Introduction
The harpacticoid copepod family Ectinosomidae was established by Sars (1904), and the name was corrected as Ectinosomatidae by Moore (1978). Most ectinosomatids inhabit sublittoral marine sediments ranging from coarse sands to flocculent muds (Clément & Moore, 2000). The family originated from marine environments where radiation and diversification were successful ( Kihara & Huys, 2009), but several ectinosomatid genera (i. e., Rangabradya Karanovic & Pesce, 2001; Ectinosoma Boeck, 1864; Microsetella Brady & Robertson, 1873; Pseudobradya G.O. Sars, 1904; Pseudectinosoma Kunz, 1935; Halectinosoma Vervoort, 1962; Arenosetella Wilson, 1932) can also be recorded from brackish and freshwater systems (Defaye & Dussart, 2011).
Currently, there are 21 valid genera within the Ectinosomatidae, and Halectinosoma and Pseudobradya are among the most diverse, each containing 65 and 43 nominal species, respectively (Clément & Moore, 2007). The name of the former genus was proposed by Lang (1944) and made available by Vervoort (1962) (Huys, 2009). Both genera are in urgent need of revision (Defaye & Dussart, 2011; Kihara & Huys, 2009) and taxonomic conflicts are still extant involving these groups. Some species of Pseudobradya resemble those currently attributed to Ectinosoma and Halectinosoma and it is probable that several nominal species have been assigned to the wrong genus (Wells, 2007). The lack of complete, detailed descriptions and the absence of types of many species have greatly contributed to the taxonomic confusion and the species-level taxonomy of these genera is still in a state of flow (Clément & Moore, 2007; Huys, Gee, Moore, & Hamond, 1996).
In the Americas, most records of Halectinosoma and Pseudobradya are from the United States, Puerto Rico, the U.S. Virgin Islands, and Brazil (Jakobi, 1954; Lang, 1965; Reid, 1998; Rouch, 1962; Suárez-Morales, De Troch, & Fiers, 2006). In Colombia, the knowledge on the composition of this harpacticoid family is still lagging; up to now, there are no previous records of species of Halectinosoma or Pseudobradya . A recent biological survey of the aquatic fauna of a protected coastal lagoon system of northern Colombia yielded several specimens of ectinosomatid harpacticoid copepods. These specimens represent 2 undescribed species, one of each of these 2 genera; both herein described, illustrated, and compared with their congeners.
Materials and methods
Biological samples of benthos and plankton were taken from the Navío Quebrado Lagoon, Colombia (11°25' N, 73°05' W) from April to December 2012, mainly in shallow littoral areas with vegetation (macrophytes and mangrove) but also from open water in areas close to oyster banks. Water salinity was measured with a WTW 3111 conductivity meter. Water samples were collected using a 25 L bucket at both vegetation areas and open water. Samples were filtered with a standard zooplankton net with a 45 μm mesh and fixed and preserved in 70% ethanol. Dissected specimens and appendages were mounted in glycerine. Drawings of the appendages were prepared at 1000X magnifications with a camera lucida mounted on a standard Olympus CX31 compound microscope. Some specimens were prepared for scanning electron microscopy, which included dehydration through graded ethanol solutions (80, 96, 100%), drying, and coating following standard methods. Observations were performed using a JEOL LV-5900 microscope at El Colegio de la Frontera Sur in Tapachula, Mexico. The specimens were measured in lateral position, from the anterior end of the rostral area to the posterior margin of the caudal rami. Morphological nomenclature follows Huys and Boxshall (1991). The following abbreviations are used in the descriptions: P1-P6, first to sixth swimming legs; EXP, exopod; ENP, endopod. The type specimens were deposited in the collections held at the Museo de Colecciones Biológicas de la Universidad del Atlántico (UARC), Barranquilla, Colombia. Additional specimens are deposited in the Collection of Zooplankton at El Colegio de la Frontera Sur, Chetumal, Mexico (ECO-CHZ).
Descriptions
Order Harpacticoida Sars, 1903
Family Ectinosomatidae Sars, 1903
Halectinosoma Vervoort, 1962
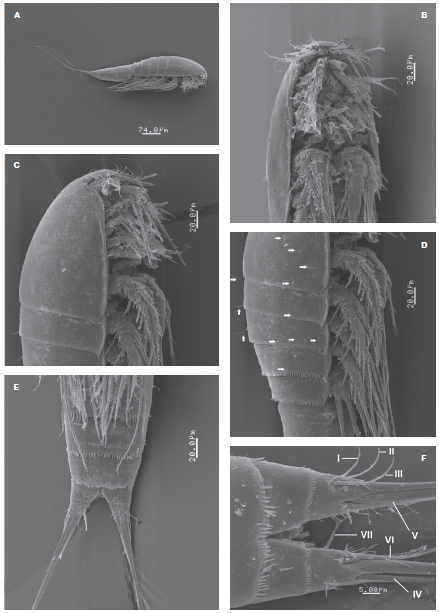
Figure 1 Halectinosoma arangureni sp. nov., adult female from Colombia, SEM-prepared specimen: A, habitus, lateral view; B, cephalosome and first thoracic somites, ventral view; C, same, lateral view; D, cephalosome and thoracic somites showing sensillae (arrowed); E, urosomites and caudal rami, ventral view; F, detail of caudal rami showing caudal setae I-VII, ventral view.
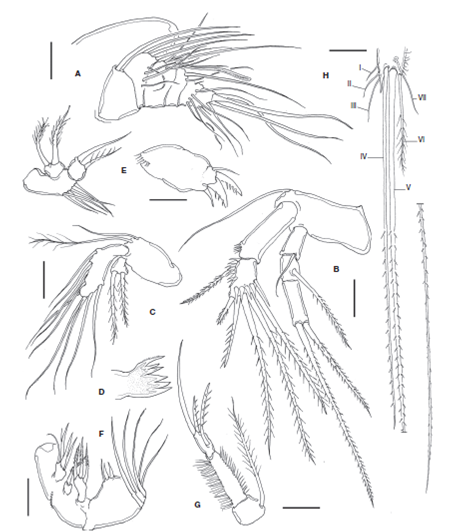
Figure 2 Halectinosoma arangureni sp. nov., adult female from Colombia: A, antennule; B, antenna; C, mandible palp; D, gnathal blade; E, maxillule; F, maxilla; G, maxilliped; H, caudal ramus, ventral view showing caudal setae I-VII. Scale bars: A-H= 20 μm.
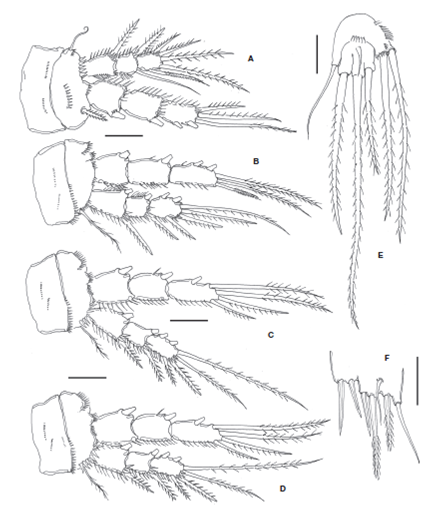
Figure 3 Halectinosoma arangureni sp. nov., adult female from Colombia: A, leg 1; B, leg 2; C, leg 3; D, leg 4; E, leg 5; adult male, F, leg 5. Scale bars: A-F=20 μm.
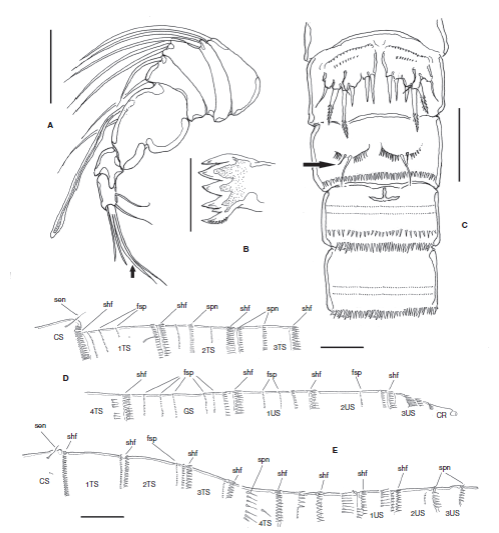
Figure 4 Halectinosoma arangureni sp. nov., adult male from Colombia: A, antennule; B, gnathal blade; C, urosomites showing P5 and P6, ventral view, outer seta of leg 6 arrowed; D, cuticular ornamentation of the female, lateral view showing subulate hyaline frills (shf), spinules (spn), sensillae (sen), fine spinules (fsp) in body somites: cephalic somite (CS), thoracic somites 1-4 (1-4TS), genital double-somite (GS), and urosomites 1-3 (US1-3); E. same, male. Scale bars: A= 25 μm; B= 100 μm; C= 20 μm; D, E= 20 μm.
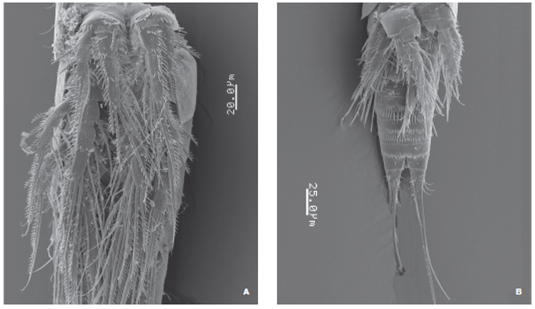
Figure 5 Halectinosoma arangureni sp. nov., adult male from Colombia, SEM-prepared specimen: A, pedigerous somites and P1-P4, ventral view; B, another specimen showing ornamentation of urosomites, ventral view.
Type material
Adult female holotype (UARC385M), male allotype (UARC386M), partially dissected, slides, Laguna Navío Quebrado, La Guajira, Colombia, coll. J. Fuentes-Reinés, April-December 2012. Paratypes: 3 females (UARC387M) and 2 males (UARC387M) from same locality, and collector. Two adult females, 2 adult males from same locality, date, and collector, specimens dissected, semi-permanent slides (ECO-CHZ-09265). Additional material examined. Ten adult females, 8 adult males in authors' (JF-R) personal collection.
Description of female
Habitus as in Figure 1A. Body fusiform in dorsal view, prosome gradually tapering anteriorly. Pedigerous somites with subacute lateral pleural projections, weakest in fifth pedigerous somite (Figs. 1C, D ). Prosome representing 32-34% of total body length. Total body length measured from tip of rostrum to posterior margin of caudal rami ranging from 448 to 518 μm (average= 499 μm, n = 15; holotype: 462 μm). Rostrum articulated at base with cephalosome (Fig. 1C), blunt in lateral view, truncate in ventral view (Fig. 1B). Labrum represented by anteriorly directed spinous projection. Cephalosome and pedigerous somites with sparsely distributed sensillae (arrowed in Fig. 1D), U-pores not observed. Posterior margin of pedigerous somites 1-3 with finely spinulated hyaline frill; somites 4 and 5 with coarser hyaline frill and with adjacent transverse rows of spinules (Fig. 1D). Genital double-somite with transverse rows of minute spinules in ventral, lateral and dorsal surfaces plus posterior coarse subulate hyaline frill (Fig. 4D). Posterior margin of first postgenital urosomite with subulate hyaline frill plus 2 rows of slender, minute spinules along ventral surface, and row of spinules (Figs. 1E, 4D ). Preanal somite with 2 rows of minute spinules on ventral surface and hyaline frill. Anal somite with posterior row of fine spinules.
Caudal rami subquadrate, about as long as wide, length/ width ratio= 1.0 (Fig. 1F), with distal acute processes arising from insertion of caudal setae. Inner distal margin of rami with a few setules. Setae I-III slender, subequal in length and width; outer caudal seta III 1.9 times as long as caudal ramus. Innermost seta IV thin, lightly setulated, inner terminal seta (V) about 1.3 times as long as outer one (seta IV), slenderer, setae IV and V spinulated distally from midlength and proximal 1/3, respectively. Dorsal seta (VII) about twice as long as caudal ramus (Fig. 2H). Ovigerous females bearing 1 sac with 8 eggs.
Antennule
Short, robust, with 6 segments (Fig. 2A).
Armature as follows, s= setae, ae= aesthetascs: 1(1s), 2 (7s), 3(3s (1+ ae)), 4(2s), 5(5s), 6(6s, 1ae).
Antenna
Coxa and basis fused, segment as long as ENP1, with abexopodal seta. ENP 2-segmented; first segment smooth except for subdistal row of spinules; ENP2 with lateral and subdistal rows of strong spinules; with 1 lateral pinnate seta and 5 apical and 1 subapical elements. EXP 3-segmented; first and second segments together about as long as third; proximal exopodal segment EXP1 with short smooth seta, EXP2 with long spinulose seta; EXP3 with 2 apical, unequally long spinulose setae (Fig. 2B). Mandible . Basis with 2 slender unequally long setae inserted at distal inner corner. ENP oval, 1-segmented, with 9 setae. EXP represented by small segment bearing 2 subequally long lightly spinulose setae (Fig. 2C). Gnathal blade with 5 acute teeth plus uniserially pinnate dorsal seta ( Fig.2D ).
Maxillule
Praecoxal arthrite broad, with proximal row of spinules, distally with 3 spines (2 of them strong) and subdistal seta. Basis with 5 slender setae inserted along inner margin. EXP represented by small subtriangular segment with 2 plumose setae. ENP 1-segmented, with 2 setae (Fig. 2E).
Maxilla
Not strongly geniculate, syncoxa with 3 endites and a spiniform seta. Proximal endite with 4 setal elements, second endite with a strong pinnate seta, third endite with 3 pinnate setae. Basis broader than syncoxa, with medial protuberant endite bearing 3 setal elements, 2 short, stout setae plus 1 slender seta. ENP 3-segmented; ENP1 and ENP2 each with 1 long slender seta; distal segment represented by broad base with 3 setal elements (Fig. 2F).
Maxilliped
Syncoxa short, subquadrate, about 0.3 times as long as basis, with 1 plumose seta reaching beyond distal end of ENP. Basis elongated, length/width ratio about 3.3; with slender outer setules, inner margin spinulose; ENP 1-segmented, short, with 3 elements (2 strong spinulose setae and 1 slender element) (Fig. 2G).
P1 coxa with rows of minute spinules on anterior surface. Basis with short, stout inner basipodal spine almost as long as ENP1 and slender outer basipodal seta. Basis with transverse rows of spinules on anterior surface and along distal margin, row of stronger spinules at insertion of endopodal ramus. ENP 3-segmented, with inner margin of segments spinulose, outer margin of segments ENP1 and ENP2 setulose, ENP1 without inner seta, ENP2 with single seta. EXP 3-segmented, shorter than ENP; EXP1 and EXP2 without inner seta; EXP1-3 with outer margin spinulose and 5 setal elements (Fig. 3A).
P2-P4 coxae with rows of minute spinules on anterior surface. Basis with spinules distally and with slender outer basipodal seta reaching beyond the distal margin of EXP1. ENP 3-segmented, longer than EXP; ENP1 and ENP2 with inner seta; ENP3 with 5 setal elements. EXP 3-segmented, EXP1 and EXP2 with inner seta; EXP3 with 6 elements (Figs. 3B- D ). Setal formula for P1-P4 is presented in Table 1.
Variability
The left P1EXP 2 of one female lacks a seta. P5 with subquadrate EXP separated from baseoendopod by suture on posterior surface; with 5-6 small spinules along suture; with 3 clearly defined naked distal lobes, each with 1 spinulose seta (innermost seta shortest, medial seta longest about 2.4 times as long as innermost seta); with slender, short, surface seta barely reaching beyond distal margin of EXP (Fig. 3E). Inner expansion of baseoendopod reaching halfway of length of inner exopodal margin, with row of inner spinules plus row of spinules at insertion of 2 spinulose setae (innermost endopodal seta slightly longer than outer seta); outer expansion of baseoendopod with long, slender basal seta (Fig. 3E).
Description of male
Body fusiform, with pleural projections of pedigerous somites less pronounced than in female; rostrum rounded; prosome representing 38-43% of total body length. Body length 298-350 μm (average=325 μm, n= 5, allotype = 329 μm).
Antennule short, robust, with 7 segments, thick at base with lobes with long setae (Fig. 4A); armature formula as follows: 1(1s), 2(2s), 3(2s), 4(2s), 5(1s+ae), 6(0), 7(4s). Last segment with distal branched seta (arrowed in Fig. 4A). A2, Mxl, Mx and Mxp (not shown) as in female. Md as in female except for wider teeth on mandibular blade (Fig. 4B).
Second and third urosomites with transverse row of minute spinules on medial ventral surface plus subdistal row of middle-sized spinules and distal subulate hyaline frill on ventral and dorsal surfaces (Figs. 4C, E , 5B ). Third urosomite with T-shaped ventral incision (Fig. 4C). Preanal somite with proximal row of minute spinules plus distal row of medium-sized spinules. Anal somite as long as preceding somite, with distal row of minute spinules at insertion of caudal rami. Caudal rami as in female. P1-P4 as in female, with spinulose segments and setae (Fig. 5A). P5. With rows of minute spinules along insertion line proximally. Inner expansion of baseoendopod with 2 subequally long robust smooth setae. Exopod partially fused to baseoendopod, seemingly without suture; exopod with 3 setal elements (innermost seta shortest, middle seta longest); surface of exopod with accessory seta located near insertion of middle exopodal seta (Figs. 3F, 4C ).
P6 consisting of a flat spinulose plate with 2 setae, inner seta about 3 times as long as outer seta (arrowed in Fig. 4C).
Etymology
The species is named after the Colombian researcher Dr. Nelson Aranguren, for his extensive work on the zooplankton from aquatic systems of Colombia and for his legacy and leadership of new generations of planktologists.
Remarks
The specimens from Colombia were identified as belonging to the genus Halectinosoma by the characters selected by Huys et al. (1996) and Wells (2007): 1) the 5-7 segmented antennule, 2) antennary exopod 3-segmented, 3) mandible basis with 3 setae, 4) maxilla not strongly geniculate, 5) slender maxilliped with long basipod and endopod bearing 3 setae, 6) female P5 with 3 setae and surface seta arising from middle of exopodal segment. Following Well's (2007) key to the genera and species of Ectinosomatidae, our specimens key down to a group of species of Halectinosoma sharing a combination of several characters including: 1) P1-4 with 5,6,6,6 setal elements on the third exopodal segment; 2) P1-P4 with 5 setae on the third endopodal segment; 3) inner seta on the first exopodal segment absent only on P1; 4) length/width ratio of the maxilliped basis between 2 and 4, and 5) the inner endopodal seta of P5 is longer than the outer one (Wells, 2007). This group of species includes H. abyssicola (Bodin, 1968), H. langi Wells 1967, and H. curticorne (Boeck, 1873).
The new species H. arangureni sp. nov. can be separated from these 3 species by: a) the length/width ratio of the basipodal segment of the maxilliped (length/width ratio about 4.0 in both H. langi (Wells, 1967) and H. abyssicola (Bodin, 1968) and 2.0 in H. curticorne (Poppe, 1885, Taf. VI, Fig. 10), but 3.3 in H. arangureni sp. nov. (Fig. 2G)); b) the armature of the exopodite of the mandibular palp (with 3 setae in H. langi (Wells, 1967, Fig. 21E) and H. curticorne (Poppe, 1885, Taf. VI, Fig. 7), but with 2 setae in H. arangureni sp. nov. and H. abyssicola (Bodin, 1968 pl. II)). According to Wells (2007), H. abyssicola is easily distinguishable from these other species by having an extremely long outer seta of the inner expansion of the P5 ENP, about 4 times as long as the inner seta. Also, H. curticorne has a distinctive pigmentary spot on the first antennulary segment, this spot is absent in the other species compared here.

Figure 6 Pseudobradya gascae sp. nov., adult female from Colombia, SEM-prepared specimen. A, rostral area showing articulation of rostrum, lateral view; B, prosome, lateral view; C, last urosomites and caudal rami, ventral view; D, cephalosome showing rostral area, ventral view.
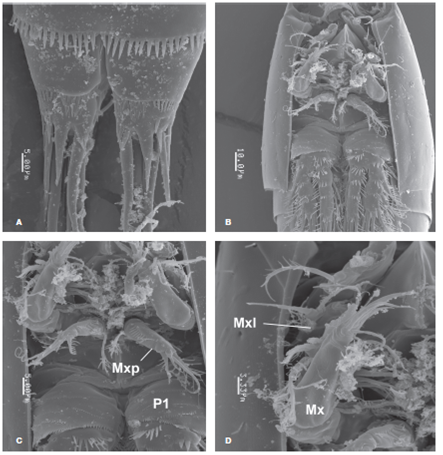
Figure 7 Pseudobradya gascae sp. nov., adult female from Colombia, SEM-prepared specimen: A, detail of caudal rami, ventral view; B, cephalosome, ventral view; C, cephalosome showing maxilliped (Mxp) and coxa and basis of P1 (P1), ventral view; D, same, showing maxilla (Mx) and exopodal segment of maxillule (Mxl), ventral view.
The first exopodal segment of the antenna is unarmed in H.langi (Wells, 1967, Fig. 21D), H. abyssicola (Bodin, 1968, pl. IX) and H. curticorne (Poppe, 1885, Taf. VI, Fig. 6) whereas a single slender seta is present in the new species, H. arangureni (Fig. 2B). The length/width ratio of the caudal rami is about 1.0 (as long as wide) in both H. langi (Wells, 1967, Fig. 21A) and H. arangureni , and reaches 1.2 in H. abyssicola (Bodin, 1968, pl. XI) and 2.0 in H. curticorne (Poppe, 1885, Taf.VI, Fig. 1). Also, the caudal seta III/caudal ramus length ratio is about 1.0 in H. langi (Wells, 1967, Fig. 21A), 0.6 in H. curticorne (Poppe, 1885, Taf.VI, Fig. 1), 0.7 in H. abyssicola (Bodin, 1968, pl. XI), but 1.9 in H. arangureni , the species with the longest such seta among these species (n = 10). The relative length of the outer endopodal setae of the female P5 with respect to the adjacent innermost exopodal seta is different in these species. In H. langi the innermost exopodal seta reaches the distal end of the outer endopodal seta (Wells, 1967, Fig. 21J), in H. curticorne (Poppe, 1885, Taf. VI, Fig. 12; Chislenko, 1967, Fig. 9) the outer endopodal seta reaches about halflength of the innermost exopodal seta, and in H. arangureni the endopodal seta reaches about 3/4 the length of the exopodal seta (Fig. 3E). Furthermore, the P5 accessory seta is shorter in the female of both the new species (Fig. 3E) and H. curticorne (Poppe, 1885, Taf. VI, Fig. 12) than in H. langi (Wells, 1967, Fig. 21J).
The male of H. arangureni sp. novdiffers from the males of the other 2 species in having a T-shaped incision in the third urosomite (Fig. 4C) which is differently shaped and in the second urosomite in H. langi (Wells, 1967, Fig. 22H) and H. curticorne (Chislenko, 1967, Fig. 9). The accessory seta of the male P5EXP is absent in H. langi (Wells, 1967, Fig. 22E), but present in the new species (Fig. 3F) and in H. curticorne (Chislenko, 1967, Fig. 9). The exopod of the male P5 is distinct from the baseoendopod in H. curticorne , but fused in the new species (Fig. 3F). The male P6 has a single seta in H. langi (Wells, 1967, Fig. 22F), but 2 in the new species (Fig. 4C). The male of H. abyssicola remains unknown and cannot be compared with the male of H. arangureni sp. nov.
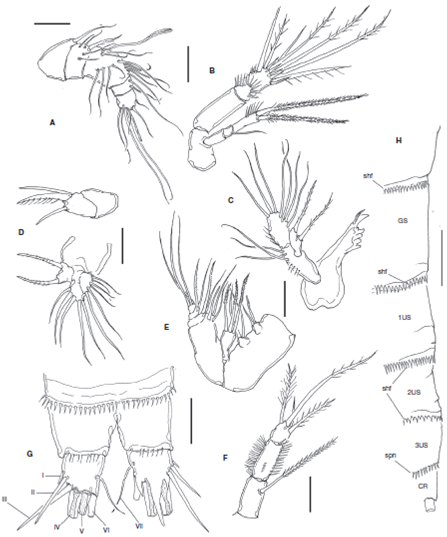
Figure 8 Pseudobradya gascae sp. nov., adult female from Colombia: A, antennule; B, antenna; C, mandible; D, maxillule; E, maxilla; F, maxilliped; G, anal somite and caudal ramus, ventral view showing setae I-VII, spinous processes omitted on left ramus; H, cuticular ornamentation of the female, lateral view showing subulate hyaline frills (shf) and spinules (spn), in genital double-somite (GS), urosomites 1-3 (US1-3) and caudal rami (CR). Scale bars: A-F= 10 μm; G, H= 20 μm.
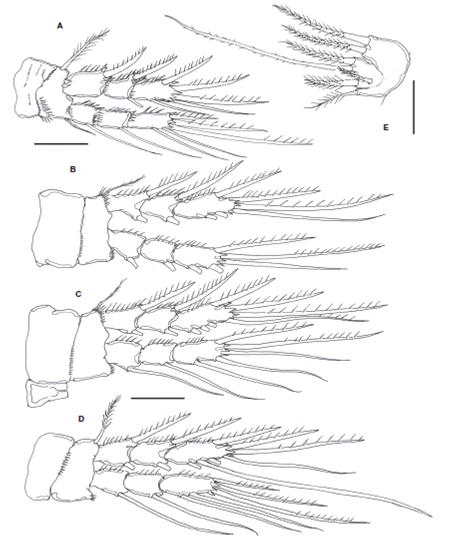
Figure 9 Pseudobradya gascae sp. nov., adult female from Colombia: A, leg 1; B, leg 2; C, leg 3; D, leg 4; E, leg 5. Scales bars: A-E= 20 μm.
Pseudobradya Sars, 1904
Type material
Adult female holotype from Laguna Navío Quebrado, La Guajira, Colombia, coll. J. Fuentes-Reinés, April-December 2012, undissected, ethanol-preserved (UARC388M). Paratypes: 1 female plus 2 females (UARC389M), partially dissected, semi-permanent slides. Additional material: 10 adult females in first author's (JF-R) personal collection. Three adult females from same locality and dates, dissected, semi-permanent slides (ECO-CHZ-09266).
Description of female
Body fusiform in dorsal view, tapering posteriorly. Total body length measured from tip of rostrum to posterior margin of caudal rami ranging from 308-350 μm (average 319.6 μm, n = 11; holotype: 308μm). Prosome representing 35% of total body length, gradually attenuating anteriorly, cephalosome robust in lateral view (Fig. 6B). Pedigerous somites with weak subacute pleural extensions (Fig. 6B). Rostrum wide, truncate, articulated at base with cephalothorax by sharp suture (Figs. 6A, B ). Cephalosome with scattered sensillae on dorsal and lateral surfaces (Fig. 7B), posterior margin of cephalosome smooth. Hyaline frill present along dorsal margin of pedigerous somites 2-4, but lateral margins unornamented. Fifth pedigerous somite with row of small spinules stretching to lateral margin and subulate hyaline frill along posterior margin (Fig. 8H). Genital double-somite and succeeding 3 urosomites each with subulate hyaline frills (Fig. 8H), but with weak ornamentation otherwise. Anal somite with row of long (inner half) and short (outer half) spinules at insertion of caudal rami (Figs. 6C, 8H ).
Caudal rami slightly broader than long, furnished with 7 setae: seta I small, stout, setae II and III about 5 times as long as seta I. Terminal setae IV and V well-developed, seta V about 1.3 times as long as seta IV, spinulose along inner margin. Seta VI 1.2 times as long as ramus. Posterior edge of rami dorsally and ventrally terminating with acuminate lappet and with adjacent distal short spinous processes (Figs. 6C, 7A , 8G ).
Antennule
Short, robust, with 5 segments. Third segment longest. Armature of antennulary segments as follows: 1(1s), 2(5s), 3(10s+(1+ae)), 4(2s), 5(5s+(2+ae)) (Fig. 8A).
Antenna
Basis short, subquadrate. ENP 2-segmented; ENP1 smooth, ENP2 with curved row of spinules proximally, lateral margin with subdistal row of spinules and 2 stout setae, segment with apical row of strong spinules and with 5 apical elements including 3 long spinulate setae, 1 short spine and 1 naked seta. EXP 3-segmented; EXP1 unadorned, unarmed, EXP2 short, with 1 seta; EXP3 as long as preceding 2 segments combined, with inner row of spinules and with 2 subequally long apical spinulose setae (Fig. 8B).
Mandible
Gnathobase well developed, with 4 small teeth and 1 long tooth on gnathal blade, plus slender dorsal seta. Basis with row of spinules and with 3 subequal setae. ENP represented by oblong segment bearing outer row of spinules plus 3 apical and 4 inner setae. EXP 1-segmented, with 2 spinulose setae (Fig. 8C).
Maxillule
Praecoxal arthrite robust, short, with 3 distal spiniform elements. Coxa short, as long as arthrite, unarmed. Basis bearing 5 setae. EXP represented by small segment with 2 stout lightly spinulated setae. ENP 1-segmented, with 6 setae (Fig. 8D).
Maxilla
Geniculate, robust; syncoxa with 3 endites; proximal and middle endites with 2 setae each; distal endite largest, with 3 setae. Allobasis with 3 setae. ENP 3-segmented, proximal segment with 2, medial segment with 2, distal segment with 3 elements (Figs. 7D, 8E ).
Maxilliped
Syncoxa cylindrical, with long spinulose seta. Basis as long as syncoxa, robust, with slender setules along outer margin, row of long spinules along opposite margin, and row of minute spinules on medial surface of segment. ENP represented by single short segment, about half the length of basis, with 4 spinulose setae (Figs. 7C, 8F ).
P1-P4
ENP and EXP 3-segmented; coxa of P1 with transverse rows of spinules on anterior surface and posterior margin, basis with semicircular row of spinules on inner proximal half, stronger spines at insertion of exopod (Figs. 7B, C ). Coxae of P2-P4 with weaker ornamentation, row of small spinules along half of distal margin. Basis of P2-P4 with distal rows of spinules near insertion of EXP and ENP (Figs. 9A- D ). Distal segments of EXP of P1-4 with distal spinous processes. Setal formula is presented in Table 2.
P5
ENP lobe almost reaching distal end of EXP, with 2 strong pinnate setae (inner seta shorter than outer seta). EXP with 4 setal elements, 3 terminal setae plus short accessory seta. Inner and outer exopodal setae pinnate, medial terminal one sparsely spinulated, noticeably long, 2.5 times as long as adjacent inner exopodal seta (Fig. 9 E).
Etymology
The species is named after Dr. Rebeca Gasca, researcher at El Colegio de la Frontera Sur-Chetumal, Mexico, for her contributions to the study of marine zooplankton of Mexico.
Remarks
These specimens were identified as belonging to the genus Pseudobradya by their possession of a combination of diagnostic characters including: 1) short antennule; 2) antennary exopod with 2-3 segments; 3) mandible basis with 2 setae, exopod with less than 5 setae; 4) geniculate, robust maxilla; 5) short maxilliped, with robust basipod; 6) legs 1-4 with 2 outer spines; 7) P5 exopod with 3 setae (Huys et al., 1996; Kihara & Huys, 2009).
We followed Wells' (2007) key as a guide to identify a group of morphologically similar species among which we can compare our specimens from Colombia. According to our observations, the setal formula of the third exopodal segment of legs 1-4 (5:6:7:7) of our specimens is shared only with P. barroisi (Richard, 1893) (see Wells, 2007). We compared additional characters with species having a similar formula (6:7:7:7), such as the presence of one seta on the second endopodal segment of P1-P4, a robust maxilliped with one syncoxal seta, and an unarmed first antennary exopodal segment. Considering these characters the new species also resembles P. robusta Sars, 1910. The new species differs from P. robusta in having 5 instead of 6 antennulary segments (Sars, 1903), in the absence of a seta on the second exopodal segment of the antenna, and the presence of 2 exopodal setae on the mandibular palp (3 in P. robusta [Sars, 1903, pl. 5]). Also, these species differ in the length of the syncoxal seta of the maxilliped; it is extremely long in P. robusta , reaching well beyond the distal margin of the appendage (Sars, 1903, pl. 5) vs. a shorter element in the new species, barely reaching the distal margin of the maxilliped. Also, P. robusta has only 3 distal elements on the maxilliped vs. 4 in the new species, although the fourth seta may be inconspicuous (Huys et al., 1996). The accessory seta on the P5 is long, naked in P. robusta (Sars, 1903, pl. 5) vs. a short, robust pinnate seta in the new species (Fig. 9E). In the new species the accessory seta arises from the exopodal surface whereas in P. robusta it is inserted more proximally, at the suture with the baseoendopod (Sars, 1903, pl. 5). In P. robusta the inner endopodal seta is clearly longer than the outer one (1.5 times) vs. a figure of 1.1 in P. gascae .
Pseudobradya gascae sp. nov. resembles also P. barroisi as redescribed by Por (1968), in the presence of 5 setal elements on P1 EXP3 (Por, 1968, Fig. 10). On the other hand, P. barroisi diverges in several characters including the presence, in P. barroisi , of a dark pigmentary spot on each of the first 2 antennulary segments (Por, 1968, Fig. 1). Such spots are absent in P. gascae sp. nov. They also differ in other important characters including the number of segments of the female antennule (7 in P. barroisi , but 5 in P. gascae ), the antennary exopod (2-segmented in P. barroisi , but 3-segmented in P. gascae sp. nov. (see footnote in Wells, 2007), the A2 EXP2 of the antenna [with strong spinules in P. gascae sp. nov. (Fig. 8B), but without ornamentation in P. barroisi (Por, 1968, Fig. 6)]. Also, the maxilliped basis is nearly as long as the syncoxa (Fig. 8F) in the new species, but about 1.5 times as long as the syncoxa in P.barroisi (Por, 1968, Fig. 9), the ornamentation of the distal margin of the P1 basis (it has a row of 2-3 strong spinules in the new species (Figs. 7B, 8A ), but it has a short row of minute spinules in P. barroisi (Por, 1968, Fig. 10)), in the caudal seta III/caudal ramus length ratio (about 1.2 in P. barroisi (Por, 1968, Fig. 3), but 2.2. in P. gascae sp. nov.), in the relative length of the P1EXP (reaching the distal margin of the P1ENP2 in P. barroisi , but of the same length in P. gascae sp. nov.), in the length of the P5EXP middle seta [2.5 times as long as adjacent inner seta in P. gascae , but noticeably shorter in P. barroisi (Por, 1968, Fig. 12)]. This character appears to be distinctive of this species, other congeners with a relatively long middle seta include P. digitata Sars, 1920 (2.2), P. pygmaea Sars, 1920 (2.0), and P. attenuata Sars, 1920 (1.8).
Distribution and ecology
Both new species, Halectinosoma arangureni and P. gascae are currently known only from the protected coastal system Navío Quebrado Lagoon, on the Caribbean coast of Colombia. This large (surface area of 10.7 km2) lagoon system is a shallow water body (depth 0.3-1.1 m), whose temperature ranges seasonally from 28 to 31 °C; pH values during the sampling period ranged from 7.8 to 8.3; salinity was 28 PSU at the time of sampling. This is the type of habitat in which these ectinosomatid genera can be recorded (Boxshall & Defaye, 2008). These species were recorded in both the limnetic region and the vegetation zones, being more frequent in the former. Pseudobradya gascae sp. nov. was particularly abundant in the surveyed area. There are only a few records of species of Halectinosoma and Pseudobradya in the Caribbean (Coull, 1970, 1971; Suárez-Morales et al., 2006). These include Halectinosoma dimorphum Coull, 1970 from Barbados, U.S. Virgin Islands, H. gothiceps (Giesbrecht, 1881) from the U.S. Virgin Island, Pseudobradya pulchera Sars, 1920 (Barbados, U.S. Virgin Islands) and Pseudobradya spec. Coull, 1971 (U.S. Virgin Islands). Both genera are recorded for the first time in Colombian waters. A total of 15 species of Harpacticoida have been recorded previously in this coastal system (Fuentes-Reinés & Suárez-Morales, 2014a, b), a figure which has increased to 17 with the addition of these 2 new species.
Acknowledgements
We are very grateful to Dr. Rony Huys (Natural History Museum, London) and Samuel Gómez (Universidad Nacional Autónoma de México, Instituto de Ciencias del Mar y Limnología, Mexico) for kindly supplying valuable taxonomic literature. Guadalupe Nieto, Laboratorio Institucional de Microscopía Electrónica at ECOSUR-Tapachula, Mexico, kindly and efficiently performed the processing of specimens for SEM analysis. Constructive comments and suggestions from two anonymous reviewers are greatly appreciated.











 nova página do texto(beta)
nova página do texto(beta)




