Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de biodiversidad
versão On-line ISSN 2007-8706versão impressa ISSN 1870-3453
Rev. Mex. Biodiv. vol.84 no.3 México Set. 2013
https://doi.org/10.7550/rmb.31913
Ecología
Nest site selection by the green turtle (Chelonia mydas) in a beach of the north of Veracruz, Mexico
Selección del sitio de anidación por la tortuga verde (Chelonia mydas) en una playa del norte de Veracruz, México
Leonel Zavaleta-Lizárraga1 and Jorge E. Morales-Mávil2*
1 Posgrado en Neuroetología, Universidad Veracruzana. Dr. Luis Castelazo Ayala s/n, Col. Industrial Ánimas, 91190 Xalapa, Veracruz, México.
2 Laboratorio Biología de la Conducta, Instituto de Neuroetología, Universidad Veracruzana. Dr. Luis Castelazo Ayala s/n, Col. Industrial Ánimas, 91190 Xalapa, Veracruz, México. *jmmavil@gmail.com
Recibido: 16 mayo 2012
Aceptado: 10 febrero 2013
Abstract
The distribution of the nests of marine turtles on beaches is one of the most important factors for hatchling success. Beaches with fine sands, moderate slopes and good humidity and drainage, are the main environmental variables for ovoposition. The objective of this investigation was to determine the nesting variations of the green turtle during which the nesting frequency per year, beach morphology and distance from the nests to the tidal line were registered. During the nesting seasons, 1 654 nests were registered on 13 beaches, among which significant differences in the number of nests per month were noted (f= 14.07; p< 0.05). Central beaches displayed major nesting probability and included beaches with short distances from the intertidal zone to the supralittoral zone. Dunes were the sites with greater preference for oviposition, being different from the sandy beaches and the intertidal zone (f=54.68; p< 0.05). Significant differences were found in the location of the nests with respect to the tidal line (t=2.33; p< 0.05), and the greatest intervals for nesting were between 10 and 24 m from the tidal line. The area displayed beaches with moderate slopes and dunes with an average distance of 22.6 m from the tidal line. This area is the most important nesting site for green turtle in the American Continent.
Key words: beach morphology, nesting density, nest distribution, sea turtles, Nautla, Veracruz, Mexico.
Resumen
La distribución y ubicación de los nidos de tortugas marinas son factores importantes para el éxito de la nidada. Las variables que facilitan la ovoposición, corresponden a playas de arena fina, pendientes moderadas y buena humedad y drenaje. El objetivo de esta investigación fue determinar las variaciones en la anidación de la tortuga verde. Se registró la frecuencia de anidación durante 3 años, así como la morfología de las playas y la distancia de los nidos a la línea de marea. Fueron registrados 1 654 nidos en 13 playas, presentando diferencias en el número de nidos por mes entre las playas (f= 14.07, p< 0.05). Las playas centrales mostraron una mayor probabilidad de anidación y corresponden a playas con distancias cortas de la zona intermareal a la zona supralitoral. Las dunas fueron los sitios con mayor ovoposición (f= 54.68, p< 0.05). Se encontraron diferencias en la ubicación de los nidos con respecto a la línea de marea (t= 2.33, p< 0.05) y el mejor intervalo para anidar fue entre 10 y 24 m. Las playas se caracterizan por pendiente moderada y dunas, con una distancia media desde la línea de marea de 22.6 m. Las playas del Raudal corresponden al sitio más importante de anidación de tortuga verde en el continente americano.
Palabras clave: morfología de playas, densidad de nidos, distribución de nidos, tortugas marinas, Nautla, Veracruz, México.
Introduction
The selection of the nesting site is defined as the laying of eggs in zones that are not chosen randomly within a certain area (Wilson, 1998). Two levels of factors affect site selection. The first is the microhabitat and includes physical factors (substrate, temperature and humidity); the second is the macrohabitat (includes factors that impact the survival of the females, reproductive success and natural or artificial depredation) (Schwarzkopf and Brooks, 1987; Janzen y Morjan, 2001; Spencer, 2002).
In the majority of oviparous species that deposit their eggs in nests, the females face a complex diversity for the election of the site. This is due, mainly, to the availability of different potential zones for nesting, which have physical factors that affect the probability of nesting success, the embryogenesis and the viability of the young to survive (Muth, 1980; Packard and Packard, 1988; Deeming and Ferguson, 1991).
The selection of a suitable nesting site is a critical aspect, which can directly influence fitness. Females that place eggs in zones with favorable characteristics for embryo development aid these to be favored by natural selection (Resetarits, 1996). In reptiles including sea turtles, that do not provide parental care, the physical conditions play a fundamental role in the success of hatchlings (Woods and Bjorndal, 2000; Broderick et al., 2003; Kamel and Morosovsy, 2005). Females may base their nest site selection on a combination of abiotic and biotic factors. Selectivity with respect to abiotic signals is evident, not only concerning the conditions tested at the site at the time of nesting, but also those signals at nearby sites (Bustard and Greenham, 1968; Ehrenfeld, 1974; Miller, 1985; Horrocks and Scott, 1991).
In the case of marine turtles, these have maintained through time a direct relation with the terrestrial environment due to the oviposition process. The nesting sites for this group, display diverse factors that influence nesting and hatching, such as beach sand physiognomy, air and sand temperature, as well as humidity and grain size (Miller, 1997; Pritchard, 1997). Green turtles are known to prefer sites with clear access from the sea and the mean particle diameter of the sand grains has been positively correlated with clutch mortality (Mortimer, 1990). Favorable positioning of the nest in the sand can reduce loss caused by inundation and depredation (Bjorndal and Bolten, 1992); also environmental factors in the surroundings are directly related to the success of hatchlings, influencing the size, growth and behavior of the neonates (Wood and Bjorndal, 2000).
Physical characteristics are important in the selection of green turtle nesting sites, as well as the relationship existing among the qualities of sand within the site (Miller, 1997). Our study identifies the importance of physical factors for the nesting success of the green turtle, as well as its preferences and the characteristics of the most relevant sites on nesting beaches. We chose the Raudal beach since it is one of the most important nesting zones on the Gulf of México and the Caribbean Sea for green turtles (Márquez, 2004). We tested the hypothesis that dunes with vegetation are the main sites selected by turtles to ensure nesting success. Female green turtles may take into account the nearby vegetation as a signal for the selection of sites with a substrate made up of fine and moist sands.
Materials and methods
Study Site. The study was conducted on the beaches of the Centro Veracruzano para la Investigación y Conservación de Tortugas Marinas del Estado de Veracruz (CVICTM), located near the town Raudal, Veracruz (Fig. 1). The area begins in its northern part at Barra de Palmas estuary (20°09' N, 96°42' W) and ends in the south at Barra Nueva estuary (20°03' N, 96°37' W). Dune vegetation is behind the beach, and consists of pioneer communities, grassland, scrub and woodlands. The vegetation consists of herbaceous and shrub species, tolerant to the drastic conditions prevailing in a mobile system where there is sand movement, wide temperature fluctuations in the sand, soil salinity and, sometimes, strong winds. Mexican laws do not yet protect the site; however, the State Government of Veracruz oversees the operation of the CVICTM. Few people live near the nesting beaches; there are only isolated houses, and the presence of poachers is not common at present. The nesting records were taken from June to October during the nesting seasons from 2005 to 2007, on 13 beaches separated by rivers and estuaries distributed along 14.5 km. These beaches were classified into 5 categories (Table 1).

We conducted beach surveys at night and during the following morning to identify those nests that we had not detected the night before (Carr, 1975; Bjorndal and Bolten, 1992). Once a nest was located it was recorded using UTM geographical coordinates using a GPS device (Garmin model 76CSx).
All the nests present along the 14.5 km of beach were counted. To improve the precision for determining beach preference, horizontal divisions were made and 3 categories were assigned: zone A, the intertidal portion of the entire coastline, ending on the last line of the tide; zone B, from the end point of the zone A, covering all the until the start of the dunes, and zone C, from the end point of zone B to the interior of the supralittoral zone. Every nest located on the beach was categorized by its position. The nesting site preference was considered according to the nest abundance in each zone. Similarly, the distance was taken from each nest to the mean high tide line and was measured with a 50 m tape. Only the years 2006 and 2007 were registered for the high tide-nest distance because this variable in the 2005 recorded nests were not considered. The nesting probability for each beach was calculated using a total nesting number. In this case, λ = 127 (mean value from the 1 654 nests) was considered for each beach. Nest density was calculated with the following equation: Nest density= No. of nests / beach area.
The beaches were classified into 5 types according to their physical form. Type I, extensive beaches from intertidal to supralittoral zone, small slope with 52m-width average, presence of costal vegetation in the supralittoral zone, beaches with a lot of tree trunks and branches. Type II, extensive beaches from intertidal to supralittoral zone, 38m-width average and low dunes with sparse vegetation. Type III, short beaches from the intertidal to supralittoral zone; moderately slop with 25m-width average and low dunes with little vegetation in the upper area. Type IV, extensive beaches from the intertidal to the supralittoral zone with high slope; 37m-width average and high dunes with vegetation into the upper area. Type V, short beaches from the intertidal to the supralittoral zone with moderate slope; 30 m-width average and high dunes covered with scrub vegetation.
All statistical analyses were made using Statistica version 6.0 (StatSoft Inc, 2001). We used Shapiro-Wilk to test data normality, all data presented normality p< 0.05. A one-way Anova was used for comparisons among monthly and annual nesting as well as the nesting site preferences. To calculate the nesting probability for each beach we used a Poisson distribution. For the beach size and the number of nest for each one the Pearson correlation was used.
Records of distances from the nests to the tidal line were grouped in 29 intervals of 2 m each, from the nearest nest registered to the farthest one. Comparisons of distances for the years 2006 and 2007 were made using t-test for independent variables and Anova for comparing between years, months and beaches.
Results
Annual nesting and density. For the period 2005-2007 we identified a total of 1 654 green turtle nests along 14.5 km of beach. The greatest number of nests appeared in 2005 with 617, whereas 501 nests appeared in 2006 and 536 nests appeared in 2007. During the 4 months in which nesting occurred (June-September), the highest number (683 nests) was registered in July, whereas the lowest number was registered in September registered with only 98 nests (Fig. 2). The annual nesting did not present statistical differences (f= 3.90, p< 0.05); however, the monthly nesting between years revealed statistical differences (f= 4.45, p< 0.05).

During the 3-year study, 65.5% of the number of nests was concentrated on 5 of the 13 beaches (Table 2). This nesting pattern showed 2 important categories, which were repeated during the 3 years of records. One zone with beaches of type 2 included extensive beaches, with a width of 38m and gradual slope, beach number 5 was in this classification. The other zone had beaches of type 5, corresponding to short beaches with a width of 30 m and a moderate slope; this classification included beaches numbers 6 and 8. It was evident that the majority of nesting activity was concentrated mainly in the center of the study area with 1 163 nests. Low nesting rates were registered on type 1 beaches (numbers 1, 2 and 13) and these correspond to extensive areas with little slope with an average width of 52m.

Statistical differences were found among the 13 nesting beaches regarding the number of nests in the 3 study years (f=14.07, p< 0.01) (Fig. 3). Four of the 13 beaches showed high nesting probabilities, and on those beaches at least 200 nests were deposited during the 3 years (Table 3). A similar probability appeared at beach 10 (0.99), although the number of nests recorded for this site was smaller. For the remaining beaches the nesting probability was more reduced, with some probabilities as low as zero.

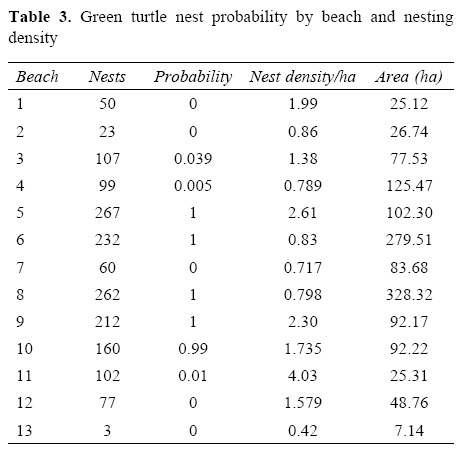
The average nesting rate over the 3 year period and the length of each beach were correlated significantly (r2= 0.701, p< 0.05). Beaches 8 and 6 had 328.32 and 279.51 nests per hectare respectively and were the beaches with the highest density in the study area. The beach with the lowest densities was 13 (Table 3). A significant positive correlation between the number of nests during the period 2005-2007 and the area of each beach in the Raudal zone was found (r = 0.55, p< 0.05).
Nesting site preferences. The females placed the greatest number of nests in the dune zone (zone C), which included 1 206 nests during the study period. For the sandy beach (zone B) the total number of nests was 419; whereas for the tidal zone area (zone A), 29 nests were located (Table 4).

There were also significant differences between green turtle nest site preferences for 2005 to 2007: (A), intertidal zone; (B), sandy beach, and (C), supralittoral (f= 56.68, p< 0.01) (Fig. 4). This can be explained by the highest preference which occurred in zone C in all months. It is important to underline that during the 3 years, zones B and C had the greatest nesting activity. The month of July had the highest number of nests in zone C, with 683, followed by August with 457. The minimum number of nests was recorded in September of each year.
The percentage of nests calculated for each zone along the beach shows that beach number 6 had 25% of the nests in zone A. Sandy beach number 5 registered 16.4%, and for zone C, beaches 5 and 8 presented 16.3% and 16.8% respectively.
All beaches showed nests in zone C, while beaches 7 and 13 no nesting was recorded in the A and B zones (Fig. 5). We found significant differences in the nesting activity by zones among the beaches (f= 54.68, p< 0.01).

Distances from nest to tidal line. During 2006 and 2007 the distances from the green turtle nests to the tidal zone were recorded. In total we recorded 1 038 nests for analysis. In both seasons, the minimum distance was 1m and the maximum was 56.8 m for 2006 and 49 m for 2007. The mean value for the distances was 20 and 19 m respectively. The distances from the nests to the tidal line during different months varied each year (t= 2.33, df = 1035, p< 0.01). August was the month with the most variation in comparison to other months (Fig. 6).
Beach 11 showed the greatest distance of 30.5m on the average, whereas beach 13 showed the maximum distance at 50 m, beach 8 had the minimum average distance with 14 m. In general, the annual average distances were 24 m for 2006 and 21 m for 2007. Most of the nests appeared within the 10 to 24 m range of distance to the tidal zone (Fig. 6). Thus, 52.8% of the nests were concentrated within 14 m in 2006 and 61.4% in 2007.
Throughout these years we found significant differences in the distances between nests (t= 2.33, df= 1035, p< 0.01). We observed differences in the average distance between nests according to the month of the year, for example in June the average distance was 17.9 m, in July and August average distance measured 20 m, whereas in September the average distance was reduced to 16 m.
Discussion
This area in the state of Veracruz on the Gulf of Mexico is one of the most important sites for green turtle nesting (Márquez, 2004). The results show that the abundance of accumulated nests in 3 years at the Raudal beach is superior to what has been reported by several authors for other sites, such as the beaches in the state of Campeche and the Yucatan Peninsula (Garcia-Vicario, 2008; National Marine Fisheries Service, 2007), and in the Caribbean Sea for sites such as the Cayman Islands and Cuba (Bell, 2007; Ferrer-Sanchez et al., 2007). The only site where the number of nests is greater corresponds to Tortuguero in Costa Rica, which has been considered one of the most important sites around the world with the greatest number of nests for this species throughout the year (Tiwari et al., 2005) (Table 5).
On the coast of the State of Florida, the number of nests of the green turtle are higher in number than those documented in this study (Meylan et al., 2006). Nevertheless, the Raudal site could exhibit nest numbers close to those registered on the western Atlantic beaches of the of the USA. This is in accordance with the numbers presented by Meylan et al. (1995, 2006) (Table 5).
In worldwide terms, the Raudal beach is also a notable place for green turtle nesting, because the number of nests is superior to those presented for the beaches of Turkey and Cyprus in the Mediterranean Sea (Broderick et al., 2002; Canbolat et al., 2002; Yalcin and Yerli, 2006), for Vietnam in Southeast Asia (Hamman et al., 2006), or even for the central Pacific Islands of Hawaii (Chaloupka et al., 2008; Balaz and Chaloupka, 2006), which are all important sites for green turtle nesting (Table 5).
The importance of the central coast of Veracruz for green turtle nesting sites may be attributed to the fact that the beaches are easily accessible from the ocean without great obstacles like rocks, pronounced slopes or coastal developments that prevent the movement of the adult females along the length and breadth of the beaches. This supports the idea that nest site selection is one of the most important factors for nesting success, since it is directly related to embryo and hatchling development (Mortimer, 1990).
Although each year an important number of nests was registered, no major variations occurred from year to year probably due to the fact that the nesting patterns in the Raudal beach area are different from those described for other important areas for this species. For example, triannual cycles on Ascension Island and in Greece are known to have one important nesting year and 2 remaining years with reductions in the nest numbers (Miller, 1997; Broderick et al., 2001).
During July and August, the months in which high temperatures (> 26° C) were recorded in the region during the 3 years of this study (Conagua 2005, 2006, 2007), presented the highest nesting numbers. This agrees with details registered at other nesting sites, such as Florida and Costa Rica in the Northern hemisphere, where the majority of Green, Loggerhead and Leatherback turtle nests were observed in the warmer months (Bjorndal et al., 1999; Weishampel et al., 2003, 2004; Antworth et al., 2006). In the case of the Southern Hemisphere, the majority of nests were recorded also in the warmer months (February to May), principally on Ascension Island in the South Atlantic (Mortimer and Carr, 1987; Godley et al., 2001; Hays et al., 2002).
For the olive ridley turtle (Lepidochelys olivacea) on the southern coasts of Baja California in the Mexican Pacific, the nesting pattern also occurs in the warmer months with most of the eggs being deposited in August and October (Garcia et al., 2003; Lopez-Castro et al., 2004). The loggerhead turtle (Caretta caretta) in Greece and Japan shows a similar pattern as well, nesting mainly in June and August (Margaritoulis, 2005; Matsuzawa et al., 2002). The foregoing pattern is probably based on favouring nesting success and embryo development during incubation (Davenport, 1997).
Therefore, the nesting patterns in the warmer months described for diverse marine turtles, among them, the green turtle of the Raudal beach area, could be related to the temperature and the humidity in the stated months, which seem to favour the development of embryos in the nest (Miller, 1985; Packard and Packard, 1988; Mortimer, 1990; Maloney et al., 1990).
In the green turtle, as in other oviparous reptile species, sex determination is influenced by the temperature in the nest (Miller and Limpus, 1981; Morreale et al., 1982). Consequently, due to the nest temperature variation during the day, temperature is an important clue that females use to select the nesting site (Mortimer, 1990).
In recent years, diverse authors have reported the sea surface temperature as a factor which also plays a part in nesting. Some authors have described the dependency of the Green and Loggerhead turtles on the surface water temperature, due to their ectothermic physiology (Sato et al., 1998). It is possible that a direct relationship between the sea surface temperature, the inter-nesting periods and the major nesting months, exists in sea turtles (Solow et al., 2002; Hays et al., 2002; Weishampel et al., 2004). In this sense, it is known that the average sea surface temperature during the months when nesting activities are highest for the green turtle and the Loggerhead is between 27-28° C (Hays et al., 2002). The preceding data indicate correlations with the sea surface temperatures registered in the study zone during these 3 years and with the major nesting months, which showed on average of 28° C (NASA, 2009).
Beaches longer than 1 300 m (except beach number 11) with gentle to medium slopes represented the most important nesting sites during the study. Specifically, for beaches 6 and 8, the dunes were found to average a height of 5 m. It is known that a positive relationship between nesting and dune beaches exists (Bouchard and Bjorndal, 2000), which means that this could be a main factor for successful nesting on our study beaches. Similarly, the afore-mentioned beaches had vegetation on their dunes, which is important for the green turtle in nest site selection (Whitmore and Dutton, 1985). The physical conditions that affect nesting areas are well known, however, there are specific conditions within them, such as width and length of the beach, the vegetation type and size of the slope of the beach that affect the probability of nesting turtle. Also, other factors may be influencing turtle decision for nesting, for example, the presence and human activity, tourism and the presence of predators, even though the physical conditions of the beaches can be appropriate for nesting.
The lowest number of nests was found on beach 7. This considerable reduction was probably due to recreational activities by the residents of nearby houses on this beach, even more so when vacation periods coincide with the major nesting months. Similar findings have been reported for green, loggerhead and leatherback turtles on the central coast of Florida, where the turtles avoid nesting on those beaches overrun with human activities (Weishampel et al., 2003).
Human activities during the day and at night, using vehicles in the nesting zones and the use of artificial light, affect the females nesting behaviour, and that of the hatchlings (Rumbold et al., 2001; Kudo et al., 2003, Peterson and Bishop, 2005). On beach 7 it was common to observe vehicles and recreational activities all day long, which could have a negative influence on the females trying to nest, thereby resulting in fewer nest numbers on the beach, even at a site having the appropriate morphological characteristics as beach length, moderate slop and dunes similar to those of beaches with high numbers of nests during the 3 years.
The low number of nests on beaches 1, 2 and 13 (with 50, 23 and 3 nests respectively) may be due to the fact that they are beaches adjacent to the estuaries of Barra de Palmas and Barra Nueva. During all the nesting periods, we observed a great amount of debris carried by the rivers, as well as vehicular traffic created by the passage of people from surrounding communities and these conditions are detrimental for females during the nesting process. On beach 12 there is an artisanal fishing camp and their activities persist until late at night and early dawn, probably causing a negative impact.
The greatest percentage of nests was located in the dune zones for all beaches. This has also been registered on the beaches of Florida, where green turtles nest in zones with dunes and beach vegetation (Witherington, 1986). Nests placed on dunes favour the embryo's development and eggs are protected from flooding; moreover the presence of roots in the nests has a direct impact on nutrients and energy interchange (Bouchard and Bjorndal, 2000).
The probability for successful nesting of the green turtle in the Raudal area is directly related to the formation of its beaches. The observations in this study show that the beach chosen by the female must have extensions of no more than 20 m in the distance from the sea to the supralittoral zone. It is also important that dunes with low slopes and vegetation are present. According to the number of nests per kilometre (ratio of nesting), the study area is also relevant. After the beaches of Ascension Island and Tortuguero, the ones at Raudal have a higher number of nests per kilometre than those recorded in Campeche, Yucatán, the Gulf of Mexico in Florida, the Cayman Islands in the Caribbean, as well as, Cyprus and Turkey in the Mediterranean. In the study zone, the nests were most frequently placed within the 10-24 m interval of distance from the tidal line. There is little information on the average distance at which green turtles nest (Hays and Speakman 1993; Hays et al., 1995). However, the nest site location is directly related to the beach characteristics. In other sea turtle species, like Caretta caretta, it is known that the nesting average is 21 m distant from the tidal zone (Wood and Bjorndal, 2000). One of the advantages of nesting at this distance is to avoid flooding due to the tides. In addition, the sand allows good nest ventilation and high moisture thus avoiding egg desiccation (Ackerman, 1980; Maloney et al., 1990).
In the case of Lepidochelys olivacea, in contrast to this study, it has been reported that nesting success occurs between 20 and 30 meters from the tidal zone (López-Castro et al., 2004). For Eretmochelys imbricata, however, nesting has been registered at an average distance of 8 m from the tidal zone (Horrocks and Scott, 1991). Thus, it was possible to observe that both the seasonal features and the beach characteristics, horizontally and vertically, are relevant factors that have a direct influence on females in the selection of nesting sites, as well as, ambient temperature, relative humidity, beach slope (Wood and Bjorndal, 2000), sand temperature (Hays et al., 2001; Matsuzawa et al., 2002; Wallace et al., 2004; Van de Merwe et al., 2006), sea surface temperature (Hays et al., 2002; Solow et al., 2002; Weishampel et al., 2004), tide behaviour (Lamont and Carthy, 2007) and human activity.
In general, our study shows the importance of the Raudal beach area for green turtle nesting sites, due to the fact that the number of nests remained similar during the 3 years of study. In spite of the fact that in Mexico the national laws protect sea turtles, the protection and surveillance programs are not achieving their purpose of protecting the nesting sites or ensuring the conservation of these reptiles. Similarly, unplanned coastal development, as well as irresponsible tourism activities have a negative impact on the nesting, hatching and population dynamics of these turtles (Arianoutson, 1988; Broderick and Godley, 1996).
Acknowledgments
We thank Jazmin Cobos-Silva and María Esther Nava-Bringas for their assistance in the field, the Coordinación General de Medio Ambiente for permission to conduct our work at Raudal and Warren Haid and Anais Horden for her generous assistance in the English review. We thank Laura T. Hernández-Salazar, Alberto González-Romero y Arturo Serrano-Solís for helpful comments on the manuscript. The first named author had a Conacyt scholarship grant No.165146 for this research. Part of the research was supported by Fomix (VER-2008-C01-109460).
Literature cited
Ackerman, R. A. 1980. Physiological and ecological aspects of gas exchange by sea turtle eggs. American Zoologist 20:575-583. [ Links ]
Antworth, R. L., D. A. Pike and J. C. Stiner. 2006. Nesting ecology, current status, and conservation of sea turtle on an uninhabited beach in Florida, USA. Biological Conservation 130:10-15. [ Links ]
Arianoutsou, M. 1988. Assessing the impacts of human activities on nesting of loggerhead sea-turtles (Caretta Caretta L.) on Zakynthos island, western Greece. Environmental Conservation 15:327-334. [ Links ]
Balazs, G. H. and M. Chaloupka. 2006. Recovery trend over 32 years at the Hawaiian Green turtle rookery of French Frigate Shoals. Atoll Research Bulletin 543:147-158. [ Links ]
Bell, C. D., J. L. Solomon, J. M. Blumenthal, T. J. Austin, G. Ebanks-Petrie, A. C. Broderick and B. J. Godley. 2007. Monitoring and conservation of critically reduced marine turtle nesting populations: lessons from the Cayman Islands. Animal Conservation 10:39-47. [ Links ]
Bjorndal, K. A. and A. B. Bolten. 1992. Spatial distribution of Green turtles (Chelonia mydas) nests at Tortuguero, Costa Rica. Copeia 1992:45-53. [ Links ]
Bjordal, K. A., J. A. Wetherall, A. B. Bolten and J. A. Mortimer. 1999. Twenty-six years of Green turtle nesting at Tortuguero, Costa Rica: an encouraging trend. Conservation Biology 13:126-134. [ Links ]
Bouchard, S. S. and K. A. Bjorndal. 2000. Sea turtles as biological transporters of nutrients and energy from marine to terrestrial ecosystems. Ecology 81:2305-2313. [ Links ]
Broderick, A. C. and B. J. Godley. 1996. Population and nesting ecology of the Green turtle (Chelonia mydas) and loggerhead turtle (Caretta caretta) in northern Cyprus. Zoology in the Middle East 13:27-46. [ Links ]
Broderick, A. C., B. J. Godley and G. C. Hays. 2001. Trophic status drives inter-annual variability in nesting numbers of marine turtles. Proceedings of the Royal Society Biological Science 268:1481-1487. [ Links ]
Broderick, A. C., F. Glen, B. J. Godley and G. C. Hays. 2002. Estimating the number of Green and loggerhead turtles nesting annually in the Mediterranean. Oryx 36:227-235 p. [ Links ]
Broderick, A. C., F. Glen, B. J. Godley and G. C. Hays. 2003. Variation in reproductive output of marine turtles. Journal Experimental Marine Biology Ecology 288:95-109. [ Links ]
Bustard, H. R. and P. M. Greenham. 1968. Physical and chemical factors affecting hatching in the Green sea turtle, Chelonia mydas. Ecology 49:269-276. [ Links ]
Canbolat, A. F. 2004. A review of sea turtle nesting activity along the Mediterranean coast of Turkey. Biological Conservation 116:81-91. [ Links ]
Carr, A. 1975 The Ascension Island Green turtle colony. Copeia 1975:547-555. [ Links ]
Chaloupka, M., T. M. Work, G. H. Balazs, S. K. K. Murakawa and R. Morris. 2008. Cause-specific temporal and spatial trends in Green sea turtle strandings in the Hawaiian Archipelago (1982-2003). Marine Biology 154:887-898. [ Links ]
Chan, E. H. 2006. Marine turtles in Malaysia: on the verge of extinction? Aquatic Ecosystems Health and Management 9:175-184. [ Links ]
Conagua (Comisión nacional del agua). 2005. Ficha temperaturas media año 2005. Xalapa, Veracruz. [ Links ]
Conagua (Comisión nacional del agua). 2006. Ficha temperaturas media año 2006. Xalapa, Veracruz. [ Links ]
Conagua (Comisión nacional del agua). 2007. Ficha temperaturas media año 2007. Xalapa, Veracruz. [ Links ]
Davenport, J. 1997. Temperature and life-history strategies of sea turtles. Journal of Thermal Biology 22:479-488. [ Links ]
Deeming, D. and M. W. J. Ferguson. 1991. Physiological effects of incubation temperature on embryonic development in reptiles and birds. In Egg incubation: its effects on embryonic development in birds and reptiles, D. C. Deeming, and M. W. J. Ferguson (eds.). Cambridge University Press, Cambridge. p. 147-172. [ Links ]
Ehrenfeld, D. 1974. Conserving the edible sea turtle: can mariculture help? American Scientist 62:23-31. [ Links ]
Ferrer-Sánchez, Y., R. Díaz-Fernández and R. Díaz-Fernández. 2007. Características de la anidación de la tortuga verde Chelonia mydas (Testudinata, Cheloniidae) en la playa Caleta de los Piojos, Cuba, a partir de marcaciones externas. Animal Biodiversity and Conservation 30:211-218. [ Links ]
García A., G. Ceballos and R. Adaya. 2003. Intensive beach management as an improved sea turtle conservation strategy in Mexico. Biological Conservation 111:253-261. [ Links ]
García-Vicario, A. 2008. Impacto de la erosión costera de las playas del litoral de Campeche, México, sobre la anidación de las tortugas marinas (Eretmochelys imbricata y Chelonia mydas). Thesis. Facultad de Ciencias Biológicas, Universidad Autónoma del Estado de Morelos. Cuernavaca. 89 p. [ Links ]
Godley, B. J., C. Broderick and G. C. Hays. 2001. Nesting of Green turtles (Chelonia mydas) at Ascension Island, South Atlantic. Biological Conservation 97:151-158. [ Links ]
Hamann, M., C. T. Cuong, N. D. Hong, P. Thuoc and B. T. Thuhien. 2006. Distribution and abundance of marine turtles in the Socialist Republic of Viet Nam. Biodiversity and Conservation 15:3703-3720. [ Links ]
Hays, G. C. and J. R. Speakman. 1993. Nest placement by loggerhead turtles Caretta caretta. Animal Behavior 45:47-53. [ Links ]
Hays, G. C., A. Mackay, C. R. Adams, J. A. Mortimer, J. R. Speakman and M. Boerema. 1995. Nest Site Selection by Sea Turtles. Journal of the Marine Biological Association of the United Kingdom 75:667-674 p. [ Links ]
Hays, G. C., J. S. Ashworth, M. J. Broderick, D. R. Emery, B. J. Godley, A. Henwood and E. L. Jones 2001. The importance of sand albedo for the thermal conditions on sea turtle nesting beaches. Oikos 93:87-94. [ Links ]
Hays, G. C., A. C. Broderick, F. Glen, B. J. Godley, J. D. R. Houghton and J. D. Metcalfe. 2002. Water temperature and internesting intervals for the loggerhead (Caretta caretta) and Green (Chelonia mydas) sea turtles. Journal of Thermal Biology 27:429-432. [ Links ]
Horrocks, J. A. and N. M. Scott. 1991. Nest site location and nest success in the hawksbill turtle (Eretmochelys imbricata) in Barbados, West Indies. Marine Ecology Progress Series 69:1-8. [ Links ]
Janzen, F. J. and C. L. Morjan. 2001. Repeatability of microenvironment- specific nesting behaviour in a turtle with environmental sex determination. Animal Behavior 62:73-82. [ Links ]
Kamel, S. J. and N. Mrosovsky. 2005. Repeatability of nesting preferences in the hawksbill sea turtle, Eretmochelys imbricata, and their fitness consequences. Animal Behaviour 70:819-828 pp. [ Links ]
Kudo, H., A. Murakami and S. Watanabe. 2003. Effects of sand hardness and human beach use on emergence success of loggerhead sea turtles on Yakushima Island, Japan. Chelonian Conservation and Biology 4:695-696. [ Links ]
Lamont, M. M. and R. R. Carthy. 2007. Response of nesting sea turtles to barrier island dynamics. Chelonian Conservation and Biology 6:206-212. [ Links ]
López-Castro, M. C., R. Carmona and W. J. Nichols. 2004. Nesting characteristics of the olive Ridley turtle (Lepidochelys olivacea) in Cabo Pulmo, southern Baja California. Marine Biology 145:811-820. [ Links ]
Maloney, J. E., C. Darian-Smith, Y. Takahashi and C. J. Limpus. 1990. The environment for development of the embryonic loggerhead turtle (Caretta caretta) in Queensland, Copeia 1990:378. [ Links ]
Margaritoulis, D. 2005. Nesting activity and reproductive output of Loggerhead Sea Turtles, Caretta caretta, over 19 Seasons (1984-2002) at Lagunas Bay, Zakynthos, Greece: the largest rookery in the Mediterranean. Chelonian Conservation and Biology 4:916-929. [ Links ]
Márquez, R. 2004. Las tortugas marinas del Golfo de México. Abundancia, distribución y protección. In Diagnostico ambiental del Golfo de México, I. M. Pisanty y E. Ezcurra (eds.). Instituto Nacional de Ecología, México, D. F. p. 173-197. [ Links ]
Matsuzawa, Y., K. Sato, W. Sakamoto and K. A. Bjorndal. 2002. Seasonal fluctuations in sand temperature: effects on the incubation period and mortality of loggerhead sea turtle (Caretta caretta) pre-emergent hatchlings in Minabe, Japan. Marine Biology 140:639-646. [ Links ]
Meylan, A., B. Schroeder and A. Mosier. 1995. Sea turtle nesting activity in the State of Florida 1979-1992. Florida Marine Research Publications 52:1-51 pp. [ Links ]
Meylan, A. B., B. E. Witherington, B. Brost, R. Rivero, and P. S. Kubilis. 2006. Sea turtle nesting in Florida, USA: assessments of abundance and trends for regionally significant populations of Caretta, Chelonia, and Dermochelys. In Book of abstracts. Twenty-sixth Annual Symposium on Sea Turtle Biology and Conservation, M. Frick, A. Panagopoulou, A. F. Rees and K. Williams (compilers). International Sea Turtle Society, Athens. p. 306-307. [ Links ]
Miller, J. D. and C. J. Limpus. 1981. Incubation period and sexual differentiation in the Green Turtle Chelonia mydas L. Proceeding of the Melbourne Herpetological Symposium, C. B. Banks and A. A. Martin (eds.). The Zoological Board of Victoria, Melbourne. p. 66-73. [ Links ]
Miller, J. D. 1985. Embryology of marine turtles. In Biology of the reptilian, volume 14, A, Gans, C. Billett and P. F. A. Madreson (eds.). Wiley-Interscience, New York. 269 p. [ Links ]
Miller, J. D. 1997. Reproduction in sea turtles. In The Biology of Sea Turtles, P. L. Lutz and J. A. Musick (eds.). CRC Press, Boca Raton. p. 51-81. [ Links ]
Morreale, S. J., G. J. Ruiz, J. R. Spotila and E. A. Standora. 1982. Temperature-dependent sex determination: current practices threaten conservation of sea turtles. Science 216:1245-1247. [ Links ]
Mortimer, J. A. and A. Carr. 1987. Reproduction and migration of the Ascension Island Green turtle (Chelonia mydas). Copeia 1987:103-113. [ Links ]
Mortimer, J. A. 1990. The influence of beach sand characteristics on the nesting behavior and clutch survival of Green turtle (Chelonia mydas). Copeia 1990:802-817. [ Links ]
Muth, A. 1980. Physiological ecology of desert iguana (Dipsosaurus dorsalis) eggs: temperature and water relations. Ecology 61:1335-1343. [ Links ]
National Marine Fisheries Service Office of Protected Resources Silver Spring, Maryland and U.S. Fish and Wildlife Service Southeast region Jacksonville Ecological Services field office Jacksonville, Florida, 2007. Green sea turtle (Chelonia mydas) 5-year review: summary and evaluation. 102 p. [ Links ]
NASA (National Aeronautics and Space Administration). 2009. Goddard Earth Sciences (GES), Data and Information Services Center (DISC). http://reason.gsfc.nasa.gov/Giovanni/; last access: 23.XII.2011. [ Links ]
Packard, G. C. and M. J. Packard. 1988. The physiological ecology of reptilian eggs and embryos. In Biology of the Reptilia, volume 16, C. Gans and R. B. Huey (eds.). Alan R. Liss, New York. p. 162-229. [ Links ]
Peterson, C. H. and M. J. Bishop. 2005. Assessing the environmental impacts of beach nourishment. BioScience 55:887-896. [ Links ]
Pritchard, P. 1997. Evolution, phylogeny and current status. In The biology of sea turtles, P. L. Lutz and J. A. Musick (eds.). CRC Press, Boca Raton. p. 1-28. [ Links ]
Resetarits, W. J. 1996. Oviposition site choice and life history evolution. American Zoologist 36:205-215. [ Links ]
Rumbold, D. G., P. W. Davis and C. Perretta. 2001. Estimating the effect of beach nourishment on Caretta caretta (loggerhead sea turtle) nesting. Restoration Ecology 9:304-310. [ Links ]
Sato, K., Y. Matsuzawa, H. Tanaka, T. Bando, T. S. Minamikawa, W. Sakamoto and Y. Naito. 1998. Internesting intervals for loggerhead turtles, Caretta caretta, and Green turtles, Chelonia mydas, are affected by temperature. Canadian Journal of Zoology 76:1651-1662. [ Links ]
Schwarzkopf, L. and R. J. Brooks.1987. Nest site selection and offspring sex ratio in painted turtles, Chrysemys picta. Copeia 1987:53-62. [ Links ]
Spencer, R. J. 2002. Experimentally testing nest site selection: fitness trade-offs and predation risk in turtles. Ecology 83:2136-2144. [ Links ]
Solow, A. R., K. A. Bjorndal and A. B. Bolten. 2002. Annual variation in nesting numbers of marine turtles. The effect of sea surface temperature on re-migrations intervals. Ecology Letters 5:742-746. [ Links ]
StatSoft, Inc. 2001. Statistica, vers. 6. Tulsa, Oklahoma, USA. [ Links ]
Tiwari, M., K. A. Bjorndal, A. B. Bolten and B. M. Bolker. 2005. Intraspecific application of the mid-domain effect model: spatial and temporal nest distributions of Green turtles, Chelonia mydas, at Tortuguero, Costa Rica. Ecology Letters 8:918-924. [ Links ]
Van De Merwe, J., K. Ibrahim and J. Whittier. 2006. Effects of nest depth, shading, and metabolic heating on nest temperature in sea turtle hatcheries. Chelonian Conservation and Biology 5:210-215. [ Links ]
Wallace, B. P., P. R. Sotherland, J. R. Spotila, R. D. Reina, B. F. Franks and F. V. Paladino. 2004. Biotic and abiotic factors affect the nest environment of embryonic leatherback turtles, Demochelys coriacea. Physiological and Biochemical Zoology 77:423-432. [ Links ]
Weishampel, J. F., D. Bagley, L. M. Ehrhart and B. L. Rodenbeck. 2003. Spatiotemporal patterns of annual sea turtles nesting behaviors along an East Central Florida beach. Biological Conservation 110:295-303. [ Links ]
Weishampel, J. F., D. Bagley and L. M. Ehrhart. 2004. Earlier nesting by Loggerhead Sea turtles following sea surface warming. Global Change Biology 10:1-4. [ Links ]
Whitmore, C. P. and P. H. Dutton. 1985. Infertility, embryonic mortality and nest-site selection in the leatherback and Green sea turtles in Suriname. Biological Conservation 34:251-272. [ Links ]
Wilson, D. 1998. Nest-site selection: microhabitat variation and its effects on the survival of turtle embryos. Ecology 79:1884-1892. [ Links ]
Witherington, B. E. 1986. Human and natural causes of marine turtle clutch and hatchling mortality and their relationship to hatchling production on an important Florida nesting beach. Masters dissertation. University of Central Florida. Orlando, Florida. 282 p. [ Links ]
Wood, D. and K. Bjordal. 2000. Relation of temperature, moisture, salinity, and slope to nest site selection in Loggerhead Sea Turtles. Copeia 2000:119-128. [ Links ]
Yalçin-Özdilek, Ü. and S. V. Yeril. 2006. Green Turtle (Chelonia mydas) nesting and habitat threats at Samandag Beach, Turkey. Chelonian Conservation and Biology 5:302-305. [ Links ]














