Introduction
The vast and unexplored Sierra Madre Occidental of Mexico (SMO) is one of the most important areas of radiation and speciation of the family Salmonidae in North America (Mayden et al., 2010) and possibly of other complexes of species belonging to the families Cyprinidae (Schonhuth et al., 2011; Schonhuth, Lozano-Vilano, Perdices, Espinosa, & Mayden, 2014; Schonhuth, Perdices, et al., 2014), Catostomidae (Siebert & Minckley, 1986) and Ictaluridae (Castañeda-Rivera, Grijalva-Chon, Gutiérrez-Millán, Ruiz-Campos & Varela-Romero, 2014; Varela-Romero, Hendrickson, Yepiz-Plascencia, Brooks, & Neely, 2011).
The SMO is a majesty orographic formation of 289,000 km2 that comprises one-sixth of Mexican territory and that is thought to be an important corridor for historical radiation and vicariance events for freshwater fishes across North America (Miller & Smith, 1986). This mountain region is known for supporting rich and unique freshwater communities. Our current understanding of the causal factors for species distributions and community assemblages is only in its early stages (Schonhuth et al., 2011; Schonhuth, Lozano-Vilano, et al., 2014; Schonhuth, Perdices, et al., 2014; Smith & Miller, 1986).
The family Catostomidae is composed of 12 genera and 72 nominal species in North America (Page et al., 2013), of which 6 genera and 16 species occur in Mexico (Miller, Minckley, & Norris, 2005). The systematics and evolutionary relationships of the members of this family has been subject of several morphological and molecular studies that have resulted in changes in the taxonomic position and nomenclature of several taxa (Chen & Mayden, 2012; Clements, Bart, & Hurley, 2012; Doosey, Bart, Saitoh, & Miya, 2010; Harris & Mayden, 2001; Smith, 1992).
In the northern SMO and Chihuahuan Desert regions at least 8 species of the genus Catostomus have been taxonomically recognized (C. bernardini Girard 1856, C. cahita Siebert and Minckley, 1986, C. clarkii Baird and Girard 1854, C. insignis Baird and Girard 1854, C. leopoldi Siebert and Minckley, 1986, C. nebuliferus Garman 1881, C. plebeius Baird and Girard 1854, and C. wigginsi Herre and Brock 1936), of which C. bernardini has a wide distribution inhabiting both drainages of the Atlantic (Río Conchos) and Pacific (rivers Mayo, Yaqui and Fuerte) (Miller et al., 2005).
Meek (1902) described a new species of sucker (Catostomus conchos) from the Río Conchos at Jiménez, Chihuahua; however, Miller et al. (2005) synonimized it with Yaqui sucker (Catostomus bernardini) based on the similarity in the number of lateral line scales, dorsal rays, body morphology and other details of the head skeleton, but pointed out the necessity of molecular genetic analysis to confirm this decision.
Hendrickson (1983) mentioned the possible occurrence of Yaqui sucker south of the Río Mayo basin, situation that was later confirmed by Hendrickson and Varela-Romero (2002) for the Río Fuerte basin. However, Catostomus has never been documented in the Río Culiacán basin.
Herein, we compare morphometric and meristic characteristics of populations of an undescribed species of Catostomus from Río Culiacán basin (Río Tamazula-Río Humaya sub-basins) with those populations of Yaqui sucker from the Río Fuerte, Río Conchos and Río Yaqui basins, in order to determine the magnitude and signification of the differences.
Materials and methods
Specimens of suckers (Catostomus bernardini and Catostomus sp.) for the comparative morphological analysis were collected in 4 basins of the Sierra Madre Occidental (rivers Culiacán, Fuerte, Yaqui and Conchos) in the states of Sinaloa, Sonora, Chihuahua and Durango, during different periods between April 2001 and February 2012 (Fig. 1). The sampling sites are situated in elevations ranging from 432 m (Arroyo Surutato at Tepehuanes, Sinaloa) to 2,356 m (Río Verde ca. Puerto Blanco, Chihuahua). Specimens were captured with AC Smith-Root 15-B POW electrofishing equipment and cast nets. In the field, recently captured specimens were preserved in 95% ethanol and fin tissue samples were obtained for future genetic analysis. All the preserved specimens were finally deposited in the fish collection of the Universidad Autónoma de Baja California (Appendix 1).
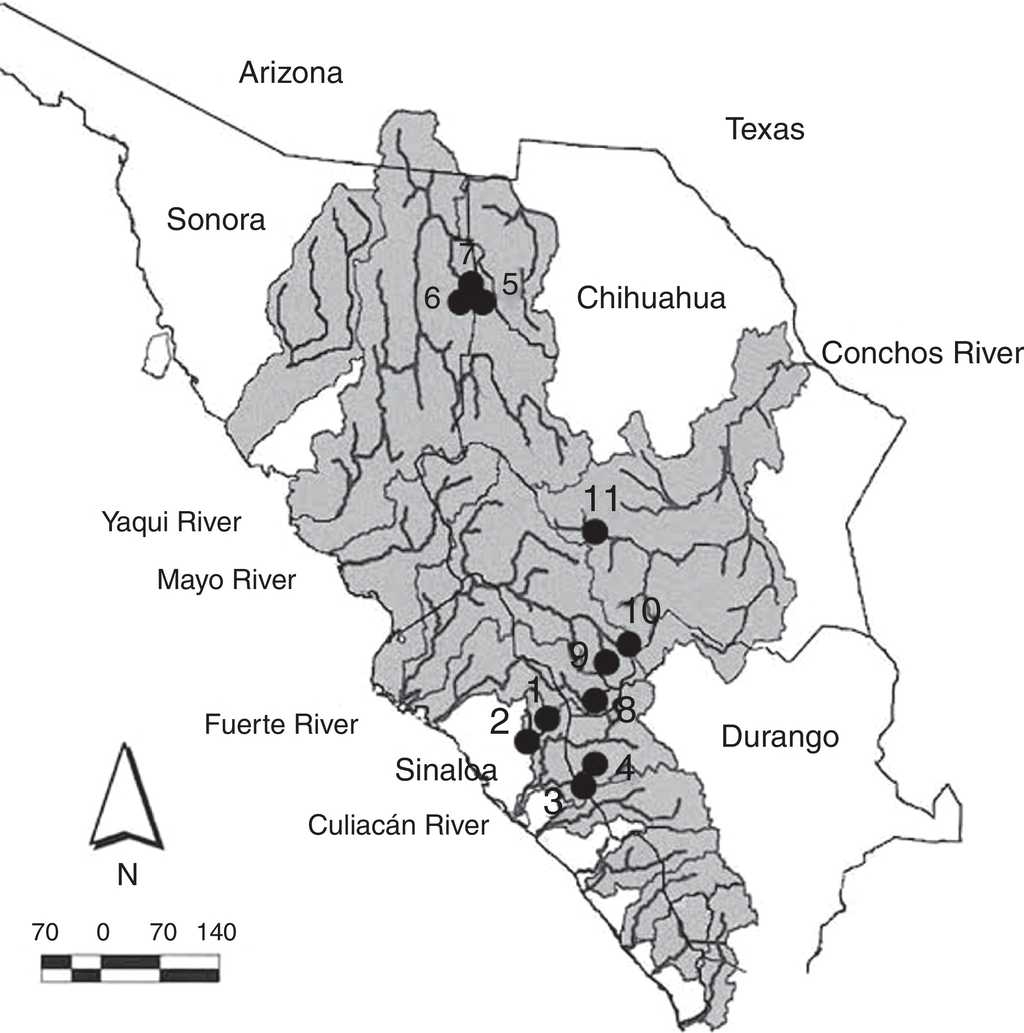
Figure 1 Collection sites for Catostomus sp. and C. bernardini in the Sierra Madre Occidental, Mexico. Numbers correpond to localities detailed in Appendix 1.
Forty-four morphological characters (37 morphometric and 7 meristic characteristics) were examined in 96 adult specimens (Río Culiacán, n = 54; Río Fuerte, n = 18; Río Yaqui, n = 12; and Río Conchos, n = 12). Morphometric characters were based on box truss protocol (Bookstein et al., 1985) and Hubbs and Lagler (1958), while the meristics was based on Hubbs and Lagler (op cit.). The morphometric variables are shown in Figure 2 and described in Appendix 2. All linear measures were taken in millimeters (mm) on the left side of each specimen using a digital caliper (precision, 0.01 mm) connected to a PC. An exploratory analysis of the original body measures was performed in order to detect aberrant or inconsistent data. This analysis consisted in a correlation between the standard length and each morphometric character of the examined specimens, presented by means of a scatterplot.

Figure 2 Landmarks for box truss protocol in populations of Catostomus sp. and C. bernardini from the Sierra Madre Occidental, Mexico.
The model for the standardization of the morphometric data of the examined specimens was the regression of Elliott, Haskard, and Koslow (1995), which removes the size component from the shape measurements (allometry) and homogenizes their variances (Jolicoeur, 1963). This model is defined by the following equation: Ms = Mo (Ls/Lo)b, where Ms = standardized measurement, Mo = measured character length (mm), Ls = overall (arithmetic) mean standard length (mm) for all individuals from all populations of each taxon, Lo = standard length (mm) of specimen, and “b” was estimated for each character from the observed data using the non-linear equation, M = a Lb. Parameter “b” was estimated as the slope of the regression of log Mo on log Lo, using every fish in every population or basin.
Both standardized measurements and meristic data of the studied sucker populations were analyzed among drainages and within the Río Culiacán drainage, and compared by means of a “forward stepwise” discriminate function analysis (DFA) using Statistica 6.0 (StatSoft, Inc., Tulsa, OK, 2002). This multivariate analysis allowed us to determine which combinations of variables best discriminated among populations and detected which populations were the most different.
The statistical significance of the discrimination among populations was determined using Wilk's lambda (λ), which oscillates from 0.0 (perfect discrimination power) to 1.0 (absence of discrimination power). The standardized coefficients of the canonical variables were determined for estimating the contribution of each variable in each canonical function; thus, the value of each standardized coefficient indicates the power of separation or discrimination of the variable into the analysis (Pires-Da Silva, Imhoff, Giarola, & Tormena, 2001).
Finally, we built tree diagrams based in the squared Mahalanobis’ distances of the morphological characters examined in order to illustrate the separation and relationships among the compared populations.
Results
Specimens examined of Catostomus sp. (n = 54) for morphometry and meristics were from the 2 sub-basins of the Río Culiacán (Humaya and Tamazula), while those of Catostomus bernardini (n = 42) were from 3 basins (Fuerte, Yaqui and Conchos). Thirty-two of the 44 meristic and morphometric variables examined entered in the stepwise forward function discriminant analysis. The global Wilks lambda (λ) was 0.00145 (p < 0000), indicating a high degree of discrimination among the populations of the compared basins. The 5 most significant variables (p < 0.001) included lateral line scales (λ = 0.00428), length of dorsal fin (λ = 0.00218), pectoral fin rays (λ = 0.00208), length of pectoral fin (λ = 0.00206) and the distance between the point of snout to occiput (M1-2, λ = 0.00194; Table 1).
Table 1 Values of lambda of Wilks and its significance (p) and tolerance for 36 meristic and morphometric variables selected by forward stepwise discriminant function analysis (DFA) for populations of Catostomus sp. (n=54) and C. bernardini (n=42) from northwestern Mexico. DFA summary; steps: 32; number of variables in model: 32; grouping: basins. Wilk's lambda: 0.00145, approx. F(96,183)=15.063, p<0.0000.
| Variable | Wilks’ lambda | Partial lambda | F-remove (3,61) | p-Level | Tolerance |
|---|---|---|---|---|---|
| Lateral line scales | 0.004275 | 0.338819 | 39.67905 | 0.000000 | 0.397361 |
| Gill-rakers | 0.001663 | 0.870822 | 3.01626 | 0.036645 | 0.350035 |
| M 7-10 | 0.001608 | 0.900605 | 2.24409 | 0.092158 | 0.311610 |
| Pectoral fin length | 0.002060 | 0.703021 | 8.58946 | 0.000077 | 0.289642 |
| Dorsal fin length | 0.002176 | 0.665555 | 10.21763 | 0.000015 | 0.318512 |
| Pectoral fin rays | 0.002076 | 0.697712 | 8.80956 | 0.000061 | 0.433354 |
| M 2-3 | 0.001752 | 0.826504 | 4.26829 | 0.008419 | 0.343242 |
| Pelvic fin length | 0.001527 | 0.948283 | 1.10893 | 0.352475 | 0.241703 |
| Dorsal fin rays | 0.001670 | 0.867263 | 3.11207 | 0.032701 | 0.479891 |
| Anal fin length | 0.001752 | 0.826702 | 4.26239 | 0.008477 | 0.229683 |
| M 9-10 | 0.001808 | 0.800863 | 5.05596 | 0.003416 | 0.064359 |
| M 1-2 | 0.001940 | 0.746541 | 6.90341 | 0.000446 | 0.091178 |
| M 1-3 | 0.001693 | 0.855513 | 3.43407 | 0.022334 | 0.222907 |
| Head length | 0.001644 | 0.881032 | 2.74565 | 0.050590 | 0.171346 |
| M 5-7 | 0.001579 | 0.917127 | 1.83735 | 0.149829 | 0.090134 |
| M 8-10 | 0.001599 | 0.905696 | 2.11718 | 0.107269 | 0.394294 |
| M 10-11 | 0.001839 | 0.787529 | 5.48581 | 0.002105 | 0.059478 |
| M 9-11 | 0.001752 | 0.826667 | 4.26342 | 0.008467 | 0.034822 |
| M 1-5 | 0.001862 | 0.777653 | 5.81373 | 0.001461 | 0.058659 |
| M 2-5 | 0.001739 | 0.832968 | 4.07737 | 0.010507 | 0.065890 |
| M 7-8 | 0.001705 | 0.849342 | 3.60676 | 0.018222 | 0.144576 |
| M 6-7 | 0.001692 | 0.856122 | 3.41717 | 0.022785 | 0.110351 |
| Interorbital distance | 0.001726 | 0.839018 | 3.90135 | 0.012901 | 0.326642 |
| M 2-4 | 0.001542 | 0.939221 | 1.31581 | 0.277446 | 0.302326 |
| M 1-4 | 0.001740 | 0.832176 | 4.10061 | 0.010227 | 0.186101 |
| M 4-7 | 0.001771 | 0.817898 | 4.52714 | 0.006246 | 0.082840 |
| M 6-8 | 0.001646 | 0.880027 | 2.77202 | 0.049023 | 0.374610 |
| M 4-6 | 0.001621 | 0.893592 | 2.42128 | 0.074551 | 0.161411 |
| M 5-8 | 0.001614 | 0.897263 | 2.32818 | 0.083336 | 0.080017 |
| Least depth | 0.001532 | 0.945359 | 1.17524 | 0.326597 | 0.337661 |
| M 5-6 | 0.001532 | 0.945162 | 1.17975 | 0.324904 | 0.124645 |
| Body depth | 0.001528 | 0.947628 | 1.12376 | 0.346529 | 0.239410 |
For the standardized coefficients for canonical variables, the canonic roots 1 and 2 explained 54.9% and 27.6% of the observed total variation, respectively (Table 2). Combined the canonical roots, accounted 82.6% of the total variation in the compared populations. In canonical root 1, 4 variables exerted the major effects: M9-11 (anterior insertion of anal fin to mid caudal base, Y = 1.23094), number of scales in lateral line (Y = −1.13661), M10-11 (posterior insertion of anal fin to mid caudal base, Y = −1.20138), and M9-10 (basal length of anal fin, Y = −0.96193).
Table 2 Standardized coefficients for canonical variables resulting from the forward stepwise discriminant function analysis for meristic and standardized morphometric data of populations of Catostomus sp. (n=54) and C. bernardini (n=42) from northwestern Mexico.
| Variable | Root 1 | Root 2 | Root 3 |
|---|---|---|---|
| Lateral line scales | −1.13661 | 0.31966 | 0.66930 |
| Gill-rakers | 0.03880 | 0.61799 | 0.19899 |
| M 7-10 | 0.24971 | 0.06303 | 0.56035 |
| Pectoral fin length | −0.37436 | 0.92310 | −0.42286 |
| Dorsal fin length | −0.01055 | −0.97613 | 0.51069 |
| Pectoral fin rays | −0.28200 | 0.51461 | −0.69071 |
| M 2-3 | −0.60280 | 0.42696 | −0.08483 |
| Pelvic fin length | 0.32650 | −0.35616 | −0.06067 |
| Dorsal fin rays | −0.10592 | 0.36754 | −0.42441 |
| Anal fin length | 0.52964 | 0.22215 | 0.74039 |
| M 9-10 | −0.96193 | 1.24873 | −1.02512 |
| M 1-2 | 0.62937 | 1.26144 | −1.11208 |
| M 1-3 | 0.23344 | −0.24049 | 0.81679 |
| Head length | −0.74827 | −0.11900 | −0.44007 |
| M 5-7 | −0.20824 | −1.00010 | −0.03597 |
| M 8-10 | −0.00671 | 0.11488 | −0.52705 |
| M 10-11 | −1.20138 | 1.51895 | −0.49221 |
| M 9-11 | 1.23094 | −1.76918 | 0.99719 |
| M 1-5 | 0.01352 | −2.04992 | 0.35320 |
| M 2-5 | 0.20864 | 1.62455 | −0.46612 |
| M 7-8 | 0.76544 | 0.23212 | −0.73890 |
| M 6-7 | −0.17612 | −1.20059 | 0.10897 |
| Interorbital distance | 0.03821 | −0.73045 | −0.16821 |
| M 2-4 | 0.26648 | 0.39172 | −0.00005 |
| M 1-4 | −0.43378 | 0.83054 | 0.38358 |
| M 4-7 | −0.74126 | 1.16220 | 0.78068 |
| M 6-8 | −0.05635 | −0.54399 | −0.26509 |
| M 4-6 | 0.34770 | −0.62538 | −0.49780 |
| M 5-8 | −0.23085 | 0.89399 | 0.80640 |
| Least depth | 0.34547 | −0.23548 | −0.04443 |
| M 5-6 | −0.19651 | −0.03600 | −0.70117 |
| Body depth | −0.31217 | 0.21872 | 0.32345 |
| Eigenval | 14.27227 | 7.17532 | 4.53000 |
| Cum. prop. | 0.54941 | 0.82562 | 1.00000 |
At the population level, the highest squared Mahalanobis’ distance (117.52) was registered between those of the Río Culiacán and Río Conchos basins, while the lowest distance (40.93) was between the Río Culiacán and Río Fuerte basins (Table 3). The tree diagram resulting from the squared Mahalanobis’ distances (Fig. 3), which indicates the degree of discrimination among populations, revealed a shorter distance between the populations of Catostomus from Río Culiacán and Río Fuerte basins, but combined they had greater distances with those of C. bernardini populations from the Río Yaqui and Río Conchos basins (Fig. 3).
Table 3 Squared Mahalanobis distances for meristic and morphometric characters among populations of Catostomus sp. (Culiacán basin, n=54) and Catostomus bernardini (Fuerte, Yaqui, and Conchos basins, n=42).
| Basin | Río Culiacán | Río Fuerte | Río Yaqui | Río Conchos |
|---|---|---|---|---|
| Río Culiacán | 0.00000 | 40.9369 | 90.3880 | 117.5248 |
| Río Fuerte | 40.9369 | 0.0000 | 72.5887 | 110.6719 |
| Río Yaqui | 90.3880 | 72.5887 | 0.00000 | 106.4698 |
| Río Conchos | 117.5248 | 110.6719 | 106.4698 | 0.00000 |
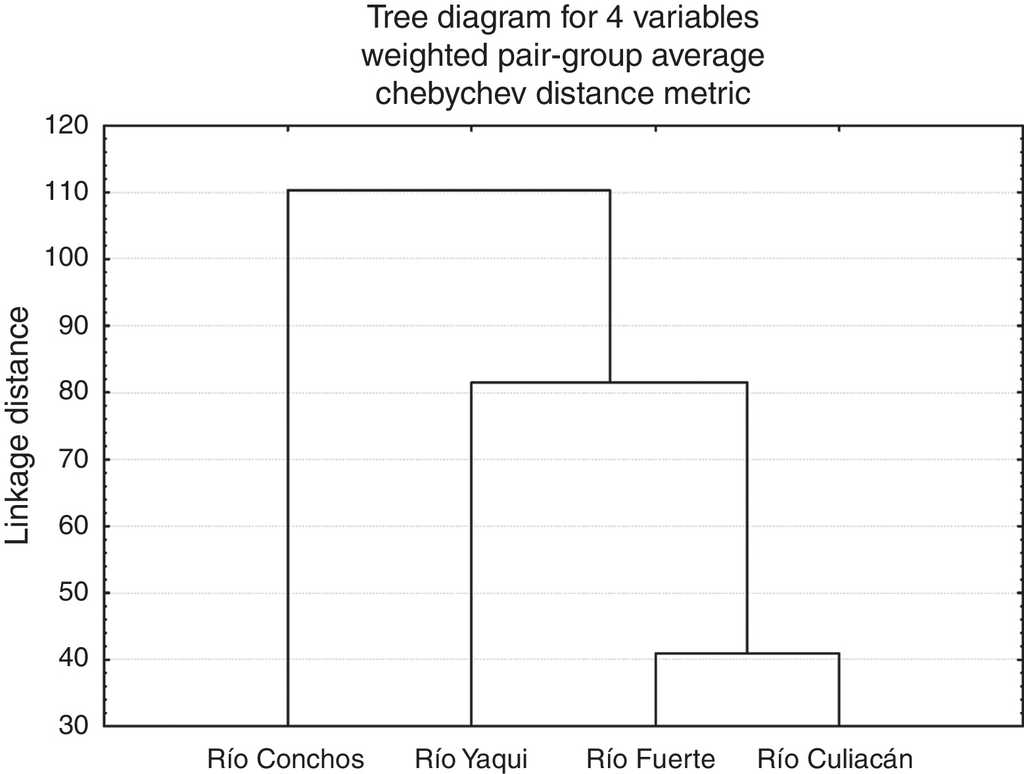
Figure 3 Tree diagram resulting from the squared Mahalanobis’ distances for populations of suckers (Catostomus sp. and C. bernardini) from 4 basins in the Sierra Madre Occidental, Mexico.
The percentage of correct classification of individuals in the examined populations by means of the discriminant function analysis was 100%, indicating that all individuals across the different drainages were correctly classified into their respective populations (see scatterplots in Fig. 4). In the scatterplot graph for roots 1 and 2 (Fig. 4A), the Río Culiacán and Río Fuerte populations appear as juxtaposed groups, while the Río Yaqui and Río Conchos populations are widely separated from each other. Likewise, in the scatterplot for the roots 1 and 3 (Fig. 4B), the Río Conchos and Río Yaqui populations are in juxtaposition, while those of the Culiacán and Río Fuerte are widely separated from each other.
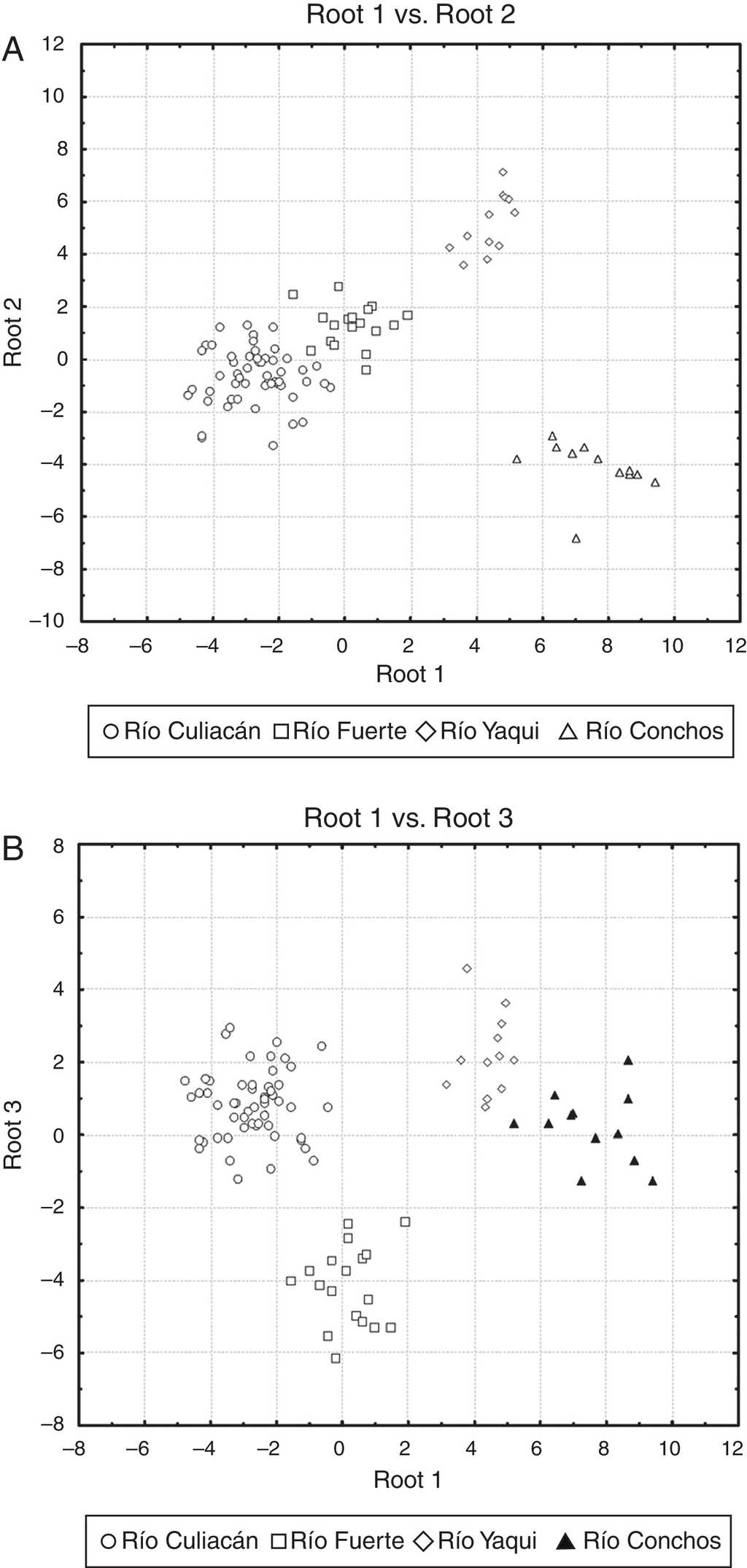
Figure 4 Scatter plots of centroids of populations for suckers (Catostomus sp. and C. bernardini) in northwestern Mexico. (A) Root 1 vs. root 2, and (B) root 1 vs. root 3 (see Table 2 for canonical coefficients).
The morphological characters separating the populations of Río Culiacán basins from those of Río Fuerte, Río Yaqui and Río Conchos basins were associated with the highest values for number of gill rakers (>34, Fig. 5I), posterior insertion of dorsal fin to posterior insertion of pelvic fin (M7-8, Fig. 5E), posterior insertion of dorsal fin to posterior insertion of anal fin (M7-10, Fig. 5F), and basal length of anal fin (M9-10, Fig. 5G). Likewise, separation of populations from the Río Culiacán basin were associated with the lowest values for predorsal distance (M1-5, Fig. 5B), soft posterior ocular margin to occiput (M2-3, Fig. 5C), and anal rays (Fig. 5H).
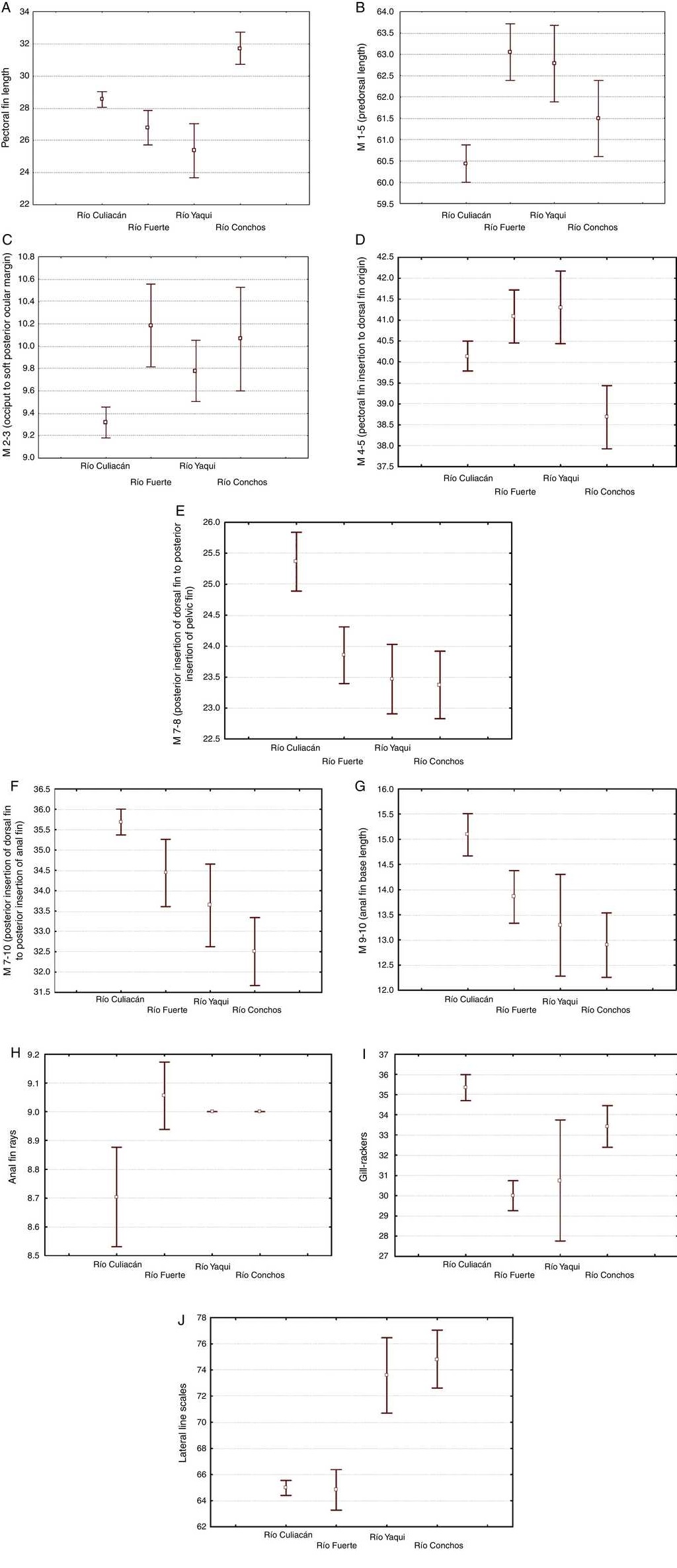
Figure 5 Box plots (average and 0.95 confidence interval) of the 10 most remarkable meristic and standardized morphometric characters for separating the populations of suckers (Catostomus sp. and C. bernardini) from 4 basins in the Sierra Madre Occidental, Mexico.
The number of scales along the lateral line for populations from either the Río Culiacán or Río Fuerte was very similar, having less than 67 scales (Fig. 5J); however, this character distinguishes populations from these 2 drainages from those of the Río Conchos and Río Yaqui basins (>70 scales, Fig. 5J). Furthermore, the length of the pectoral fin in specimens from the Río Culiacán basin is greater than that of specimens from the Río Fuerte and Río Yaqui basins, although to a lesser extent in comparison with specimens from the Río Conchos (Fig. 5A). Additionally, the average number and standard deviation of pharyngeal teeth is notably higher in specimens of Río Conchos (41 ± 5.9) and Río Culiacán (39 ± 11.2) in comparison with those of Río Fuerte (30 ± 1.34) and Río Yaqui (26 ± 2.60).
The discriminant function analysis (forward stepwise) for populations grouped by subbasins also had a significant Wilk's lambda value (λ = 0.0000024, p < 0.0000). In this analysis 34 variables were considered in the model, of which 22 variables resulted to be significant (p < 0.05). The 5 most significant variables (p < 0.001) were scales of lateral line (λ = 0.000007), number of gill-rakers (λ = 0.000005), dorsal fin length (λ = 0.000005), pectoral fin rays (λ = 0.000004) and pectoral fin length (λ = 0.000004).
In the predicted classification of individuals for the populations of Catostomus of the different subbasins compared, all the individuals were correctly assignated into their corresponding populations. Canonical root 1 explained 48.07% of the total variation, while the roots 2, 3 and 4 explained, in an accumulative manner 68.64%, 79.91%, and 88.16%, respectively. The tree diagram resulting from squared Mahalanobis’ distance displayed 2 main groups, one formed by the populations [subbasins] of the Río Culiacán, and other formed by the populations [subbasins] from the rivers Conchos, Yaqui and Fuerte, including one population from upper Río Culiacán (Arroyo La Mesa) (Fig. 6).
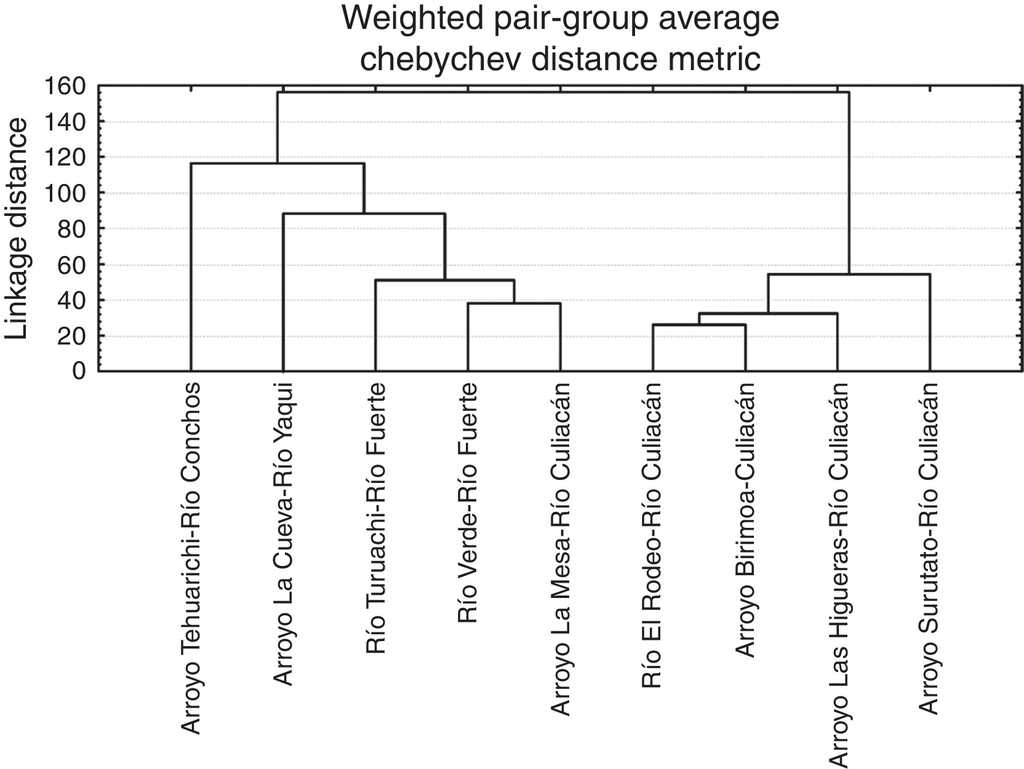
Figure 6 Tree diagram resulting from the squared Mahalanobis’ distances for populations of suckers (Catostomus sp.) from 4 basins in the Sierra Madre Occidental, Mexico.
The breeding coloration of suckers from Arroyo El Rodeo in December (Fig. 7A), a tributary to Río Tamazula (Río Culiacán basin sucker) is dark brown on the dorsum and sides, contrasting with the shiny white in the ventral region. Coloration of fins, except for caudal, is orange-yellow. Lips and cheeks are also orange-yellow. A whitish band borders the anal, while the dorsal fin is tipped with black; the caudal fin is dark brown. Specimens of suckers from Arroyo Las Higueras (a tributary to Río Humaya, Río Culiacán basin) showed a dark brown dorsum contrasting with the shiny white coloration of sides and belly speckled with orange spots (Fig. 7C). In the case of the Río Conchos sucker, the live body coloration of ripe adults is greenish gray on dorsum and sides with a shiny white venter (Fig. 7E). Specimens from Arroyo La Presita (Río Yaqui basin) showed a yellowish brown coloration on dorsum and sides with irregular dark gray blotches (Fig. 7G), as well as an orange-yellow anal fin, and the venter and areas around the insertion of pectoral and pelvic fins has a pearl white tonality.

Figure 7 Life coloration of suckers (Catostomus sp. and Catostomus bernardini) from the Sierra Madre Occidental, México. (A) Catostomus sp., Arroyo El Rodeo (Río Tamazula subbasin), (B) Catostomus sp., Río Birimoa (Río Tamazula subbasin), (C) Catostomus sp., Arroyo Las Higueras (Río Humaya subbasin), (D) Catostomus sp., Arroyo Surutato (Río Humaya subbasin), (E) Catostomus bernardini, Río Conchos basin, (F) Catostomus bernardini, Arroyo Verde (Río Fuerte basin), and (G) Catostomus bernardini, Arroyo La Presita (Río Yaqui basin). Photographs by Sergio Sánchez-Gonzáles (A-D), Gorgonio Ruiz-Campos (E and F), and Alejandro Varela-Romero (G). Bar length=1cm.
Discussion
The complex of Catostomus inhabiting the different basins draining the Sierra Madre Occidental (SMO) has possibly resulted from an evolutionary radiation within the Catostomus lineage that began from the Miocene, and that included episodic orographic events, periods of progressive aridity, and most recently the Pleistocene pluvial cycles (Schonhuth, Doadrio, & Mayden, 2006; Schonhuth et al., 2011; Schonhuth, Perdices, et al., 2014; Smith et al., 2002). The long-term isolation of major basins has resulted in the differentiation of the forms of Catostomus inhabiting the SMO. Other complexes of populations or species that have experimented adaptative radiations into the SMO are those described for the genera Gila (Schonhuth, Perdices, et al., 2014), Codoma (Schonhuth, Lozano-Vilano, et al., 2014), and Ictalurus (Varela-Romero et al., 2011).
The population of Yaqui sucker from the Río Conchos basin was originally described as Catostomus conchos (Meek, 1902) and later synonimized with C. bernardini by Miller et al. (2005) due to the similarities of some morphological characters (number of scales in lateral line, dorsal fin rays, body morphology and details of the head skeleton). Our analysis showed that at least 3 characters distinguish C. conchos from other C. bernardini populations in the Sierra Madre Occidental; these characters include a greater length of pectoral fin, shorter distance between the insertion of pectoral fin to dorsal fin origin, and higher number of pharyngeal teeth (>34). The average number of pharyngeal teeth was higher in specimens from Río Conchos (41), in comparison with those from Río Culiacán (39), Río Fuerte (31) and Río Yaqui (26).
The populations of Catostomus sp. from Río Humaya (Sinaloa) and Río Tamazula (Durango) sub-basins reported herein, were all recorded at altitudes ranging from 432 m (Arroyo Surutato at Tepehuanes, Sinaloa) to 480 m (Arroyo Birimoa at Tamazula, Durango), representing in both cases the lowest elevation ranges for populations within the Yaqui sucker complex.
Finally, a molecular genetic analysis of the metapopulations of Catostomus “bernardini” through the Sierra Madre Occidental, including the undescribed sucker of the Río Culiacán, may clarify the taxonomical and evolutionary relationships of the populations studied here, as well as their degree of biological differentiation.











 nueva página del texto (beta)
nueva página del texto (beta)


