Notas científicas
First record of Batrachochytrium dendrobatidis infecting threatened populations of Tandilean Red-belly toad (Melanophryniscus aff. montevidensis) in Argentina
Primer registro de Batrachochytrium dendrobatidis infectando poblaciones amenazadas del sapito de panza roja de Tandil (Melanophryniscus aff. montevidensis) en Argentina
María Gabriela Agostinia
⁎
Agustina Cortelezzib
Igor Berkunskyb
Gabriela Solerc
Patricia Burrowesd
aGrupo de Estudios sobre Biodiversidad en Agroecosistemas, Instituto de Ecología, Genética y Evolución de Buenos Aires, Universidad de Buenos Aires, Consejo Nacional de Investigaciones Científicas y Técnicas, Pabellón II Ciudad Universitaria C1428EHA CABA, Argentina
bInstituto Multidisciplinario sobre Ecosistemas y Desarrollo Sustentable, Universidad Nacional del Centro, Campus Universitario, Paraje Arroyo Seco s/n, (B7000GHG), Tandil, Argentina
cInstituto Superior de Formación Docente 10 “Osvaldo Zarini”, Belgrano 1619 (B7000KBB), Tandil, Argentina
dDepartment of Biology, University of Puerto Rico, P.O Box 70377, San Juan, Puerto Rico
ABSTRACT
We present the first record of Batrachochytrium dendrobatidis (Bd ) infecting endangered populations of the Tandilean Red-belly toad (Melanophryniscus aff. montevidensis ). We obtained skin swab samples of 32 individuals. The prevalence was 35.5% and the infection levels varied between 0.34 and 915 Bd -genomic equivalents. This finding represents a new threat that could be affecting, in conjunction with the high habitat fragmentation, the viability of the studied populations.
Keywords Chytridiomycosis; Amphibians; Population decline; Highland grassland
RESUMEN
Presentamos el primer registro de Batrachochytrium dendrobatidis infectando poblaciones amenazadas del sapito de panza roja de Tandil (Melanophryniscus aff. montevidensis ). Obtuvimos muestras de hisopados de piel de 32 individuos cuyos resultados indicaron una prevalencia del 35.5% y niveles de infección que variaron entre 0.34-915 equivalentes genómicos de Batrachochytrium dendrobatidis . Este hallazgo representa una nueva amenaza que, conjuntamente con la alta fragmentación del hábitat, podría estar afectando la viabilidad de las poblaciones estudiadas.
Palabras clave Quitridiomicosis; Anfibios; Declinación poblacional; Pastizales de altura
Chytridiomycosis is an emerging infectious disease caused by the chytrid fungus Batrachochytrium dendrobatidis (Bd ), which has contributed to amphibian population declines and extinctions worldwide (Blaustein et al., 2011). In Argentina, up to 16 species from different biogeographic regions (e.g., Paranaense, Patagonica, Pampeana, del Monte, Chaqueña, Espinal provinces) have been positive for Bd (Ghirardi, Perotti, Steciow, Arellano, & Natale, 2011; Lescano, Longo, & Robledo, 2013). Few studies have been conducted on threatened species; Telmatobius pisanoi , T. atacamensis (Barrionuevo & Mangione, 2006), and Atelognathus patagonicus (Fox, Greer, Torres-Cervantes, & Collins, 2006) were positive for Bd , but none of these studies conclude that chytridiomycosis is responsible for the population declines.
The Red-belly toad belongs to the Melanophryniscus stelzneri group. The taxonomy of the species in this group has not yet been adequately resolved (Kwet, Maneyro, Zillikens, & Mebs, 2005). Consequently, we refer to the population that inhabits Tandilean and Ventania mountains ridges as Melanophryniscus aff. montevidensis (Vaira et al., 2012). However, it is important to note that Ventania and Tandilia populations are isolated and they are probably different lineages.
The Tandilean Red-belly toad is an endemic species restricted to the remnants of highland grasslands in the Tandilean Mountains (Soler, Cortelezzi, Berkunsky, Kacoliris, & Bettina, 2014). These mountains belong to the Pampa eco-region, considered a maximum priority for conservation due to its great alteration, biological uniqueness, and the absence of protected areas (Bilenca & Miñarro, 2004). The Pampa eco-region has been predicted to have the highest suitability for Bd (Ghirardi et al., 2011). However, the presence of Bd has not yet been studied in these particular habitats of highland grassland.
The threatened Melanophryniscus populations are a high conservation priority (Vaira et al., 2012; Zank, Becker, Abadie, Maneyro, & Borges-Martins, 2014). In the next decades the populations of Tandilean Red-belly toad are predicted to lose climatic suitability in more than 60% of their present range (Zank et al., 2014). The fragmentation and modification of natural grasslands is causing mortality, affecting the dispersal of toads and the connection between reproductive ponds (Cairo & Zalba, 2007). Several studies demonstrated that abiotic factors and habitat loss can affect Bd -amphibian dynamics (Longo, Burrowes, & Joglar, 2010; Piotrowski, Annis, & Longcore, 2004; Rohr, Raffela, Romansic, McCallumb, & Hudson, 2008). Under scenarios of climate change and habitat fragmentation, Bd infections would represent an additional serious threat to the Tandilean Red-belly toad populations. Therefore, detection and quantification of Bd in remnants of highland grasslands will contribute to establish conservation strategies.
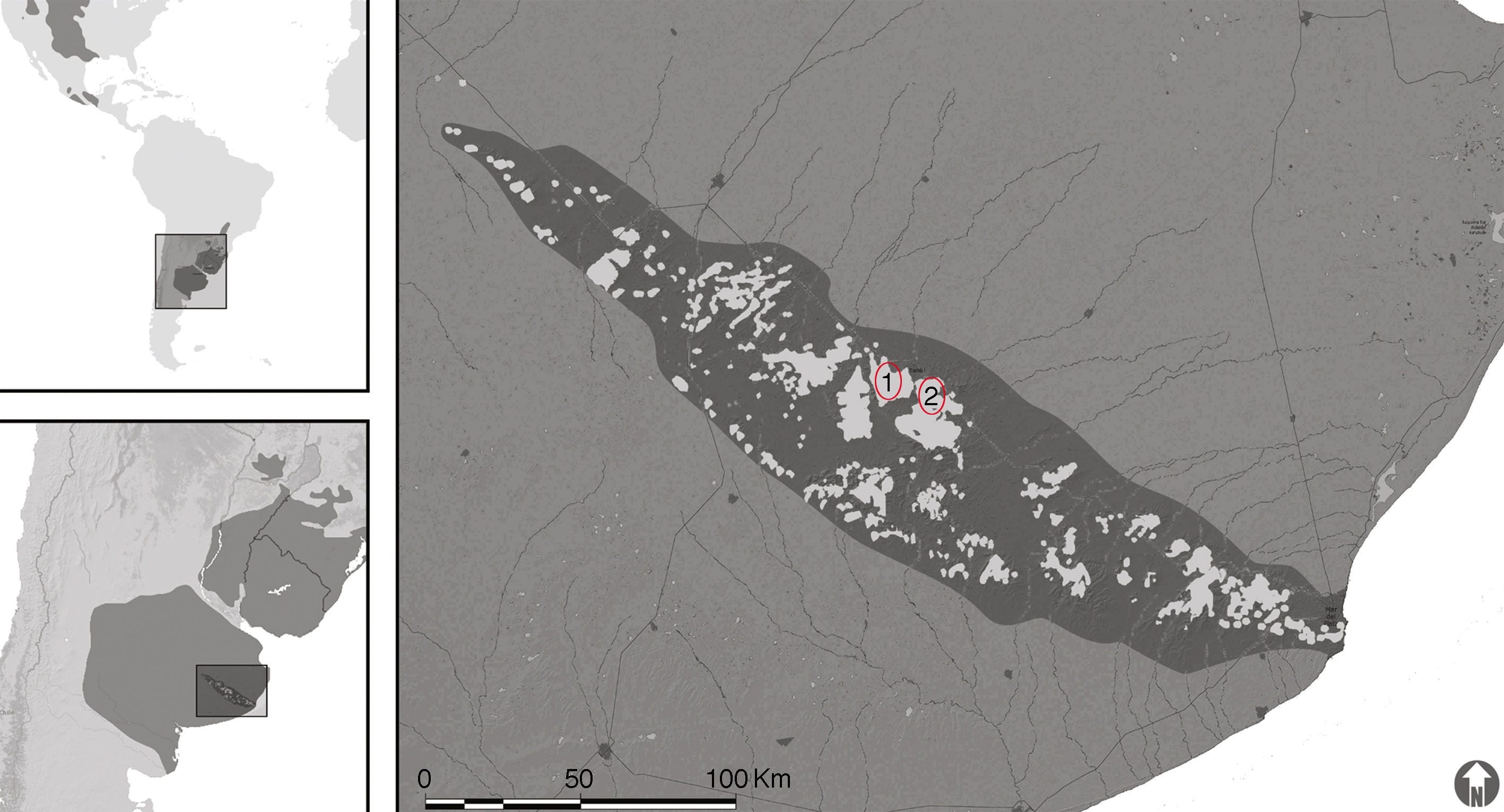
We surveyed temporary ponds in highland grasslands of the Tandilean Mountains (Fig. 1). We captured 32 adult toads in 2 different sites (Sierra del Tigre and Las Ánimas). Toads were captured by using visual-encounter surveys during 2 reproductive seasons (2012 and 2013). We identified each individual by photographs to prevent taking multiple samples from the same individual. We collected tissue from toads by swabbing (10 strokes) the ventral pelvic-patch region, thighs, and ventral surface (palmar) of hands and feet. DNA extraction from swab samples was done using 50 μl of PrepMan Ultra (Hyatt et al., 2007). Detection and quantification of Bd was done with the Taqman PCR method in an Applied Biosystems 7500 Real-Time PCR system according to Boyle, Boyle, Olsen, Morgan, and Hyatt (2004). Infection intensity was calculated as the number of Bd zoospore genomic equivalents in each swab sample.
Overall, 11 of 32 (35.5%) toads were Bd -positive. The infection was detected in both study sites. Infected individuals showed Bd -zoospore genomic equivalents ranging from 0.34 to 915 (mean = 103.2; SE = 253.5). All individuals examined were apparently healthy, and no sick, dying, or dead individuals were found.
We report the first record of chytridiomycosis on M. aff. montevidensis in the highland grasslands of the Tandilean Mountains, where at least 5 other species of amphibians occur. While this is the only threatened species, Bd could be affecting other species.
The decline of Red-belly toad is well documented. Populations from Ventania Mountains have been predicted to become extinct in 100 years (Cairo, 2010), while populations near Tandil City reported a decline and a complete extinction in some areas where this toad used to be abundant (Cortelezzi, com. pers.). Since these populations appear to be experiencing mostly sub-lethal levels of infection, we cannot assume a direct link between the population decline and Bd disease. But other studies have shown that amphibians persisting with Bd have significantly lower survival probabilities, and can die from chytridiomycosis (Longo & Burrowes, 2010; Longo, Ossiboff, Zamudio, & Burrowes, 2013).
Although this study presents Bd infection as another plausible cause for Red-belly toad population decline, the individual contribution of the disease in a multiple-causal scenario remains a challenge in need of investigation. The variation in distribution and prevalence of Bd across the fragmented landscape should be assessed taking into account an amphibian community perspective, in order to define the status and trend of chytridiomycosis in this relict of highland grasslands.
We thank M. Tejerina and C. Barletta for helping in fieldwork. We also thank to the "Scouts de la Ciencia" program for their assistance during the fieldwork.
References
Barrionuevo and Mangione, 2006 S. Barrionuevo, S. Mangione. Chytridiomycosis in two species of Telmatobius (Anura: Leptodactylidae) from Argentina. Diseases of Aquatic Organisms. 2006; 73:171p
[ Links ]
Bilenca and Miñarro, 2004 D. Bilenca, F. Miñarro. Identificación de áreas valiosas de pastizal (AVP) en las pampas y campos de Argentina. Uruguay y sur de Brasil. Buenos Aires: Fundación Vida Silvestre Argentina; 2004.
[ Links ]
Blaustein et al., 2011 A.R. Blaustein, B. Han, R.A. Relyea, P.T.J. Johnson, J.C. Buck, S.S. Gervasi. The complexity of amphibian population declines: understanding the role of cofactors in driving amphibian losses. Annals of the New York Academy of Sciences. 2011; 1223:108p
[ Links ]
Boyle et al., 2004 D.G. Boyle, D.B. Boyle, V. Olsen, J.A.T. Morgan, A.D. Hyatt. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Disease Aquatic Organism. 2004; 60:141p
[ Links ]
Cairo, 2010 S.L. Cairo. Historia de vida, demografía y conservación de las poblaciones más australes del género Melanophryniscus (Anura: Bufonidae). Bahía Blanca, Buenos Aires, Argentina: Universidad Nacional del Sur; 2010.
[ Links ]
Cairo and Zalba, 2007 S.L. Cairo, S.M. Zalba. Effects of a paved road on mortality and mobility of Red Bellied toads (Melanophryniscus sp.) in Argentinean grasslands. Amphibia-Reptilia. 2007; 28:377p
[ Links ]
Fox et al., 2006 S.F. Fox, A.L. Greer, R. Torres-Cervantes, J.P. Collins. First case of Ranavirus-associated morbidity and mortality in natural populations of the South American frog Atelognathus patagonicus. Diseases of Aquatic Organisms. 2006; 72:87p
[ Links ]
Ghirardi et al., 2011 R. Ghirardi, M.G. Perotti, M.M. Steciow, M.L. Arellano, G.S. Natale. Potential distribution of Batrachochytrium dendrobatidis in Argentina: implications in amphibian conservation. Hydrobiologia. 2011; 659:111p
[ Links ]
Hyatt et al., 2007 A.D. Hyatt, D.G. Boyle, V. Olsen, D.B. Boyle, L. Berger, D. Obendorf. Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Diseases of Aquatic Organisms. 2007; 73:175p
[ Links ]
Kwet et al., 2005 A. Kwet, R. Maneyro, A. Zillikens, D. Mebs. Advertisement calls of Melanophryniscus dorsalis (Mertens, 1933) and M. montevidensis (Philippi, 1902), two parapatric species from southern Brazil and Uruguay, with comments on morphological variation in the Melanophryniscus stelzneri group (Anura: Bufonidae). Salamandra. 2005; 41:3p
[ Links ]
Lescano et al., 2013 J.N. Lescano, S. Longo, G. Robledo. Chytridiomycosis in endemic amphibians of the mountain tops of the Córdoba and San Luis ranges, Argentina. Diseases of Aquatic Organisms. 2013; 102:249p
[ Links ]
Longo and Burrowes, 2010 A.V. Longo, P.A. Burrowes. Persistence with Chytridiomycosis does not assure survival of direct-developing frogs. Ecohealth. 2010; 7:185p
[ Links ]
Longo et al., 2010 A.V. Longo, P.A. Burrowes, R.L. Joglar. Seasonal patterns of Batrachochytrium dendrobatidis infection in direct-developing frogs suggest a mechanism for persistence in enzootic conditions. Diseases of Aquatic Organisms. 2010; 92:253p
[ Links ]
Longo et al., 2013 A.V. Longo, R.J. Ossiboff, K.R. Zamudio, P.A. Burrowes. Lability in host defenses: terrestrial frogs die from chytridiomycosis under enzootic conditions. Journal of Wildlife Diseases. 2013; 49:197p
[ Links ]
Piotrowski et al., 2004 J.S. Piotrowski, S.L. Annis, J.E. Longcore. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia. 2004; 96:9p
[ Links ]
Rohr et al., 2008 J.R. Rohr, T.R. Raffela, J.M. Romansic, H. McCallumb, P.J. Hudson. Evaluating the links between climate, disease spread, and amphibian declines. Proceedings of National Academy of Sciences USA. 2008; 105:17436p
[ Links ]
Soler et al., 2014 G. Soler, A. Cortelezzi, I. Berkunsky, F. Kacoliris, B. Bettina. Primer registro de depredación de huevos de anuros por sanguijuelas en Argentina. Cuadernos de Herpetología. 2014; 28:39p
[ Links ]
Vaira et al., 2012 M. Vaira, M. Akmentins, M. Attademo, D. Baldo, D. Barrasso, S. Barrionuevo. Categorización del estado de conservación de los anfibios de la República Argentina. Cuadernos de Herpetología. 2012; 26:151p
[ Links ]
Zank et al., 2014 C. Zank, F.G. Becker, M. Abadie, R. Maneyro, M. Borges-Martins. Climate change and the distribution of neotropical Red-Bellied Toads (Melanophryniscus, Anura, Amphibia): how to prioritize species and populations?. PLOS ONE. 2014; 9:e94625p
[ Links ]











 nueva página del texto (beta)
nueva página del texto (beta)


