Introduction
During a collaborative study of the helminths of marine fishes of the southern Pacific coast of Mexico, specimens of a monogenean generally similar to members of EuzetiaChisholm and Whittington, 2001 were collected from the gills of Rhinoptera steindachneri Evermann and Jenkins, 1891. Similar to Euzetia , these specimens had 10 peripheral loculi and an additional loculus on either side of the central loculus; however, the central loculus was reduced in size, with the additional loculi on either side of central loculus in contact with each other. Further study revealed that the specimens had two accessory structures on the dorsal surface of the haptor, also lacking in Euzetia . Considering these characters, among others mentioned below, a new genus is created; the description of the new genus and species is provided herein and the subfamily is emended to accommodate this species. The key of Chisholm, Wheeler, and Beverley-Burton (1995) to the subfamilies of Monocotylidae is revised to include Euzetiinae Chisholm and Whittington, 2001, and a key to the genera and species of this subfamily is provided. Based on the characteristics of members of Heterocotylinae, Decacotylinae, and Euzetiinae, a phylogenetic hypothesis is presented for the relationships of the three subfamilies and the new taxa described herein.
Materials and methods
Eighteen specimens of Rhinoptera steindachneri were collected in coastal marine waters in Bahía de Acapulco, Guerrero, Mexico (Playa Las Hamacas: 16°51′10.80″ N, 99°53′59.02″ W) between February and June, 2011. The external body surface of each stingray was examined using a magnifying glass and each gill arch was excised, placed in a Petri dish with seawater, and examined using a stereomicroscope. Monogeneans were removed from gill lamellae and transferred temporarily to dishes containing seawater. When all worms had been collected they were killed with hot water, fixed with Alcohol-Formalin-Acetic Acid (AFA) at room temperature for at least 12 h, and transferred for storage to 70% ethyl alcohol. Specimens were stained with Gomori's trichrome, Mayer's carmalum, or Delafield's hematoxylin, dehydrated in an ethanol series, cleared in methyl salicylate, and mounted in Canada balsam. Specimens were examined using a compound photomicroscope equipped with normal light and differential interference contrast microscopy (DIC or Nomarski) optics and drawings were made with the aid of a drawing tube. Measurements were made using an ocular micrometer; all measurements are given in micrometers as the mean followed in parentheses by the range and the number of structures measured. Terminology for structures of the haptor follows Chisholm et al. (1995), and Chisholm and Whittington (1998a, 2001). Specimens were deposited in the Colección Nacional de Helmintos, Instituto de Biología, Universidad Nacional Autónoma de México, Ciudad de México, México (CNHE); the Harold W. Manter Laboratory, Division of Parasitology, University of Nebraska State Museum, Lincoln, Nebraska (HWML). Material examined for this study included: Decacotyle floridana (Pratt, 1910) Chisholm and Whittington, 1998 (CNHE-327, 4368, 4369; USNPC-49447; 36968; 39582; 87548; 90354); D. lymmae (Young, 1967) Chisholm and Whittington, 1998 (HWML-1386; USNPC-61747); D. octona (Young, 1967) Chisholm and Whittington, 1998 (HWML-1389; USNPC-61759; 87549); D. tetrakordyle Chisholm and Whittington, 1998 (USNPC-87550); and Euzetia lamotheiPulido-Flores and Monks, 2008 (CHNE-6067; 6068, HWML-48817, 48818, CHE P-6-00056). Other species of stingrays were collected in the same locality but were negative for the species described herein: Rhinobatos glaucostigma Jordan and Gilbert, 1883 (1 individual), Urotrygon rogersi (Jordan and Starks, 1895) (2), Narcine entemador Jordan and Starks, 1895 (3), Aetobatus cf. A. narinari (Euphrasen, 1790) (1), and Dasyatis longa (Garman, 1880) (3).
Description
Monocotylidae Taschenberg, 1879 Euzetiinae Chisholm and Whittington, 2001 (emended) Type-genus: Euzetia Chisholm and Whittington, 2001
Revised diagnosis
Monocotylidae (sensuChisholm et al., 1995). Haptor with one central loculus, one additional loculus on either side of central loculus, and 10 peripheral loculi. Marginal valve present. Sclerotized sinuous septal ridge and septal sclerites absent. Hamuli with distinct handle and guard. Fourteen hooklets distributed in marginal valve. Unsclerotized accessory structures present or absent on dorsal surface of haptor. Eye-spots present in form of dispersed pigment granules. Intestinal caeca without diverticula, ending blindly in posterior portion of body. Testis single. Male copulatory organ sclerotized; accessory piece absent. Two spherical internal chambers (sensuChisholm & Whittington, 2001; Pulido-Flores & Monks, 2008) of ejaculatory bulb present or absent. Vaginal pore unarmed, single, opening on left side of body at level of common genital pore; vagina not sclerotized. Common genital pore unarmed. Parasites of Rhinopteridae.
Remarks
The revised diagnosis follows Chisholm and Whittington (2001) with the following emendations: unsclerotized accessory structures are present or absent on the dorsal surface of haptor and internal chambers are present or absent in the ejaculatory bulb. Chisholm and Whittington (2001) noted the resemblance of members of Euzetiinae (at that time, only Euzetia occultumChisholm and Whittington, 2001 was assigned to the subfamily) with members of Decacotylinae Chisholm, Wheeler and Beverley Burton, 1995 in having 10 peripheral loculi. The discovery of the new genus described below reinforces this shared similitude. The presence of dorsal unsclerotized haptoral accessory structures in Euzetiinae also suggests a close relationship between the two subfamilies. These structures, called dorsal haptoral protuberances (dhp) by Chisholm et al. (1995) and dorsal unsclerotized haptoral accessory structures (dhas) by Chisholm and Whittington (1998b), are features shared with members of Decacotylinae and Heterocotylinae Chisholm, Wheeler and Beverley Burton, 1995.
Denarycotyle n. gen. (Fig. 1A-C)

Figure 1 Line drawings of Denarycotyle gardneri n. gen., n. sp. (A) holotype, ventral view; (B) detailed view of male and female reproductive systems, ventral view (CNHE-9558); and (C) hamuli (r, right; l, left). Scale bars: A, B=50μm; C=20μm. Abbreviations: ar, anterior ridge; asp, accessory sclerotized piece; cgp, common gonopore; dhas, dorsal haptoral accessory structure; e, eyespots as dispersed pigment; eb, ejaculatory bulb; h, hooks; ha, hamulus; i, intestine; m, mouth; mco, male copulatory organ; mg, Mehlis’ gland; mv, marginal valve; o, ovary; oot, oötype; p, pharynx; s, spherical reservoir; sr, seminal receptacle; sv, seminal vesicle; t, testis; tvd, transverse vitelline duct; v, vagina; vp, vaginal pore.
Diagnosis
With characters of the subfamily Euzetiinae as emended above. Mouth surrounded by distinct ridges. Haptor with one central loculus and 10 peripheral loculi (Fig. 1A); one additional loculus on either side of central loculus, in contact with each other anterior to central loculus. Two unsclerotized accessory structures present on dorsal surface of haptor associated with posteriormost loculi. Hamuli with accessory sclerotized piece (asp) (Fig. 1C). Ejaculatory bulb without spherical internal chambers (Fig. 1B).
Taxonomic summary
Type-species . Denarycotyle gardneri n. gen., n. sp.
Etymology. The generic epithet refers to the 10 peripheral loculi, to draw attention to the similarity with members of Decacotylinae.
Remarks
The only monocotylid reported to have an accessory piece on the hamuli that is sclerotized (asp, sensuYoung, 1967) is Troglocephalus rhinobatidisYoung, 1967 (Dasybatotreminae). However, the shape is different (Chisholm et al., 1995; Young, 1967). The accessory sclerotized piece (asp; sensuYoung, 1967) of the hamuli of members of the new genus is a single-pointed structure that is narrowly semicrescentic (Fig. 1C) (see Clopton, 2004 for a comprehensive discussion and tables of standard terminology for shapes); the asp of T. rhinobatidis is not concave, but blade-like with more than one point on the posterior end of the structure (Chisholm et al., 1995; Young, 1967). Species of Euzetia do not have hamuli with an asp. Members of Euzetia possess a haptor with one central loculus, with an additional loculus on either side, and 10 peripheral loculi, features also shared with Denarycotyle n. gen., although the three central loculi are of different shapes in each. In Euzetia , the three loculi are approximately the same size and shape, and the two additional loculi are entirely lateral to the central loculus (Chisholm & Whittington, 2001; Pulido-Flores & Monks, 2008). In contrast, in Denarycotyle n. gen., the central loculus is smaller than the loculi on either side of the central loculus, and in contact with each other anterior to the central loculus (Fig. 1A). Members of Euzetia have two internal chambers in the ejaculatory bulb, a feature not present in the new genus (Fig. 1B). A suite of three characters, the presence and shape of the accessory sclerotized piece on the hamuli, the presence of two unsclerotized accessory structures on the dorsal surface of the haptor, and the absence of internal chambers in the ejaculatory bulb distinguish Denarycotyle n. gen. from Euzetia , the only other genus currently assigned to the subfamily.
Denarycotyle gardneri n. sp. (Fig. 1A-C)
Diagnosis . Measurements based on 10 specimens. Body (excluding haptor) 439 (390-490, n = 10) long by 178 (155-210, n = 10) wide at level of anterior margin of testis. Haptor very broadly elliptoid, 185 (60-225, n = 10) long and 235 (215-270, n = 10) wide, with one central loculus and 10 peripheral loculi (Fig. 1A); one additional loculus on either side of central loculus, larger in size than central loculus, in contact with each other anterior to central loculus. Two unsclerotized accessory structures present on dorsal surface of haptor, associated with posteriormost loculi (Fig. 1A). Marginal valve with 14 hooklets, 9 (7-10, n = 18) long distributed as illustrated (Fig. 1A). Hamuli with accessory sclerotized piece, 41 (38-45, n = 16) long, with handle 14 (13-20, n = 15) long, associated with posterolateral septa (Fig. 1A). One anterior gland present on each side, lateral to pharynx, ascending ducts connected by a lateral commissure located anterior to mouth, ducts continuing to openings on anterior margin of head; contents of glands not observed. Anteromedian gland not observed. Eye-spots in form of dispersed pigment granules located anterodorsal to pharynx (Fig. 1B). Mouth ventral, subterminal, anterior and posterior muscular ridges present around mouth; esophagus absent (Fig. 1A). Pharynx muscular 64 (55-80, n = 9) long, 54 (45-65, n = 9) wide, with 6-8 transversal muscular packets (Fig. 1A and B). Intestinal ceca without diverticula, extending laterally to posterior portion of body proper, not confluent posteriorly. Cecal bifurcation 129 (115-162, n = 9) from anterior end of body. Testis single, spherical to oval, 112 (80-170, n = 9) long and 114 (95-148, n = 9) wide (Fig. 1A and B). Vas deferens arising from left side of testis, enlarged to form spherical reservoir, 60 (47-80, n = 9) long by 18 (17-20, n = 9) wide, leading to a smaller reservoir curved toward left side of body; loosely coiled narrow duct ascending dorsally, posterior to genital pore, to connect to seminal vesicle. Seminal vesicle 74 (53-107, n = 9) long by 42 (33-50, n = 9) wide (Fig. 1A and B), connected to spherical ejaculatory bulb, 19 (16-23, n = 9) long and 20 (18-23, n = 9) wide, by short, narrow duct (Fig. 1B). Chambers within ejaculatory bulb absent. Accessory glands associated with ejaculatory bulb not observed. Male copulatory organ short sclerotized tube 19 (15-22, n = 7) long and 3 (3-4, n = 7) wide (Fig. 1B); accessory piece absent. Ovary elongate, V-shaped, with lateral arm of "V" encircling right intestinal cecum dorsoventrally and narrowing to form oviduct. Longitudinal length of ovarian mass 94 (81-116, n = 9) (Fig. 1B). Oviduct receives duct from seminal receptacle and common vitelline duct and joins oötype (Fig. 1B). Mehlis' gland not prominent (Fig. 1B). Oötype 115 (95-150, n = 9) long and 40 (30-55, n = 9) wide, opening medially at unarmed common genital pore. Vaginal pore unarmed, opening ventrally on left side slightly posterior to common genital pore located 137 (122-160, n = 6) from anterior end of body. Vagina muscular, unsclerotized, in shape of an elongate sack, 35 (30-40, n = 8) long and 11 (8-17, n = 8) wide. Proximal portion of vagina connected to seminal receptacle by a narrow duct. Seminal receptacle 39 (32-45, n = 9) long and 21 (15-32, n = 9) wide (Fig. 1B). Vitellarium extending from level of posterior portion of pharynx to posterior of body proper. Transverse vitelline duct common, arising from dorsal vitelline glands to join oviduct. Egg tetrahedral, side length 40 (n = 1) by 52 wide (n = 1) (egg measured within oötype); short appendage present at one pole.
Taxonomic summary
Type host. Rhinoptera steindachneri Evermann and Jenkins, 1891 (Myliobatidae).
Type locality. Playa Las Hamacas, Acapulco, Guerrero, México (16°51′10.80″ N, 99°53′59.02″ W).
Prevalence and intensity. 4 of 18 (22%) individuals of Rhinoptera steindachneri infected (mean intensity = 11.5).
Specimens deposited. Holotype CHNE-9558; paratypes CHNE-9559 (six specimens), paratypes HWML-75364 to 75367 (four specimens).
Site of infection . Gills.
Etymology. The specific epithet honors Dr. Scott Lyell Gardner, Harold W. Manter Laboratory of Parasitology, University of Nebraska-Lincoln, Lincoln, Nebraska, U.S.A. for his years of friendship, both personal and professional, and his help in obtaining the grant that gave the authors the opportunity for this study.
Remarks . Of the Monocotylidae, only representatives of Euzetia , DecacotyleYoung, 1967 and Denarycotyle n. gen. have a haptor with 10 peripheral loculi (Fig. 1A) (Chisholm & Whittington, 1998b, 2001; Pulido-Flores & Monks, 2008). Both Decacotyle , type genus of Decacotylinae, and Denarycotyle n. gen. have unsclerotized accessory structures on the dorsal surface of the haptor. The presence of an additional loculus on either side of the central loculus in Denarycotyle and the presence of an accessory sclerotized piece on the hamuli, features not present in Decacotyle , clearly distinguishes between the two genera. The new species shares lateral loculi (on either side) of the central loculus with members of Euzetia , type genus of Euzetiinae, but it can be distinguished by having two unsclerotized accessory structures on the dorsal surface of the haptor, a sclerotized accessory piece on each hamulus, the absence of the two internal chambers in the ejaculatory bulb, features which are not present in Euzetia . Furthermore, in the only species of Denarycotyle n. gen. that is presently known, the additional loculi on either side of the central loculus are larger than the central loculus, and they are in contact with each other anterior to the central loculus (Fig. 1A), but in Euzetia the three loculi are approximately the same shape and size, and the central loculus completely separates the loculi on either side (Chisholm & Whittington, 2001; Pulido-Flores & Monks, 2008).
Key to the subfamilies of the Monocotylidae.
Key to the genera and species of the Euzetiinae.
Discussion
Since the formal conception of Monocotylidae by Taschenberg (1879) (at the subfamilial level), the interest in the group has grown. Price (1938), Dawes (1946), and Sproston (1946) recognized Monocotylidae as a family with five subfamilies, three of which, Monocotylinae Taschenberg, 1879, Calicotylinae Monticelli, 1903, and Merizocotylinae Johnston and Tiegs, 1922, are still considered to be part of the family, with Dasybatotreminae Bychowsky, 1957 as a later addition (Bychowsky, 1957). In the only comprehensive cladistic analysis of the phylogenetic relationships of putative members of the family using morphological characters, Chisholm et al. (1995) recognized the aforementioned subfamilies and proposed the addition of Decacotylinae Chisholm, Wheeler, and Beverly-Burton, 1995 and Heterocotylinae Chisholm, Wheeler, and Beverly-Burton, 1995. However, that study did not resolve the relationships between the subfamilies. Subsequently, Euzetiinae Chisholm and Whittington, 2001, was added as a seventh subfamily (Chisholm & Whittington, 2001); members of Euzetiinae have not been included in any formal analysis to date.
Recently studies using molecular data have not been able to determine completely these relationships, but the interpretations of those data suggest that Heterocotylinae and Decacotylinae are closely related (Chisholm, Morgan, Adlard, & Whittington, 2001; Fehlauer-Ale & Littlewood, 2011; Olson & Littlewood, 2002). Members of the two subfamilies share some structural features (Chisholm & Whittington, 1996, 1998b; Chisholm et al., 1995); among these, the presence of accessory structures on the dorsal surface of the haptor is a putative synapomorphy. Presence or absence of sclerotization of the dorsal haptoral accessory structures (dhas) has been used to distinguish between members of the two subfamilies, but recent work (Chisholm, 2013; Vaughan & Chisholm, 2010a, 2010b) has cast doubt on the ability to recognize sclerotization in some structures.
Chisholm et al. (1995) did not explain why they considered the dorsal protuberance with no evident sclerotization to not be homologous with the dorsal haptoral accessory sclerites (dhas), which also are rounded protuberances on the dorsal haptor of some species of Heterocotylinae (Chisholm & Whittington, 1996, 1998b); the dhas also is referred to as a dorsal haptoral "bump" in some works (Chisholm, 1995). In many species of both subfamilies the dhas are broadly to narrowly rounded, although some are spine-like (Vaughan & Chisholm, 2010a, 2010b). The similarity between the rounded forms of dhas suggests that they are homologous, with sclerotization being a separate character (Fig. 2).
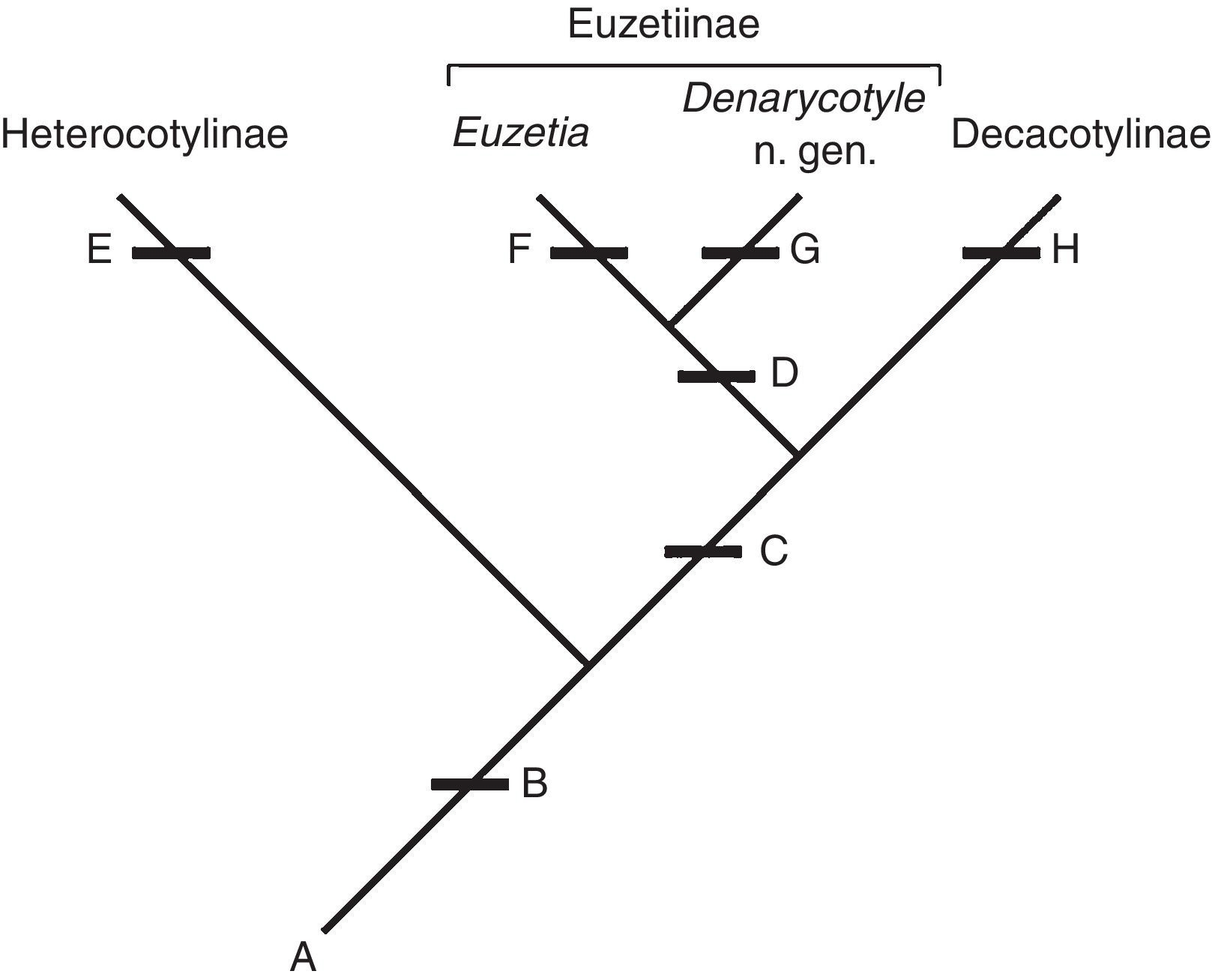
Figure 2 Characters supporting the relationship between Heterocotylinae, Decacotylinae, and Euzetiinae, and assignment of Denarycotyle n. gen. to Euzetiinae: (A) characters plesiomorphic for these taxa as given in Chisholm et al. (1995); (B) presence of dorsal haptoral accessory structures (dhas); (C) 10 peripheral loculi; (D) additional loculus on each side of the central loculus; (E) seven to nine peripheral loculi and the presence of sclerotization of the dhas; (F) presence of internal chambers in the ejaculatory bulb and secondary absence of dhas; (G) additional loculi larger than the central loculus and the presence of an accessory structure each hamulus; and (H) secondary absence of sclerotization of the dhas.
The presence and form of oral ridges were used by Domingues and Marques (2011) as diagnostic characters in a phylogenetic analysis. Oral grooves and oral ridges, in several forms, have been illustrated and described by many authors (Chisholm, 1994, 1995, 2013; Chisholm & Whittington, 1995, 2000; Domingues & Marques, 2007, 2011; Domingues, Pancera, & Marques, 2007; Santos, Santos, Cunha, & Chisholm, 2012; Vaughan & Chisholm, 2009, 2010a, 2010b; Vaughan, Chisholm, & Christison, 2008; Whittington, Barton, & Lester, 1989), but a complete interpretation is still lacking.
Domingues and Marques (2011) listed 12 species of Potamotrygonocotyle Mayes, Brooks and Thorson, 1981 (Heterocotylinae) that have sclerotized oral ridges and Chisholm (2013) listed an additional 12 species that have muscular oral ridges. However, Chisholm (2013) commented on the difficulty of distinguishing between oral ridges that are muscular and those that are sclerotized. This argument was supported by citing studies of sclerotized structures in monocotylids carried out by Vaughan and Chisholm (2010a, 2010b) in which proteolytic digestion of the haptor of specimens of H. tokoloshei Vaughan and Chisholm, 2010 and N. robii Vaughan and Chisholm, 2010, in which the degree of digestion of the sinuous ridge on the septa was equal to that of other non-sclerotized parts of the haptor; this was interpreted as casting doubt on the determination of presence of sclerotized structures using microscopy. In spite of this, we used microscopy (with DIC illumination) as the basis for our characterization of the oral ridges of D. gardneri n. gen., n. sp. as muscular and to characterize the accessory sclerotized piece on the hamuli as sclerotized.
Studies of morphological and of molecular data separately have supported the value of the number of haptoral loculi in the recognition of taxa within the family (Chisholm, 2013; Chisholm & Whittington, 1998a; Chisholm et al., 1995, 2001; Fehlauer-Ale & Littlewood, 2011; Olson & Littlewood, 2002). These features are useful for distinguishing between the subfamilies and are important for differentiation of the assigned genera.
Of the seven subfamilies, Decacotylinae and Euzetiinae have 10 peripheral haptoral loculi (see key). Members of Euzetiinae are distinguished by the presence of an additional loculus on either side of the central loculus. In light of these characters, and others included in previous studies mentioned above, the presence of dorsal haptoral accessory structures is a putative synapomorphy for Heterocotylinae, Decacotylinae, and Euzetiinae (dhas secondarily absent in Euzetia ). Members of Heterocotylinae have 7-9 peripheral loculi; having 10 peripheral loculi is a synapomorphy for Euzetiinae and Decacotylinae. Euzetiinae is distinguished from Decacotylinae by the additional loculus on each side of the central loculus and two chambers in the ejaculatory bulb, features absent in Decacotylinae (Chisholm & Whittington, 2001; Pulido-Flores & Monks, 2008). Denarycotyle n. gen. is assigned to Euzetiinae because it shares having 10 peripheral loculi and an additional loculus on each side of the central loculus. Denarycotyle n. gen. can be distinguished from Euzetia by the absence of chambers in the ejaculatory bulb, presence of dhas, and the additional loculi being much larger than the central loculus. This hypothesis is presented in Fig. 2.
Chisholm and Whittington (2001) created Euzetiinae to accommodate Euzetia occultum , the first monocotylid to be described from the gills of a species of Rhinoptera Cuvier (on R. neglecta Ogilby, 1912 from Australia). Later, E. lamothei from R. bonasus (Mitchill, 1815) from Campeche, Gulf of México, was the second member of Euzetia , to be described (Pulido-Flores & Monks, 2008). Denarycotyle gardneri n. gen., n. sp., parasite of the gills of R. steindachneri from the Pacific coast of México, is the third published record of a monocotylid collected from stingrays of this genus.
Acknowledgments
The authors thank all those who made possible the examination of specimens, especially Scott L. Gardner, Curator, and Gabor R. Racz, Collection Manager, at the HWML. Mary Hanson Pritchard, Affiliate of the HWML, provided access to literature in the laboratory archives. Part of this manuscript was prepared during a research visit to the HWML, which provided office and laboratory space, access to computers, the reprint collection, and microscopes. Several reviewers provided comments. This study was supported by funds from the Patronato Universitario (Gerardo Soza Castelán, President), Universidad Autónoma del Estado de Hidalgo (UAEH), the Consorcio de Universidades Mexicanas (CUMEX) and the Programa de Mejoramiento del Profesorado (PROMEP) (Project title-Red temática: Calidad ambiental y desarrollo sustentable).











 nueva página del texto (beta)
nueva página del texto (beta)




