until necropsy. Each fish had its external surface scraped with a knife to remove mucus, scales and to detach the monogeneans. The gills were removed, put in a jar and shaken at least 50 times with formalin solution 1:4,000. The monogeneans found were processed and the description of their terminology and measurements was conducted according to Gallas, Calegaro-Marques, and Amato (2014). Measurements are shown in micrometers (µm) and represent the range followed by the mean, the standard deviation, and the sample size (in parenthesis).
Line drawings were made with a drawing tube using a Nikon E-200 microscope, scanned and prepared using CorelDraw X4® and Adobe's Photoshop® CS2. Ecological parameters follow Bush, Lafferty, Lotz, and Shostak (1997). Holotype and paratypes were deposited in the ‘Coleção Helmintológica do Instituto Oswaldo Cruz’ (CHIOC), Rio de Janeiro, State of Rio de Janeiro, Brazil, and voucher specimens were deposited in the ‘Coleção Helmintológica do Laboratório de Helmintologia’ (CHDZ), Departamento de Zoologia, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, State of Rio Grande do Sul, Brazil. Hosts were identified following Bertaco and Lucena (2010). Representative specimens of the hosts were deposited in the ‘Coleção Ictiológica’, Departamento de Zoologia, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, State of Rio Grande do Sul, Brazil.
Description
Characithecium triprolatum n. sp.(Figs. 1,2,3,4,5,6,7,8,9)
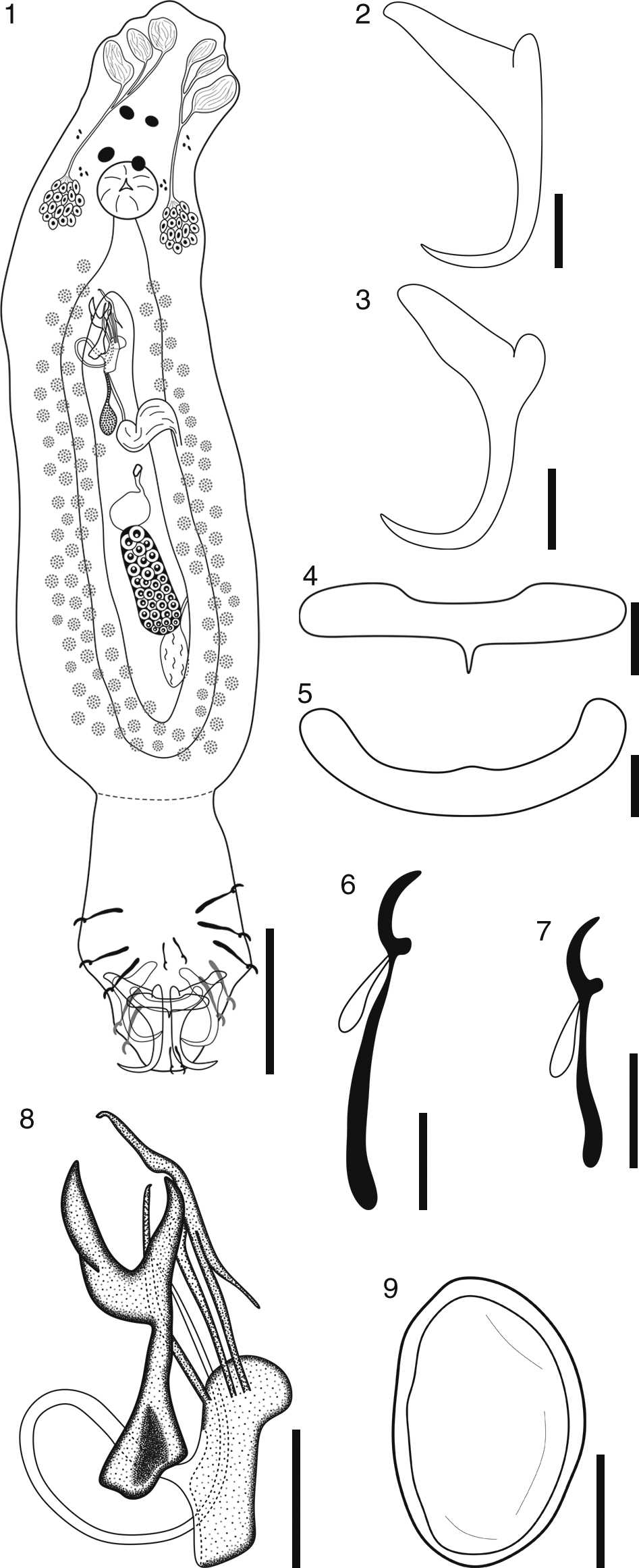
Figures 1-9 Diagrams of Characithecium triprolatum n. sp. 1, Composite (ventral view) of entire specimen. Scale bar=50µm.; 2, ventral anchor. Scale bar=10µm.; 3, dorsal anchor. Scale bar=10µm.; 4, ventral bar. Scale bar=5µm.; 5, dorsal bar. Scale bar=5µm.; 6, hook pair 4. Scale bar=5µm.; 7, hook pair 1. Scale bar=5µm.; 8, male copulatory organ. Scale bar=10µm.; 9, egg. Scale bar=20µm.
Description (based on 22 specimens): Dactylogyridae, Ancyrocephalinae. Body 322-555 (426 ± 87; n = 11) long, 60-122 (88 ± 20; n = 11) wide at gonads level. Moderately developed cephalic lobes, 1 pair terminal and the other lateral; cephalic glands clustered in 2 groups in the region of the pharynx. Two pairs of eyes, the anterior pair slightly closer than the posterior one; few accessory granules in the cephalic area. Spherical pharynx, 15-25 (20 ± 3; n = 11) in diameter; caeca without branches forming a cyclocoel posterior to the gonads. Haptor separated from the body by a peduncle, 55-125 (73 ± 22; n = 10) long, 35-60 (51 ± 7; n = 10) wide. Ventral anchor containing an elongate superficial root and a short deep root, both lacking protuberances; slightly straight shaft and elongate point. Ventral anchor 32-40 (37 ± 3; n = 12) long, base 15-22 (18 ± 3; n = 12) wide. Ventral bar 5-10 (7 ± 1; n = 10) long, 17-25 (21 ± 2; n = 10) wide, showing a regular anterior margin and a posterior margin containing a median projection and expanded ends. Dorsal anchor containing an elongate superficial root and a short deep root both lacking protuberances; slightly straight shaft and elongate point. Dorsal anchor 22-35 (28 ± 4; n = 12) long, base 10-15 (12 ± 2; n = 12) wide. Dorsal bar 7.5-12 (10 ± 2; n = 12) long, 22-35 (27 ± 3; n = 12) wide, U-shaped, usually showing a small elevation on the anterior margin and a regular posterior margin. Hooks with protruding thumb and dilated shank. Hook pairs 1 and 5, 10-12 (12 ± 1; n = 20) long, filamentous hook (FH) loop 2-5 (3 ± 1; n = 20) long. Hook pairs 2, 3, 4, 6, and 7, 15-22 (18 ± 2; n = 56) long, filamentous hook (FH) loop 4-10 (6 ± 1; n = 56) long. Slightly overlapped gonads. Testis posterior to the ovary, 22-40 (29 ± 7; n = 5) long, 10-20 (13 ± 3; n = 8) wide. Copulatory complex composed of male copulatory organ (MCO) and an accessory piece containing 2 subunits, the dorsal one apparently articulated directly to the MCO. MCO showing a counterclockwise ring coil, ring diameter 12-17 (14 ± 2; n = 8); base differentiated, possibly fused to the proximal portion of the dorsal subunit of the accessory piece. Accessory piece containing a pincer-shaped ventral subunit on the distal portion, 20-35 (26 ± 4; n = 10) long; dorsal subunit containing 3 elongations that arise from a rod-shaped proximal portion on the distal portion, 27-45 (36 ± 7; n = 11) long. One prostatic reservoir, posterior to the base of the MCO. Seminal vesicle present, a dilation of vas deferens; vas deferens looping left caeca, anterior to ovary. Slightly sclerotized mid-ventral vaginal opening. Seminal receptacle present, anterior to ovary. Ovary 37-75 (56 ± 10; n = 9) long, 12-30 (19 ± 6; n = 9) wide. Oviduct, ootype, and uterus not observed. Vitelline folicles found from pharynx to the end of cyclocoel. Rounded eggs, filament not observed, 35 and 55 (n = 2) long, 32 and 37 (n = 2) wide.
Taxonomic summary
Type host: Astyanax aff. fasciatus (Cuvier, 1819).
Other host: Astyanax jacuhiensis Cope, 1894.
Hosts specimens deposited: A. aff. fasciatus UFRGS 19121 (male); UFRGS 19122 (female) and A. jacuhiensis UFRGS 19123 (male); UFRGS 19124 (female).
Type locality: Lake Guaíba, Municipality of Barra do Ribeiro (30°17′11″S, 51°18′01″W), State of Rio Grande do Sul, Brazil.
Other locality: Lake Guaíba, Municipality of Guaíba (30°08′28″S, 51°18′53″W), State of Rio Grande do Sul, Brazil.
Site of infestation: external surface and gills.
Prevalence: 57.1% (A. aff. fasciatus) and 46.7% (A. jacuhiensis).
Mean intensity of infestation: 1.90 helminths/host (A. aff. fasciatus) and 1.82 helminths/host (A. jacuhiensis).
Mean abundance of infestation: 1.09 helminths/host (A. aff. fasciatus) and 0.85 helminth/host (A. jacuhiensis).
Amplitude of intensity of infestation: 1-5 helminths (A. aff. fasciatus and A. jacuhiensis).
Material deposited: CHIOC n° 38286 (holotype); 38287 and 38288 (paratypes).
Etymology (L. triprolatum - composite adjective of 1st class (triform) - tri = 3 + prolatus = extended, elongated). The specific epithet refers to the 3 elongations characteristic of the dorsal subunit of the accessory piece of the male copulatory complex.
Remarks
Rossin and Timi (2014) emended the diagnosis of the genus Characithecium proposed by Mendoza-Franco et al. (2009) to include species showing the vagina in different positions in addition to the original medio-ventral, the ventral bar showing or missing a median posterior projection, the MCO presenting a joint between the base of the MCO and the accessory piece, and the accessory piece apparently lacking subunits. The new species was identified as belonging to Characithecium based on the mid-ventral vagina, the ventral anchor larger than the dorsal one, the ventral bar showing a posterior margin containing a median projection, and the accessory piece of the MCO composed of 2 subunits.
Characithecium triprolatum n. sp. differs from C. chascomusensis, C. longianchoratum, C. robustum, C. quadratum, and C. chelatum by the morphology of its ventral bar (expanded ends and median projection on the posterior margin), the mid-ventral vagina (except in C. longianchoratum that also shows a mid-ventral vagina), and the shape of accessory piece, a pincer-shaped ventral one and a dorsal containing 3 elongated projections. Characithecium triprolatum n. sp. is similar to C. costaricensis, differing by (1) having a haptor separated from the body by a long peduncle (short in C. costaricensis), (2) the presence of an accessory piece composed of 2 subunits, 1 pincer-shaped ventral at the distal end, and 1 dorsal with an expanded rod-shaped proximal end and the distal end containing 3 elongated projections, possibly serving as a guide for the MCO, according to Mendoza-Franco et al. (2009) the accessory piece in C. costaricensis shows 2 subunits connected to the base by a small joint, but the illustration provided in its description does not allow to clearly distinguish them, and (3) the presence of a small median elevation in the anterior margin of the dorsal bar (the dorsal bar shows smooth margins in C. costaricensis).
In addition, the difference in the body length of C. costaricensis and Characithecium triprolatum n. sp. could be related to body shape. Whereas it is fusiform, wider in the gonadal region and with a haptor with a short peduncle, in C. costaricensis, it is fusiform and elongated with a nearly homogeneous width throughout the trunk and with a haptor with a longer peduncle in Characithecium triprolatum n. sp.
Characithecium costaricensis has been recorded in the following hosts and locations: A. fasciatus in Mexico (Mendoza-Franco, Scholz, Vivas-Rodríguez, & Vargas-Vázquez, 1999), Nicaragua (Mendoza-Franco, Posel, & Dumailo, 2003), Costa Rica (Kritsky & Leiby, 1972; Price & Bussing, 1967), Panama (Mendoza-Franco et al., 2009), and Colombia (Kritsky & Thatcher, 1974); Astyanax aeneus (Günther, 1860) in Mexico (Mendoza-Franco et al., 2009); Astyanax ruberrimus Eigenmann, 1913 in Panama (Mendoza-Franco et al., 2009); A. bimaculatus and Curimata argentea (= Steindachnerina argentea (Gill, 1858)) in Trinidad and Tobago (Molnar, Hanek, & Fernando, 1974). In Brazil, C. costaricensis was recorded in A. fasciatus (Acosta, Queiroz, Brandão, & Silva, 2015; Gioia et al., 1988), A. scabripinnis (Gioia et al., 1988), and A. altiparanae (Azevedo et al., 2007). The other congeners (C. chascomusensis, C. longianchoratum, C. robustum, C. quadratum, and C. chelatum) were recorded in Oligosarcus jenynsii (Günther, 1864) in Argentina (Rossin & Timi, 2014).
Data on ecological parameters of infestations are only available from Mendoza-Franco et al. (2003), Rossin and Timi (2014) and Acosta et al. (2015). The low sample size (n = 2 and n = 1) of C. costaricensis parasitizing A. fasciatus collected in 2 rivers in Nicaragua by Mendoza-Franco et al. (2003) prevents the comparison with the findings of the current study. Rossin and Timi (2014) report the ecological parameters of Characithecium spp. from a subsample (n = 10) of the 54 collected O. jenynsii. Acosta et al. (2015) reported the ecological parameters of C. costaricensis in A. fasciatus collected in 2 environments. In a lotic ecosystem (n = 30) prevalence was 83.3%, the average intensity of infestation, 2.3, and the average abundance, 1.9, whereas these figures were 66.7%, 1.3, and 0.9, respectively, in a lentic ecosystem (n = 30). Therefore, these authors recorded higher prevalences than those of Characithecium triprolatum n. sp. in A. fasciatus and A. jacuhiensis reported in the present study. Habitat characteristics and host population density are potential causes of such differences (Acosta et al., 2015; Paraguassú & Luque, 2007).
The range of the intensity of infestation of Characithecium triprolatum n. sp. in A. aff. fasciatus and A. jacuhiensis was 1-5 helminths. This range is similar to those recorded for C. robustum (1-7 helminths) and C. quadratum (1-4 helminths), but much lower than those found for C. chascomusensis (2-163 helminths), C. longianchoratum (2-98 helminths), and C. chelatum (2-65 helminths) in O. jenynsii in Argentina (Rossin & Timi, 2014). It is possible that these differences are mediated by host size (O. jenynsii, 33.9 cm > A. aff. fasciatus, 16.4 cm and A. jacuhiensis, 14.3 cm; Luz-Agostinho, Latini, Abujanra, Gomes, & Agostinho, 2010; Marques, Braun, & Fontoura, 2007) as larger fish may have larger gills for the establishment of oncomiracidia, thereby allowing an increase in the intensity of infestation (Ferrari-Hoeinghaus, Takemoto, Oliveira, Makrakis, & Baumgartner, 2006). Studies relating environmental characteristics and species co-occurrence and other factors may shed light onto the observed high intensity of infestation found in these species.
Finally, although Cohen, Justo, and Kohn (2013) contend that dactylogyrid biodiversity is high in Brazilian fish, there are only a handful of records in Astyanax spp. This study increased our knowledge on the diversity of monogeneans parasitizing A. aff. fasciatus and A. jacuhiensis in southern Brazil. It also lends support to the hypotheses of Mendoza-Franco, Caspeta-Mandujano, and Salgado-Maldonado (2013) and Gallas et al. (2014) that Astyanax species host a high diversity of poorly known monogeneans.











 nueva página del texto (beta)
nueva página del texto (beta)


