Introduction
Research focusing on the dynamics of the communities of helminthic parasites has traditionally paid more attention to vertebrate definitive hosts (Esch, Bush, & Aho, 1990) and recently on larval stages of mollusks (Scholz, Aguirre-Macedo, Díaz-de León, & Ditrich, 2000). Few projects have been carried out regarding intermediate hosts. These hosts because of the predator-prey pathways, which lead to parasite transmission, have a closer ecological association with the definitive host (Wetzel & Esch, 1996). Many Digenea (Platyhelmintha: Trematoda) use freshwater crayfish as second host intermediate (Lefebvre & Poulin, 2005). Cambarellus montezumae (Decapoda: Astacidae: Cambarinae) is an endemic species of the benthos of the reservoirs in the Mexican Altiplano (Arredondo-Figueroa, Vásquez-González, Nuñez-García, Barriga-Sosa, & Ponce-Palafox, 2011; Villalobos, 1955). Abundant in Salazar Lagoon, it serves as food source for birds, fish and humans in the region (Rodríguez & Carmona, 2002). It is unknown whether this species acts as an intermediate host or definitive host and if it represents a risk to human health (Moctezuma, 1996). Different environmental factors such as the increase of tourism on the lakeshore (Vargas, 1997), overgrazing, and pollution of soil and water by solid waste have made a strong impact on native aquatic fauna. The later including C. montezumae , considered by the National Commission of Natural Protected Areas (Conanp) as a focal species of economical and biological importance in Mexico. Some studies have registered the presence of digeneans in freshwater crayfish (Edgerton, Evans, Stephen, & Overstreet, 2000). On the other hand, Sogandares-Bernal (1965) reported parasitized crayfish in Louisiana and McAllister, Robison and Font (2011) reported 3 species of crayfish parasitized by Alloglossidium corti in Arkansas and Oklahoma. Lane et al. (2009) described human paragonimiasis by ingestion of raw crayfish in North America. Mexican studies on C. montezumae include population ecology (Álvarez & Rangel, 2007; Moctezuma, 1996; Villalobos, 1955), physiology (Rodríguez & Carmona, 2002), feeding behavior and epibionts (López-Ochoterena & Ochoa-Gasca, 1971; Rioja, 1940) among others. In spite of this species being a source of human consumption in many places (Álvarez & Rangel, 2007), there are no parasitological studies.
Haematoloechus (Looss, 1899) (Digenea: Plagiorchioidea) has a worldwide distribution with at least 50 different species described, 12 of which have been reported for Mexico (León-Règagnon, 2010; León-Règagnon & Brooks, 2003). Among them, Haematoloechus pulcher (Bravo, 1943) was found in the Central Altiplano (Bravo, 1943; Mata-López, García-Prieto, & León-Règagnon, 2002; León-Règagnon & Brooks, 2003; Paredes-León, García-Prieto, Guzmán-Cornejo, León-Règagnon, & Pérez, 2008). Adult Haematoloechus spp. parasitize the lungs of lower vertebrates (frogs and caudates). Nevertheless, presence of H. pulcher metacercariae in crayfish has not been documented. It is unknown whether C. montezumae is an intermediate host of human parasites (Moctezuma, 1996). This paper shows the temporal dynamics of the metacercariae parasitosis in crayfish C. montezumae and its determining factors, as well as the risk involved in the development of this crayfish population species.
Materials and methods
Salazar Lagoon is located in the Miguel Hidalgo y Costilla National Park in the municipality of Lerma, Estado de México (19°18′13″ N, 99°23′11″ W). The major axis of the lagoon is around 1 km and the lower (north-south) about 300 m. The climate of the area is temperate sub-humid with an average temperature of 12-18 °C with summer rains, where rainfall exceeds 800 mm (Moctezuma, 1996).
Thirteen monthly samplings were carried out (from February 2008 to February 2009) with a 60 × 30 cm rectangular dredge (mesh size of 0.5 mm). Samples were collected along a 3 m wide band at a water depth of 90 cm. Temperature and oxygen saturation, as well as transparency and pH were recorded.
A total of 221 crayfish were obtained during the 13 months. Each month the crayfish were brought to the laboratory and maintained in a 20 L aquarium containing lagoon water at 20 °C, with continuous aeration. Each specimen was anesthetized with chloroform (0.7 or 1% depending of the size) and submitted to external examination, measuring and sex determination. The metacercariae number per crayfish and per anatomical unit was registered. The prevalence, intensity and abundance (Margolis, Esch, Holmes, Kuris, & Shad, 1982) were calculated each month. In order to calculate and demonstrate a difference of parasitosis between both sexes the statistical U -Mann-Whitney test (Zar, 2010) was performed. In addition, the principal component analysis (PCA) was centered and standardized (Pielou, 1984) to evaluate the involvement of the abiotic variables in the presence of metacercariae. PCA results were used to determine which of the abiotic variables have more impact on metacercariae parasitization. Prevalence and abundance of metacercarie were related with temperature and oxygen concentration rates by linear regression using Excell to highlight the months of highest infection (Zar, 2010).
In order to identify the parasites, the crayfish were dissected and each anatomical unit (antennules, antennas, maxillipeds, mandibles, maxils, periopods, pleopods, uropods, telson, gills and internal organs) was examined under stereo and compound microscopes. Once metacercaria cysts were identified and extracted from each anatomical unit, excystation was induced in water at room temperature and by occasional shaking. Photographs of the metarceriae were taken with a digital camera (Pentax-Optio 33 L) and the parasites were fixed for 2 weeks in 4% formaldehyde and then transferred to 70% ethanol. (Scholz et al., 2000; Vidal-Martínez, Aguirre-Macedo, Scholz, González-Solis, & Mendoza-Franco, 2002). Some metacercarie were flattened between 2 slide glasses and stained with Delafield's hematoxylin or alcoholic hydrochloric carmine, cleared in clove oil and mounted in synthetic resin (Vidal-Martínez et al., 2002). The metacercariae were observed under a photomicroscope Axiophot Zeiss.
For scanning electron microscopy (SEM), 10 metacercariae were washed twice with PBS and fixed with 2.5% glutaraldehyde in PBS for 1 h. Afterwards, the parasites were washed with PBS, post-fixed with 1% osmium tetroxide solution for 1 h, and dehydrated through a graded ethanol series (50-100%). Samples were dried using the critical point method, covered with gold and scanned and analyzed under a Jeol JSM-5800 LV.
To confirm the identification of Haematolechus metacercariae species, 10 specimens were fixed in 90% ethanol and stock DNA was extracted using the DNeasy Blood & Tissue Kit (Qiagen). Nineteen 28S Ribosomal RNA gene sequences from different species or isolates (Haematolechus complexus AF133104, sp. AF133114, Haematolechus illimis AF133109, Haematolechus medioplexus AF133113, Haematolechus cf complexus AF532138, H. pulcher AF531866, Haematolechus coloradensis AF133108, Haematolechus longiplexus AF133110, sp. RML California 4 GU191159, California 1 GU191156, California 3 GU191158, Haematolechus abbreviatus AF184251, Haematolechus variegatus AF151916, Haematolechus floedae AY672126, Haematolechus breviplexus AF387800, Haematolechus meridionalis AF531864, Haematolechus danbrooksi AF479652, Haematolechus parviplexus AF479653 and Haematolechus exoterorchis AF531858) were used to perform a multiple sequence alignment using the Clustal-W program (Larkin et al., 2007) to obtain a set of primers for PCR amplification. A consensus sequence was generated by the alignment of a conservative region flanking a variable region. The primers 28S-F 5′ GAGGGTGAAAGGCCCGTGGG and 28S-R 5′ACGCATGCACACACCTCRAGCCG 3′ were designed. The 28S gene fragment was amplified in a 50 μl reaction containing 100 ng of DNA template, 8 μM forward and reverse primers and Master mix 2X (ROCHE). The cycling was performed as follows: initial denaturing step 94 °C/5 min followed by 40 cycles: denaturing 94 °C/45 s, annealing 60 °C/30 s and extension 72 °C/45 s and a final extension 72 °C/2 min. The PCR product (613 bp) was analyzed in a 1% agarose gel. The product was sequenced in both strands in the Unidad de Proteogenómica, UNAM, Juriquilla, Mexico. The sequence was viewed in the Chromas program Lite 2.01 and refined by hand. The final sequence (461nt) was analyzed by Blast in the NCBI server. The sequence was registered in GenBank (accession number KM821049.1).
The sequences of 28S Ribosomal RNA gene used for primers design were refined by hand and aligned using Clustas X 1.8 (Larkin et al., 2007) and visualized in Seaview (Gouy et al., 2010). Maximal likelihood analysis was performed using PhyML (Guindon et al., 2010).
Results
The metacercariae were oval (462 ± 104 × 208 ± 42 μm) with spinosed tegument; oral sucker sub apical (93 ± 17 μm) and acetabulum post equatorial (66 ± 12 μm diameter); pharynx lightly oval (47 ± 10 × 42 ± 10 μm) and intestinal ceca ending blindly and extended to the half 1/3 the posterior end of the body, the excretory vesicle was Y shaped forming 2 collecting tubes reaching the pharynx and the distance between the oral sucker and the acetabulum was 249.19 μm (Figs. 1 and 2): it was not possible to observe other structures.
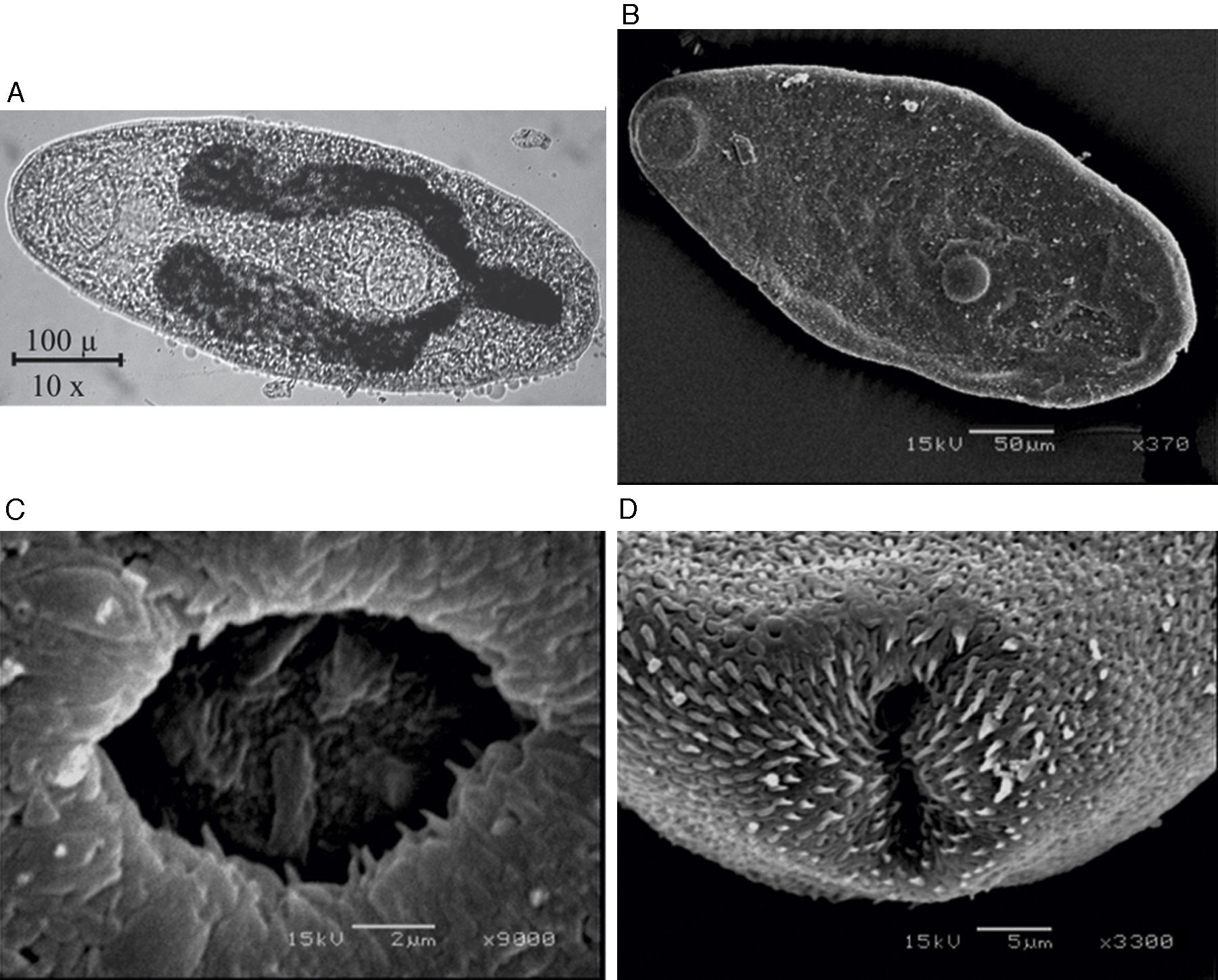
Figure 1 Metacercariae de Haematoloechus pulcher. (A) Light microscopy image of excysted metacercaria, scale bar=100μ; (B) SEM image of excysted metacercaria, scale bar=50μm; (C) SEM image of the acetabulum scale bar=2μm; (D) SEM image of the excretory pore scale bar=5μm.

Figure 2 Metacercariae de Haematoloechus pulcher showing the distance between the oral sucker and the acetabulum.
The blast analysis revealed a 99% identity (457/461) with Haematoloechus pulcher. The Fig. 3 shows the maximum likelihood phylogram tree generated. The phylogenetic relationship among the experimental and H. pulcher sequence confirm the identification. The phenogram showed 2 main branches. In the upper branch are clustered the California, experimental, H. pulcher, H. complexus H. variegatus, H. abbbreviatus , H. exoterorchis and H. longiplexus.
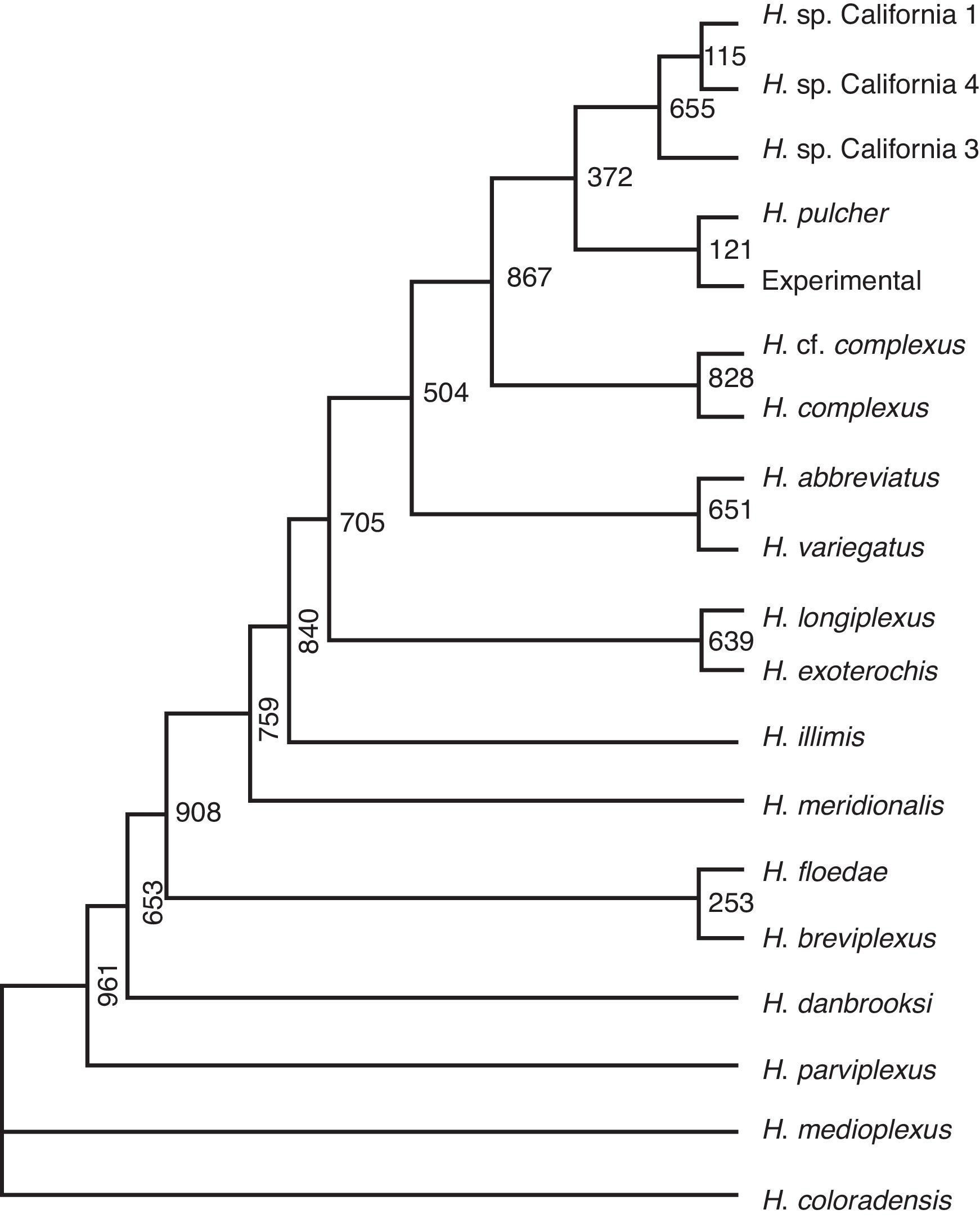
Figure 3 Maximal likelihood phylogram reconstructed from 28S rRNA fragment gene sequences of the distinct isolates.
The prevalence of the metacercariae in crayfish was 42.5% (94/221). The maximum intensity of metacercariae (26) was recorded in a male crayfish with a size of 25.7 mm, collected in September 2008. The metacercarie were located mainly in the gills, podites and telson. Other anatomical units include: front, exoskeleton and internal organs (Fig. 4). The average intensity was 6 metacercariae by crayfish in both sexes. Metacercarie abundance was higher in females (2.6%) than in males (2.4%), however, the difference was not significant (U -Mann-Whitney p = 0.93). The sizes of crayfish analyzed ranged from 11.4 to 35 mm of length. The size at which the presence of parasites was detected in females was 13.94 mm, furthermore, an alteration in the intensity of infestation by cohorts was found. The parasite number in females increased gradually with size up to 32 mm (Fig. 5a). The male size range was 11.76-30.49 mm. The smallest parasitized specimen was 13.78 mm and the peek intensity of parasites was found in the size class from 26 to 30 mm of length (Fig. 5b).
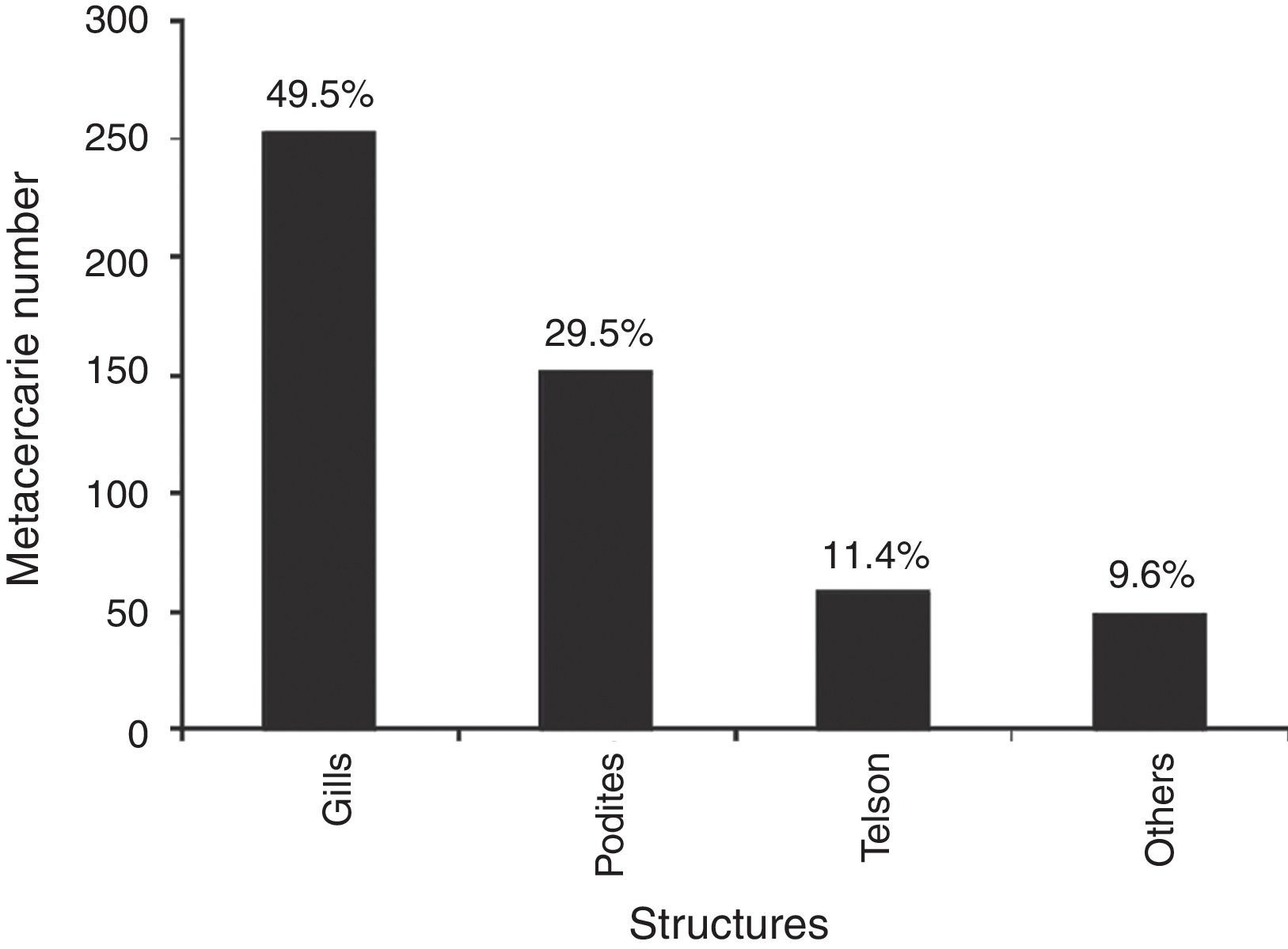
Figure 4 Number of Haematoloechus pulcher metacercariae per anatomical unit of Cambarellus montezumae.
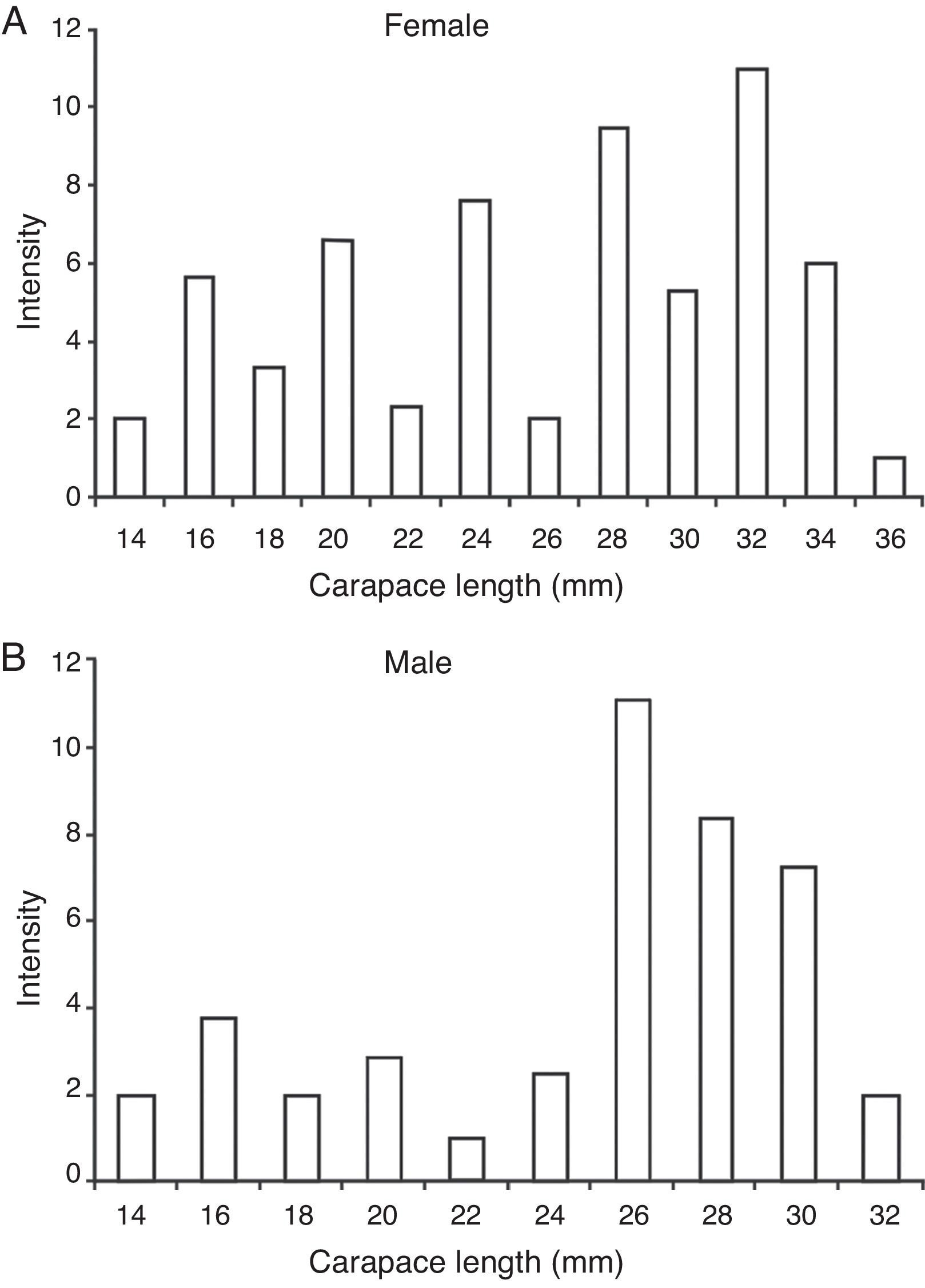
Figure 5 Intensity of Haematoloechus pulcher metacercariae versus carapace length (mm) in Cambarellus montezumae. (A) Females; (B) males.
Both male and female crayfish were parasitized throughout the year (except January) (Fig. 6). The abundance of metacercariae per crayfish was higher from March to June (Fig. 6a). The prevalence was higher from March to June (Fig. 6b). This results in a potentially active and ongoing infection. The average of the intensity increased from February to June (Fig. 6c).
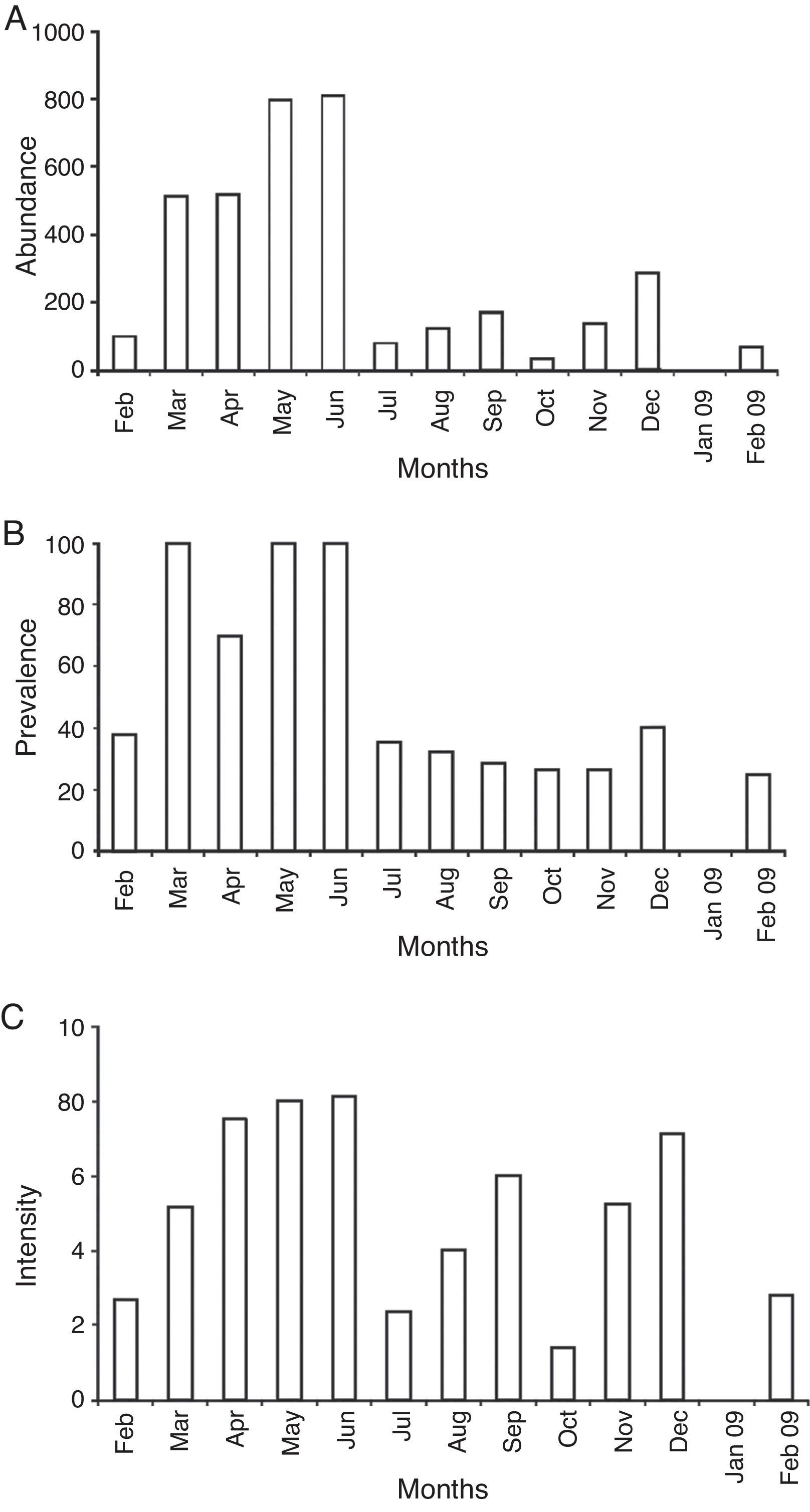
Figure 6 Presence of Haematoloechus pulcher metacercariae during months of sampling collection. (A) Abundance; (B) prevalence; (C) intensity.
The abiotic factors recorded were as follows: the water temperature ranged from 10 °C in November and December, to 24 °C in May (at 15 h), with a global average of 16 °C. The pH ranged from 5 (December 2008) to 9.3 (February 2010), the average being 6.4. On the other hand, the oxygen concentration range was 4.4 mg/l (October) to 11.7 mg/l (May) with an average of 6.75 mg/l. The minimum transparency was 1.1 m (August and September) and the maximum was 3.67 m (February 2009). The catch per unit effort (CPUE) ranged from 3.5 (June and July) to 45 (August), with an average of 12 crayfish per sampling unit. The increase in the number of metacercariae in crayfish was related to the increase in temperature (Fig. 7a and c) and concentration of dissolved oxygen in water (Fig. 7b and d), present in warmer months (March to June). The correlation between temperature and abundance was not significant at 95% confidence level (p = 0.059).

Figure 7 Association of abiotic variables and the presence of metacercariae in the crayfish Cambarellus montezumae. (A) Abundance of metacercariae (%) and association with the water temperature (°C); (B) abundance of metacercariae (%) and association with oxygen concentration (ppm); C: prevalence of metacercariae (%) and association with the water temperature (°C); D: prevalence of metacercariae (%) and association with oxygen concentration (ppm).
Discussion
The purpose of this project was to analyze the presence of metacercariae infection in crayfish. Furthermore, if it is considered that almost 49.5% of the metacercariae were found in the gills, it is possible to conclude that the crayfish are parasited through the gill epithelium. The presence of metacercariae in such proportion, as epizoic organisms in gills of decapods, could reduce respiratory efficiency and result in a reduction of potential utilization, particularly in the case of crayfish (Moctezuma, 1996). Since the podites and telson structures had a similar parasite load intensity as the gills, another possibility of penetration by metacercarie presents itself in the joining membranes and the rectum, as happens in Odonata nymphs (Anisoptera) (Yamaguti, 1975). The maximum intensity of 26 metacercariae found in a male crayfish of 25.7 mm in length was mainly located in the cephalothorax, pereiopods, and antennules. Therefore, as well as the joint membranes, the exoskeleton can be considered as an alternative pathway, such as in the case of dragonflies (Zygoptera) infected by H. complexus (Yamaguti, 1975).
The highest parasitemia was observed in the warmer months, providing suitable conditions for the development of crayfish: temperature of 21 ± 2 °C and O2 concentration of 4-6 mg/l (Latournerié, Osorio, Cárdenas, & Romero, 2006). This time interval is characterized by high ecdysis that results in the softening of the exoskeleton of the crayfish, rendering them subject to infestation. In addition, the lack of rain may increase the concentration of infective stages. On the other hand, metacercariae mainly parasitized crayfish structures which are in contact with the substrate. Apparently, the crayfish are excellent hosts for metacercariae since their benthic habits promote the infection (Wetzel & Esch, 1996). The crayfish cohabit with the first intermediate host, the snail Physa (Mata-López et al., 2002).
The alternation in the intensity of infestation observed in cohorts could be related with the release of cercariae from the first host by pulses and the transition phase to a new host characterized by intermittent activity. This intermittent behavior is a mechanism used by many phyla whose advantage is to conserve energy and reduce predation (Sukhdeo & Sukhdeo, 2004).
The direct relation between an increase of parasite intensity and the size of crayfish may be related to the body bulk "available" for the metacercariae, indeed, individuals with a total length smaller than 13.8 mm showed no metarcercariae, but an accumulation over time.
The average values of temperature (16 °C) and dissolved oxygen (6.75 mg/l) recorded in the Salazar Lagoon, suggest a lentic environment, cooler and more oxygenated for C. montezumae , as the one recorded in Xochimilco, D.F. (Álvarez & Rangel, 2007), within the tolerance limits of this species (10-35 °C and 2 mg/l dissolved oxygen as minimum) (Moctezuma, 1996).
Bolek (2006) demonstrated that Rana pipiens was infected with Haematoloechus coloradensis and H. complexus by feeding on non-odonate arthropods that act as secondary intermediate hosts. The adult stage of H. pulcher was registered in lungs of: Ambystoma lermaensis (Mata-López et al., 2002), Ambystoma tigrinum and Lithobates montezumae (=Rana montezumae ) (Pérez-Ponce De León, García-Prieto, & Mendoza-Garfias, 2007) in the Cienega of Lerma, Estado de México (Bravo, 1943). Additionally, L. montezumae consumes mollusks and crustacean (Mendoza, Lara, & Castro, 2008), therefore both species could be the definitive host and the crayfish C. montezumae , the second intermediate host of the digenean H. pulcher .
H. pulcher was identified by taxonomic characters and DNA sequencing. Taking into account the variations that resulted from the abiotic factors, we conclude that C. montezumae should be considered a second intermediate host of the digenean in the Salazar Lagoon in Mexico due to the prevalence and presence of this parasite throughout the year.











 nueva página del texto (beta)
nueva página del texto (beta)


