Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de biodiversidad
versão On-line ISSN 2007-8706versão impressa ISSN 1870-3453
Rev. Mex. Biodiv. vol.84 no.3 México Set. 2013
https://doi.org/10.7550/rmb.33019
Ecología
Fish community structure dynamics in cenotes of the Biosphere Reserve of Sian Ka'an, Yucatán Peninsula, Mexico
La estructura de la comunidad de peces en cenotes de la Reserva de la Biosfera de Sian Ka'an, península de Yucatán, México
Teodiceldo Camargo-Guerra, Luis H. Escalera-Vázquez and Luis Zambrano*
Departamento de Zoología, Instituto de Biología, Universidad Nacional Autónoma de México. Apartado postal 70-153, 04510 México, D. F., México. *zambrano@ibiologia.unam.mx
Recibido: 03 septiembre 2012
Aceptado: 11 marzo 2013
Abstract
Cenotes are permanent aquatic systems formed by geomorphologic processes (karst), located in the Yucatan Peninsula. Many of these oligotrophic systems are connected superficially to wetlands during the wet season. We describe the fish community structure in 4 cenotes during the rainy and dry seasons over a 4-year period and relate it to limnetic dynamics in each cenote. We sampled cenotes to describe morphology, water physicochemical characteristics, primary production, and invertebrates and fish abundance and richness. We did not find differences in physicochemical variables between seasons but we did find differences among years and among cenotes. We found 11 fish species (25% of the total richness reported for the Biosphere Reserve of Sian Ka'an) from 5 families: Cichlidae, Poeciliidae, Characidae, Pimelodidae and Synbranchidae. We noted little seasonal or inter-annual variation of fish species richness. But there were higher differences of fish species richness and abundances among cenotes. Fish community structure was correlated with temperature, transparency, pH, dissolved oxygen, chlorophyll a, periphyton and zooplankton abundance. Results showed that physicochemical variables and fish community structure are not affected by seasonal hydrology dynamics, suggesting that cenotes are stable aquatic systems compared to the surrounding wetlands. Other limnetic factors such as cenote morphology are also related to fish community structure.
Key words: karstic systems, wetlands, geomorphology, macrophytes.
Resumen
Los cenotes son sistemas acuáticos formados por procesos geomorfológicos cársticos en la península de Yucatán. Muchos de estos sistemas oligotróficos se conectan con los humedales a nivel superficial en la temporada de lluvia. Describimos la estructura de la comunidad de peces en 4 cenotes durante las temporadas de lluvias y secas en un periodo de 4 años y lo relacionamos con su dinámica. Describimos la morfología, características fisicoquímicas, producción primaria, abundancia y riqueza de invertebrados y peces. No encontramos diferencias en las variables fisicoquímicas entre temporadas, pero sí entre años y cenotes. Encontramos 11 especies de peces (25% de la riqueza total registrada para la Reserva de la Biosfera de Sian Ka'an) de 5 familias: Cichlidae, Poeciliidae, Characidae, Pimelodidae and Synbranchidae. No existe ninguna variación interanual o temporal en la riqueza de especies, pero hubo diferencias mayores en su abundancia y su riqueza entre cenotes. La estructura de la comunidad de peces está correlacionada con temperatura, transparencia, pH, oxígeno disuelto, clorofila a, abundancia de perifíton y zooplancton. Los resultados indican que las variables fisicoquímicas no afectan la estructura de la comunidad de peces en cada cenote, ni tampoco están relacionados con la dinámica hidrológica de las temporadas, comparados con los humedales. Esto sugiere que son sistemas acuáticos más estables. La morfología del cenote también está altamente relacionada con la estructura de la comunidad de peces.
Palabras clave: sistemas cársticos, humedales, geomorfología, macrofitas.
Introduction
Cenotes are the most abundant permanent aquatic systems in the Yucatán Peninsula, Mexico. Cenotes are systems formed by geomorphological processes (karst). Subterranean rivers slowly dilute the limestone formed by CaCO3 in particular areas, until the roof collapses, generating a new lake (Perry et al., 1995; Steinich, 1996). Carbonates are captured and cause precipitation of phosphorous diluted in the water column to the sediment. This process produces a lack of nutrients for phytoplankton, making cenotes oligotrophic. Cenotes can have different sizes and external morphologies. Many of them are bucket-shaped, and have vertical limestone walls without shore areas (Schmitter-Soto et al., 2002). Other cenotes have shapes resembling a typical deep lake with a shore area, allowing primary producers to establish on the perimeter in shallow areas (Schmitter-Soto et al., 2002).
Cenotes are located from the central region to the coastal area of the Yucatán Peninsula, many of them on the western Caribbean wetlands. This area is characterized by periodic drying and flooding events (Collinson et al., 1995; Schwartz and Jenkins, 2000), which can cause extensive mortality of fish in the dry season (Loftus and Kushlan, 1987; Trexler et al., 2005), but in wet seasons the expansion of the aquatic habitat can create new habitats for fish survivors (Galacatos et al., 2004).
Seasonal changes in wetland hydrology are related to the fish community structure (Escalera-Vázquez and Zambrano, 2010). The most abundant fish in the Reserve are Poeciliids (e.g., Gambusia sp., Poecilia sp., and Xiphophorus maculatus) and Cichlids (e.g., Cichlasoma friedrichsthalii and C. octofasciatum). Other species are abundant, such as the Caracid (Astyanax aeneus) and 2 of the top predators in terms of biomass, Pimelodid (Rhamdia guatemalensis) and the Synbranchid (Ophisternon aenigmaticum). All of these species can survive in highly variable environments, and some of them (i.e., most of the poeciliids) are primary consumers with the capacity to invade new aquatic systems during the early rainy season. All of these factors create a dynamic fish community highly related to changes on environmental factors (i.e. temperature, depth, pH and macrophytes coverage) during the wet and dry seasons (Escalera-Vázquez and Zambrano, 2010).
Unlike wetlands, cenotes seem to be more hydrologically stable, with low water level fluctuations between wet and dry seasons, and they never become dry (Schmitter-Soto et al., 2002). Changes in fish community structure related to environmental variables have been mentioned in the aquatic literature, regarding seasonal aquatic habitats. However, little attention has been given to stable aquatic systems, regarding the factors structuring fish communities in these habitats.
Therefore, the fish community structure in stable aquatic habitats (such as cenotes) can be highly related to morphological variables such as the shore slope and the composition of the walls of aquatic systems, which normally are not considered in these studies. These variables have a direct influence on the water column in abiotic factor such as temperature, pH, turbidity, and dissolved oxygen. In addition, the shore slope influences habitat complexity for fishes (Willis et al., 2005).
The morphology also affects the survival capacities of primary producers. The presence of the primary producers is capable of modifying the food web structure (Cohen, 1989). Macrophytes are not only a source for zooplankton, snails and aquatic insects, but also refuge providers for animals, including fish (Grenouillet et al., 2001; Grenouillet et al., 2002). The phytoplankton plays a central role in the pelagic food chain, which has been described in theories involving dynamics controlled by bottom-up or top-down processes (Lampert and Sommer, 1997; Currie et al., 1999). These dynamics suggest that algae can be the central driving force of processes in fish community structure. The morphological contrasts between cenotes can help us to understand differences in fish community structure. These systems are within the same area that is constantly inundated in rainy seasons. Therefore, all fish species from a particular region are capable of colonizing all the aquatic systems (Bedoya, 2005).
Morphologically, cenotes are shaped by geological processes (Perry et al., 1995; Steinich, 1996), combining mechanisms spanning thousands of years, starting with the formation of a cave by CaCO3 dissolution from a subterranean river. The roof collapses due to the constant dissolution of CaCO3, generating a cenote with no shore area. After continuous sediment infilling from the surroundings, the cenote shape becomes more similar to a typical lake (Perry et al., 1995; Stoessell et al., 1993; Steinich, 1996). Consequently, cenotes may have similar size but have different shapes and contrasting morphologies, a characteristic which depends on the age of the cenote (Perry et al., 1995; Steinich, 1996). Also, fish community structure in wetlands is also influenced to inter-annual variation, caused by meteorological process such as hurricane periodicity or extremely long rainy periods. It is possible that these large-scale processes have also an influence on the fish community structure in cenotes.
The aim of this study is to describe fish community structure in 4 cenotes over a 4 year period, discussing the possible implications of the hydrological stability within and among years in this structure. We will discuss the results of the fish community structure based on the differences in morphology of the cenotes.
Materials and methods
The study was conducted at the Biosphere Reserve of Sian Ka'an (18°54'00" N, 87°24'35" W) and at the Santa María locality (21°06'48.39" N, 87°10'37.32" W) Quintana Roo, Mexico (Fig. 1), at a maximum elevation of 10 m asl. Annual temperature is 24-28° C, and annual precipitation is 1 300-2 000 mm. We sampled 4 cenotes: Cenote Tres Reyes (TR); Cenote Santa María (SM); Cenote Límite (CL) and Cenote Norte (CN) (Table 1) from 2004 to 2008. Each cenote was visited 3 times per year (except for the years 2004 and 2008, which were sampled only once), covering each rainy (June to November) and dry (January to April) season, during 4 years. Eleven visits were made to each cenote, except in the Santa María cenote with only 9 visits. From a morphological perspective sampled cenotes are characteristic of Sian Ka'an Biosphere Reserve: 2 of the sampled cenotes had a typical lake-shape and had a shore area, and 2 had a typical bucket-shape with vertical walls from the surface to the bottom. Cenotes, which have characteristics such as rocky edges and direct communication with the water table, are considered to be in early stages of formation. Cenotes, formed by the accumulation of organic matter and a reduced communication with the water table, are considered in their final stage (Steinich, 1996). In this aging process of cenotes, the biotic and abiotic conditions change significantly. Variables, such as nutrient concentration, temperature and macrophytes coverage can change, depending on the presence and length of the shore area. For example, in non-shored cenotes nutrients settle directly to the bottom, out of reach from the primary producers. As a result, the autochthonous energy flow could be smaller than in other tropical systems (Schmitter-Soto et al., 2002). In shored cenotes, precipitated phosphorous moves to the shallow silt and can be used by periphyton, macroalgae and aquatic plants (Schmitter-Soto et al., 2002).
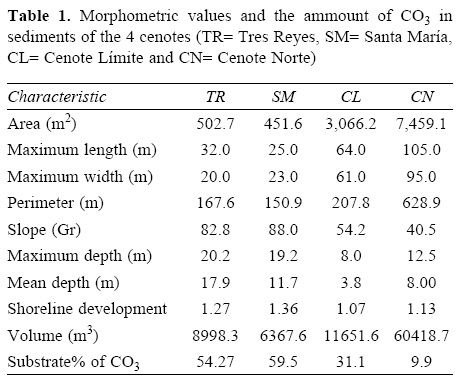
Each cenote was morphologically characterized based on methods described by Torres-Orozco and García-Calderón (1995), using the area (m2), maximum length of the cenote (m), maximum width (m), perimeter (m), maximum depth (m), mean depth (m), shoreline development and volume (m3). Slope shape of the cenote was obtained by calculating the means of 2 perpendicular bathymetric profiles.
In each visit 2 profiles of water column variables (e.g., temperature °C, dissolved oxygen mg 1-1, pH, salinity and turbidity mS cm-1) were obtained in situ at each cenote with a multi-parameter sensor (YSI 6600, Yellow Springs, USA). Transparency (m) values were obtained using a Secchi disk. A water sample was obtained from each site per season to determine the nutrient concentrations of the phosphates (using the technique of ascorbic acid reduction), nitrates (using the technique of zinc reduction), ammonium (using Nesslerization) and silica (using molybdate; Apha, 1998) with a field colorimeter (La Motte Smart, Chestertown, USA). Organic material and concentration of CaCO3 were obtained using the lost matter through ignition method (Bengtsson and Enell, 1986).
We measured phytoplankton abundance by determining the concentrations of chlorophyll a concentrations in situ at: 1, 3 and 5 meters depth (3 samples per each depth) using the field fluorine meter (Turner Designs Aquafluor). During the same sampling period, the benthic periphyton productivity in the water column was quantified with 5 wooden charts (2 x10 x 10 cm) positioned at each cenote. Two charts were placed in the center of the cenote at depths of one and 3 meters depth, and the third chart was positioned close to the shore edge at a depth of 0.7 m. These charts were checked after 4 months by collecting and weighing the periphyton-wet mass according to the method described in Biggs (2000). To quantify the surface covered by primary producers (periphyton, macroalgae and aquatic plants), 5 transects were established per cenote over the 2 sampling seasons. Since macrophyte communities did not vary in the first 2 years, we assumed this variable as a constant and did not sample during the following years. These transects were positioned in a radial configuration from the edge to the center of the cenote. The transect size varied depending on the dimensions of each cenote. On each transect, quadrats (1 m2) were used to measure the percent coverage of macrophytes. The number of quadrants per transect varied depending on the cenote dimensions. Plants were identified using Lot et al. (1998).
At each cenote, we acquired 5 compound samples to determine zooplankton abundance. These samplings were obtained by filtering 12 liters of water with 43 |i mesh size nets of at 3 different depths (1, 3 and 5 m). The samples were fixed with 4% formalin. A subsample of 3 ml was used to count and classify the organisms into rotifers, copepods, cladocerans, ostracods and insects according to Barnes (1996).
The aquatic insects were sampled over a 3-year sampling period with a spoon net (0.25 mm mesh size), randomly dipping 12 times per cenote for each season in the shore area. The samples were counted and classified to genus using the taxonomic keys of Cranston and Daly (2008), Courtney and Merritt (2008), Ferrington et al. (2008), McCafferty et al. (1997), Merritt and Webb (2008), Novelo-Gutiérrez (1997a, 1997b), Polhemus (2008), Tennessen (2008), Wallace and Walker (2008), Waltz and Burian (2008) and White and Roughley (2008).
On each visit, fish were sampled in both wet and dry seasons. To increase the capture probabilities, 2 types of minnow traps were used: 6 traps (20 x 20 x 40 cm, 5 cm funnel size opening) and 6 traps of 20 cm x 30 cm, 2.5cm funnel size opening. In each cenote, 12 traps were placed in the water for 6-hours and checked every 2 hours. The traps were set in the morning (8-11 am) and in the afternoon (4-7 pm) when fish are more active (personal observation). The traps were placed at 2 depths, 10 cm and 40 to 70 cm, in different areas of the cenote. A pilot study suggested that the fish were more likely to be captured near the shore area; therefore, most of the traps were placed there. However, 25% of the traps were placed in areas close to the center of the cenotes. Other fishing techniques were used, such as gill nets (2.4 m in width x 38 m in length) of different mesh sizes (1, 1.5, 2, 2.5 and 3 inches). Fishhooks were also used. These last 2 techniques represented less than 3.4% of the total number of fish caught and less than 6.1% of the total weight of the fish captured. Based on these proportions these data were excluded from the analyses. All of the sampled fish were preserved in a 10% formalin solution (Schreck and Moyle, 1990) and identified according to Schmitter-Soto (1998) and Greenfield and Thomerson (1997).
We used multivariate analysis of variance (MANOVA) to test for differences between cenotes and seasonality in terms of physiocochemical (temperature, conductivity, dissolved oxygen, pH, ammonium, nitrate, phosphate, silica and transparency) and biological (chlorophyll a, periphyton productivity, macrophytes, zooplankton, aquatic insects and fish) parameters. We also used pairwise multiple comparisons (Tukey test) to separate cenotes according to these parameters. To ordinate the abiotic data of the cenotes for all of the samples, we used principal component analysis (PCA).
To analyze fish community structure, we normalized all of the relative abundance data using the fourth square root transformation. Non-metric multidimensional scaling (NMDS) was used as an ordination procedure for fish communities, using ranked Bray-Curtis dissimilarity distances. This procedure is not susceptible to problems associated with zero truncation. We used the fish abundance in each cenote to evaluate the percentage contribution of each species to the fish community, and ANOSIM to test differences between fish communities among cenotes, seasons and years (Zar, 1999).
To understand the relationship between abiotic variables and fish community structure, we correlated the PCA scores with the NMDS axis values. We correlated these axis values for each of the abiotic and biotic variables. In addition, a multiple regression was performed using total fish abundance and species of each sampling from the 4 cenotes with its corresponding biotic and abiotic variables. PCA, NMDS and species contribution analyses were performed with the software Primer 5.2.9 for Windows (Primer-Ltd, Plymouth, UK.). Although the samples were in different months, we clustered these into seasons to generate a time series analysis, which tests trends along sampled years of fish abundance with Statistics 10.
Results
Studied cenotes were small (< 1ha) and their maximum depths were greater than 10 m. They had conductivity values < 3.5 μS/cm and salinity values < 1.5 ppm, suggesting a lack of influence of marine water, which is relatively close to the sampling area. These cenotes were oligotrophic, based on their low concentrations of chlorophyll a (< 3.5 μ/1) and phosphate (< 0.6 ppm) in addition to their transparency values (> 1.9 m). The bottom composition was at least 10% CaCO3 reaching up to almost 60%, with the rest of the substrate being organic matter (Table 1). Physicochemical variables did not show any differences between the dry and rainy seasons (MANOVA, Wilks test= 0.743; F= 0.559; p= 0.859), but they did present differences among years for variables: temperature, dissolved oxygen, pH, PO4 and transparency (MANOVA, Wilks test= 0.0468; F= 3.9872; p< 0.001). There were differences among cenotes in oxygen values, transparency and pH. They did not differ in terms of conductivity and nutrient concentrations. CL and CN were significantly warmer (> 4.5° C) and had larger temperature variations between the surface and the bottom. These tropical cenotes did not present a thermocline, which is different to other similar systems (Gutiérrez et al., 2007). On the contrary, CTR and CSM presented an oxycline at 5.5 m depth. This oxycline reduced the average oxygen values, which were significantly lower than those of CL and CN. Transparency was significantly higher in cenotes TR and SM (average= 8.2 m) compared to CL and CN (average= 3m), which had higher pH values (average = 8.1) compared to TR and SM (average= 7) (Table 2).
Primary producer abundance did not change among seasons. But chlorophyll a concentration was significantly higher in CL and CN. Other primary producers, including periphyton, macroalgae and aquatic plants, covered close to 24.8 and 33% of the total area of CN and CL, while these producers covered 1.5 and 0% of the area in TR and SM cenotes. In CL and CN cenotes, Cladium sp. and Eleocharis sp. were the most abundant, while Nympha sp. and Gibba sp. dominated TR as rooted plants on the walls (Table 2).
Zooplankton abundance was significantly lower in TR and SM than CL and CN (Table 2). In all cenotes, copepods (68.6 to 90.2%) were the most abundant and cladocerans were the second largest group. The aquatic insect abundance was not significantly different among cenotes, but the insect species richness values were significantly higher in CL and CN.
A total of 1 350 fish were caught and classified into 11 species from 5 families (Table 3). Time series did not show any clear trend in the fish abundance or richness among the 4 years of sampling or between seasons. SM had a greater abundance of fish (37-62%) than the other cenotes (Fig. 2); and rarefaction curve analysis showed that species richness was not affected by abundance in all cenotes. CL had 9 species; CN had 8, while SM had 3 species and TR only 2.
The predominant fish species differed according to the type of cenote. In TR and SM, dominant fish species were Cichlasoma friedrichsthalii (this abundance reached 76.2% in the TR cenote) and Gambusia Yucatdna (the abundance reached 88.4% in the SM cenote). Rhamdia guatemalensis was found exclusively in non-shored cenotes. In cenotes CN and CL Astyanax aeneus and Cichlasoma urophthalmus were dominant fish species, being the first one with Ophisternon aenigmaticum found exclusively in these cenotes.
PCA separated cenotes into 2 groups by their biotic and abiotic values (Fig. 3), explaining 72% of the total variation in the first 3 components (Table 4). The first component revealed strong associations with macrophyte coverage, transparency, dissolved oxygen, chlorophyll a and pH. The second component showed strong associations with all of the nutrient concentrations. The third component showed associations with conductivity and the ammonia concentration (Table 4).

NMDS analyses grouped CL and CN as one group, and TR and SM as another group (Fig. 4). ANOSIM showed differences among cenotes in terms of species richness (p< 0.001) and fish abundance (p< 0.001). A post-hoc test in species richness revealed differences among all cenotes except for TR and SM (p> 0.01). The post-hoc test on fish abundance showed differences between SM vs. TR and CN (p< 0.01). However, there were no differences between seasons (p> 0.6) and years (p> 0.8). The correlations between the PCA scores and the NMDS axes revealed negative correlations in PCA score 1 (NMDS axis 1: r= -0.64, p= 0.001) and positive correlations in PCA score 1 (NMDS axis 2: r= 0.66, p< 0.05).
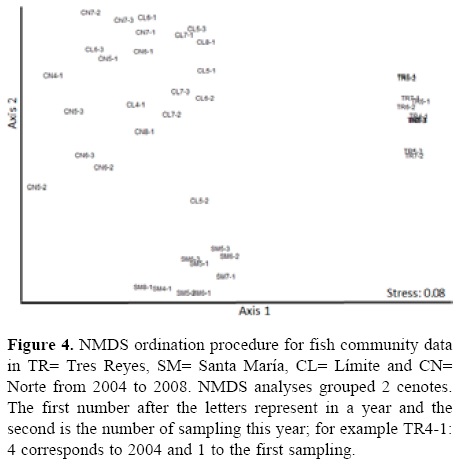
The correlations between the abiotic variables and NMDS axis 1 indicated that most important variables were dissolved oxygen (r= 0.60, p< 0.001), transparency (r= -0.58, p< 0.001), chlorophyll a (r= 0.62, p= 0.004), periphyton (r= 0.65, p= 0.003) and aquatic insects abundance (r= 0.743, p= 0.02). Other variables, such as temperature (r= 0.62, p< 0.001), dissolved oxygen (r= 0.59, p< 0.001), pH (r= 0.55, p< 0.001) and zooplankton abundance (r= 0.61, p= 0.012), were correlated to the second axis.
Multiple regression shows positive relationship between fish richness and abiotic variables winch dissolved oxygen (DO), temperature, pH and PO4+3 (R2= 0.64). Using an individual analysis of each species showed that only A. aeneus abundance was positively correlated with pH, PO4+3 and DO (R2= 0.45).
Discussion
Although cenotes are the most important aquatic system in Yucatán Peninsula, most of their abiotic dynamics and the factors related to fish community structure are poorly understood. This research is a base line to elucidate those drivers that directly affect the fish community structure in oligotrophic small systems. The high influence that allochthonous variables play on cenotes lacking a shore area suggests there are aquatic dynamics in these peculiar systems that remain undiscovered, compared with other systems with shore areas. Results presented in this manuscript raise the necessity of increased ecological research on the region.
The fish species found in these cenotes are widely distributed in the wetlands of the RBSK, pools and other cenotes in the Yucatán Peninsula. Species found in the studied cenotes represent 52% of all of the continental fish species in the Sian Ka'an Reserve (Zambrano et al., 2006).
The small difference on the most abiotic variables across seasons and years suggests that fish which live within these kind of aquatic systems can be related to an equilibrium life history strategy (Southwood, 1977; Townsend and Hildrew, 1994). Fish species with an equilibrium strategy have parental care and prolonged breeding seasons and live in deeper and more stable habitats, such as slow-flowing ponds, river channels and most lakes (Lamouroux et al., 2002; Vila-Gispert et al., 2002). This strategy is used by some species of the Cichlidae family (e.g., C. urophtlhalmus, C. friedrichthali and P. splendida) (Greenfield and Thomerson 1997) and other families, such as Pimelodidae (R. guatemalensis) and Symbranchidae (O. aenigmaticum) (Schmitter-Soto, 1998), which are abundant in the permanent pools of the RBSK (Escalera-Vázquez and Zambrano, 2010).
Also, the stability on the fish community structure over the 4-year sampling period suggests a low change in the hydrological dynamics and water physicochemical variables in a meteorological highly variable landscape. Rainy and dry periods do not yield measurable impacts on the physiocochemical dynamics on the studied cenotes. Contrary to wetlands, cenotes are not subjected to abrupt changes in the water level (Escalera-Vázquez and Zambrano, 2010). This difference in hydrological dynamics between 2 types of systems, which are located in a region with the same environmental dynamics can be explained by the karstic soil. This type of soil absorbs the rainwater within few days, provoking a fast change in the level of water table (Gaona-Vizcayano et al., 1980). In lowlands the water table rises above the surface, generating the temporal wetlands (= 1.5 m depth). In dry season water table retreats, drying out most of the wetlands, with only scattered small permanent pools. Cenotes are deeper systems (> 5 m) than wetlands which are able to buffer any local change in the water table in rainy and dry season (between 0.5 to 1.2 m). Therefore, wetlands and permanent pools are submitted to seasonal hydraulic changes in the 2 periods, changing variables such as temperature and dissolved oxygen concentrations (Escalera-Vázquez and Zambrano, 2010). On the contrary, our results show that most of the physiocochemical variables in cenotes are relatively constant.
Factors affecting fish community structure in wetlands include temperature, depth, pH and macrophytes coverage (Escalera-Vázquez and Zambrano, 2010). Some of these variables also affect fish community structure in cenotes such as temperature and pH. It seems that these variables have a strong relation with fish community structure among the entire aquatic system. However, variables in cenotes that affect fish community structure also include dissolved oxygen and phosphorous concentrations. The DO concentration seems to limit fish richness but not their abundance. This finding can be explained by the low capacity of some species to survive in harsh conditions, surviving only species such as R. guatemalensis and G. yucatana. Phosphorus concentrations have a major role in the ecosystem dynamics (see above), influencing fish community structure depending on system productivity.
To understand the fish community structure within a stable system, it is necessary to analyze the lake trophic structure energy and variables such as nutrients concentration and food sources availability (Sterner et al., 1997). Some of these variables, such as primary production and insect abundance, proved to be highly related to fish community structure. Primary production is related directly to nutrients concentration (Dillon and Rigler, 1974). The low nutrient concentrations and the N/P ratio (> 12.3) in all of the cenotes suggests that phosphorous is the limiting resource for the primary producers. Phosphorus enters the system by rock dissolution and by erosion of nearby areas (Beddows et al., 2007). Most of the phosphorus entering the water column precipitates to the bottom due to the high concentration of CaCO3, a transition that is characteristic of these karstic systems (Roldán, 1992). Phosphorous precipitation has a variety of consequences, depending on the cenote morphology. In deep cenotes with steep walls, phosphorus moves away from the primary producers and precipitates directly to the anoxic and light-limited bottom (Roldán, 1992). In cenotes with a shore area, the phosphorus precipitates to shallower depths that can be used by periphyton, macroalgaes and aquatic plants. These systems may have higher return rates of phosphorous with a stronger autochthonous energy flow (Schmitter-Soto et al., 2002).
The low abundance of food in cenotes without a shore, such as TR and SM, increases the proportion of external food sources. In most lakes, aquatic primary producers support a significant part (> 84%) of the heterotrophic production (Jansson et al., 1999). However, other aquatic systems exist that have higher proportions of allochthonous production supporting the food webs (Cole et al., 1994; Jansson et al., 1999), such as CL and CN. In previous studies in the region we found similar results (Zambrano et al., 2006); fish species richness was significantly higher in cenotes with vegetation and a shore area than cenotes without vegetation and no-shore area.
Allochthonous sources are more important when the aquatic system is small (Mehner et al., 2007 ), is oligotrophic or/and is surrounded by forests (Hodgson and Hansen, 2005; Hodgson et al., 1993), as which was the case with these cenotes, particularly for the non-shore ones. Important food sources are terrestrial insects, which form the basis of some aquatic system food webs (Nakano and Murakami, 2001; Sabo and Power, 2002). A large number of insects live at the cenote edge and are available to fish once they enter the water (pers. observation). Therefore, there are a high proportion of top predators, mainly consuming insects of terrestrial origin and with the typical top predator morphology, such big eyes, big mouth and teeth (Magoulick, 2000). The major species of fish (i.e., P. splendida, C. urophthalmus, C. friedrichsthalii, O. aenigmaticum, B. belizanus and A. aeneus) are predators (Neil, 1984), and they comprise 42% to 66.7% of the total species in cenotes. These species can also be predatory toward grazers, such as C. meeki, P. mexicana, G. yucatana and P. orri (Reznick and Miles, 1989).
Those cenotes with a shore area may have higher autochthons food sources due to the quantity and variety of primary producers that are part of the periphyton, algae and aquatic plants (Schmitter-Soto et al., 2002). The amount of energy generated from the variety of producers could increase the possibility of having a higher richness of fish top predators as happened in both shore area cenotes.
Results suggest that physicochemical variables and fish community structure are not affected by seasonal hydrology dynamics, as happens in the nearby wetlands. Fish community structure in cenotes did not vary between seasons and had a small variation among years. This is completely different from wetlands that have a highly dynamic fish community structure even within seasons (Escalera-Vázquez and Zambrano, 2010). However, similar variables influencing wetlands also are related to fish community structure in cenotes such as dissolved oxygen, transparency, chlorophyll a, periphyton and aquatic insect abundance. These variables change with the morphology of cenotes, and therefore the fish community structure seems to be shaped by the presence or absence of shore areas. In cenotes without a shore area the low quantity of food and the lack of spatial heterogeneity seem to explain why these systems have lower fish richness values than those cenotes with a shore area. The capacity of shore plants to establish at the edge of the cenotes increases the habitat heterogeneity for different types of fish to survive, increasing the amount of refuges against predation and providing more food for the community.
These initial results suggest that shore areas increase the fish richness, but more cenotes must be sampled to analyze this relationship.
Acknowledgements
Financial support of this research was provided by Semarnat-Conacyt for the project C01-202-0082 and PAPIITIN 230007. We extend thanks to Angel Omar Ortiz-Moreno of the Biosphere Reserve of Sian Ka'an and Santa María Ranch, where the sampled cenotes are located. Sampling permit: FAUT0112. Daniel Bedoya G., Patricia Santos R., Nancy Calderón and Filemón Melo for their collaboration. Julio Díaz for providing information on aquatic insects studied in the cenotes. Dirección General de Estudios de Posgrado, Posgrado en Ciencias Biologicas, UNAM for their support. This manuscript was written while LHEV was a postdoctoral researcher at National Institute for Environmental Studies, Japan. Thanks to Conacyt for the fellowship No. 174717.
Literature cited
Apha. 1998. American public health association. American water works association. Water environment federation. Standard methods for examination of water and wastewater. Apha, Washington, D. C. [ Links ]
Barnes, R. D. 1996. Zoología de los invertebrados. 6ª ed. Nueva Editorial Interamericana. México, D. F. 826 p. [ Links ]
Beddows, P. A., P. Blanchon, E. Escobar and O. Torres-Talamante. 2007. Los cenotes de la península de Yucatán. Arqueología Mexicana 83:32-35. [ Links ]
Bedoya, D. G. 2005. Distribución de la familia Poeciliidae a nivel regional en la reserva de la Biosfera de Sian Ka'an. Master thesis, Posgrado en Ciencias Biológicas. Instituto de Biología, UNAM. Mexico, D. F. 44 p. [ Links ]
Bengtsson, L. and M. Enell, 1986. Chemical analysis. In Handbook of Holocene palaeoecology and palaeo-hydrology, B. E. Berglund (ed.). John Wiley and Sons Ldt., Chichester, 423-451. [ Links ]
Biggs, B. J. F. and C. Kilroy. 2000. Stream periphyton monitoring manual. New Zealand Ministry for the Environment/NIWA. Christchurch. 222 p. [ Links ]
Cohen, J. E., 1989. Food webs and community structure. In Perspectives in ecological theory, J. Roughgarden, R. M. May and S. Levin (eds.). Levin, S. A. Princeton University Press, Priceton. p. 181-202. [ Links ]
Cole, J. J, N. F. Caraco, G. W. Kling and T. K. Kratz. 1994. Carbon dioxide supersaturation in the surface waters of lakes. Science 265, 5178:1568-1570. [ Links ]
Collinson, N. H., J. Biggs, A. Corfield, M. J. Hodson, D. Walker, M. Whitfield and P. J. Williams. 1995. Temporary and permanent ponds: an assessment of the effects of drying out on the conservation value of aquatic macroinvertebrate communities. Biological Conservation 74:125-133. [ Links ]
Courtney, G. W. and R.W. Merritt. 2008. Aquatic Diptera, larvae of aquatic diptera. Part one. In An introduction to the aquatic insects of North America, R. W. Merritt, K. W. Cummins and M. B. Berg (eds.). Kendall/Hunt Publishing Company, Dubuque, Iowa. p. 687-722. [ Links ]
Cranston, P. S. and H. V. Daly. 2008. General classification and key to the orders of aquatic and semiaquatic. In An introduction to the aquatic insects of North America, R. W. Merritt, K. W. Cummins and M. B. Berg (eds.). Kendall/Hunt Publishing Company, Dubuque, Iowa. p. 157164. [ Links ]
Currie, D. J., P. Dilworth-Christie and F. Chapleau, 1999. Assessing the strength of top-down influences on plankton abundance in unmanipulated lakes. Canadian Journal of Fisheries and Aquatic Sciences 56: 427-436. [ Links ]
Dillon, P. J. and F. H. Rigler. 1974. The phosphorus-chlorophyll relation ship in lakes. Limnology and Oceanography 19: 767-773. [ Links ]
Escalera-Vázquez, L. H. and L. Zambrano. 2010. The effect of seasonal variation in abiotic factors on fish community structure in temporary and permanent pools in a tropical wetland. Freshwater Biology 55:2557-2569. [ Links ]
Ferrington, J. L. C., M. B. Berg and W. P. Coffman. 2008. Chironomidae. In An introduction to the aquatic insects of North America, R. W. Merritt, K. W. Cummins and M. B. Berg (eds.). Kendall/Hunt Publishing Company, Dubuque, Iowa. p. 847-989. [ Links ]
Galacatos, K., R. Barriga-Salazar and D. J. Stewart. 2004. Seasonal and Habitat Influences on Fish Communities within the Lower Yasuni River Basin of the Ecuadorian Amazon. Environmental Biology of Fishes 71:33-51. [ Links ]
Gutiérrez, E. M., A. Cervantes-Martínez, M. Gutiérrez-Aguirre and A. M. Arce-Ibarra. 2007. Los cenotes y lagunas del centro y sur de la península de Yucatán. In Las aguas interiores de México: conceptos y casos, G. De la Lanza-Espino (Comp.). AGT Editor. México. p. 423-446. [ Links ]
Greenfield, D. W. and J. E.Thomerson. 1997. Fishes of the continental waters of Belize. University Press of Florida. p. 310. [ Links ]
Grenouillet, G. and D. Pont. 2001. Juvenile fishes in macrophyte beds: influence of food resources, habitat structure and body size. Journal of Fish Biology 59:939-959. [ Links ]
Grenouillet, G, D. Pont and K. L. Seip. 2002. Abundance and species richness as a function of food resources and vegetation structure: juvenile fish assemblages in rivers. Ecography 25:641-650. [ Links ]
Hodgson, J. R. and E. M. Hansen. 2005. Terrestrial prey in the diet of largemouth bass, Micropterus salmoides, in a small north temperate lake. Journal of Freshwater Ecology 20:793-794. [ Links ]
Hodgson, J. R., X. He and J. F. Kitchell. 1993. The fish populations. In The trophic cascade in lakes, S. R. Carpenter and J. F. Kitchell (eds.). Cambridge University Press, Cambridge. p. 43-68. [ Links ]
Jansson, M, A. K.Bergstrom, M. P. Blomqvist, A. Isaksson and A. Jonsson. 1999. Impact of allochthonous organic carbon on microbial food web carbon dynamics and structure in Lake Örträsket. Archives for Hydrobiology 144:409-428. [ Links ]
Kobza, R. M., J. C.Trexler, W. F. Loftus and S. A. Perry. 2004. Community structure of fishes inhabiting aquatic refuges in a threatened Karst wetland and its implications for ecosystem management. Biological Conservation 116:153-165. [ Links ]
Lamouroux, N, N. L. Poff and P. L. Angermeier. 2002. Intercontinental convergence of stream fish community traits along geomorphic and hydraulic gradients Ecology 83:1792-1807. [ Links ]
Lampert, W. and U. Sommer. 1997. Limnoecology: the ecology of lakes and streams. Oxford University Press. New York. 382 p. [ Links ]
Loftus, W. F. and J. A. Kushlan. 1987. Freshwaters fishes of southern Florida. Bulletin of the Florida Museum ofNatural History 31:147-344. [ Links ]
Lot, A, R. A. Novelo and G. P. Ramírez. 1998. Diversidad de la flora acuática mexicana. In Diversidad biológica de México. Origenes y distribución, T. P. Ramamoorthy, R. Bye, A. Lot y J. Fa (comps.). Instituto de Biologia. UNAM, México, D. F. p. 563-578. [ Links ]
Magoulick, D. D. 2000. Spatial and temporal variation in fish assemblages of drying stream pools: The role of abiotic and biotic factors. Aquatic Ecology 34:29-41. [ Links ]
McCafferty, W. P., C. R. Lugo-Ortiz, A. V. Provonsha and T. Q. Wang. 1997. Los efemeropteros de México: clasificación superior, diagnosis de familias y composición. Dugesiana 4:1-29. [ Links ]
Merritt, R. W. and D. W. Webb. 2008. Aquatic diptera (part two): pupae and adults of aquatic diptera. In An introduction to the aquatic insects of North America, R. W. Merritt, K. W. Cummins and M. B. Berg (eds.). Kendall/Hunt Publishing Company, Dubuque, Iowa. p. 723-800. [ Links ]
Miller, R. R. 2005. Freshwater fishes of Mexico. The University of Chicago Press. Chicago and London. p. 507. [ Links ]
Nakano, S. and M. Murakami. 2001. Reciprocal subsidies: dynamic interdependence between terrestrial and aquatic food webs. Procedures of the National Academy of Sciences, USA 98:166-179. [ Links ]
Neil, S. 1984. Field studies of the behavioral ecology and agonistic behavior of Cichlasoma meeki (Pisces: Cichlidae). Environmental Biology of Fishes 10:59-68. [ Links ]
Novelo-Gutiérrez, R. 1997a. Clave para la determinación de familias y generos de las nayades de odonata de México. Parte II. Anisoptera. Dugesiana 4:31-40. [ Links ]
Novelo-Gutiérrez, R. 1997b. Clave para la separación de familias y generos de las neyades de odonata de Mexico. Parte I. Zygoptera. Dugesiana 4:1-10. [ Links ]
Perry, E. L., L. Marín, J. McClain and G. Velásquez. 1995. Ring of cenotes (cenotes), northwest Yucatán, Mexico: its hydrogeologic characteristics and possible association with the Chicxulub impact crater. Geology 23:17-20. [ Links ]
Polhemus, J. T. 2008. Aquatic and semiaquatic hemiptera. In An introduction to the aquatic insects of North America, R. W. Merritt, K. W. Cummins and M. B. Berg (eds.). Kendall/Hunt Publishing Company, Dubuque, Iowa. p. 385-423. [ Links ]
Reznick, D. E. and D. B. Miles. 1989. Systematic overview of the poeciliidae. In Ecology and evolution of livebearing fishes (Poeciliidae), G. K. Meffe and F. F. J. Snelson (eds.). Prentice-Hall, Inc., Englewood Cliffs, New Jersey. p. 453. [ Links ]
Roldán, G. P. 1992. Fundamentos de limnologia neotropical. Ciencia y Tecnología. Universidad de Antioquia, Antioquia. 529 p. [ Links ]
Sabo, J. L. and M. E. Power. 2002. River-watershed exchange: effects of riverine subsidies on riparian lizards and their terrestrial prey. Ecology 83:1860-1869. [ Links ]
Schmitter-Soto, J. J. 1998. Catálogo de los peces de Quintana Roo. El Colegio de La Frontera Sur, Unidad Chetumal, México, D. F. 239 p. [ Links ]
Schmitter-Soto, J. J, F. A. Comin, E. Escobar-Briones, J. Herrera-Silveira, J. Alcocer, E. Suárez-Morales, M. Elías-Gutiérrez, V. Díaz-Arce, L. E. Marín and B. Steinich. 2002. Hydrogeochemical and biological characteristics of cenotes in the Yucatán Peninsula (SE Mexico). Hydrobiologia 467:215-228. [ Links ]
Schreck, B. C. and R. B. Moyle 1990. Methods for fish biology. American Fishery Society, Bethesda, Maryland. p. 363-387. [ Links ]
Schwartz, S. S. and D.G. Jenkins. 2000. Temporary aquatic habitats: constraints and opportunities. Aquatic Ecology 34:3-8. [ Links ]
Steinich, B. 1996. Investigaciones geofísicas e hidrogeológicas en el noroeste de la Península de Yucatán, México (Geophysical and hydrogeological investigations in the northwest peninsula of Yucatán, Mexico), Ph. D. thesis, Instituto de Geofísica, Universidad Nacional Autónoma de México. México, D. F. 94 p. [ Links ]
Sterner, R. W, J. J. Elser, E. J. Fee, S. J. Guildford and T. H. Chrzanowski. 1997. The light: nutrient ratio in lakes: the balance of energy and materials affects ecosystem structure and process. The American Naturalist 150:663-684. [ Links ]
Stoessell, R. K, Y. H. Moore and J. G. Coke.1993. The occurrence and effect of sulfate reduction and sulfide oxidation on coastal limestone dissolution in Yucatán cenotes. Ground Water 31:566-575. [ Links ]
Tennessen, K. J. 2008. Odonata. In An introduction to the aquatic insects of North America, R. W. Merritt, K.W. Cummins and M. B. Berg (eds.). Kendall/Hunt Publishing Company, Dubuque, Iowa. p. 237-294. [ Links ]
Torres-Orozco, R. and J .L. García-Calderón. 1995. Introducción al manejo de datos limnológicos. Universidad Autónoma Metropolitana, México, D. F. 130 p. [ Links ]
Trexler, J., W. Loftus and S. Perry. 2005. Disturbance frequency and community structure in a twenty-five year intervention study. Oecologia 145:140-152. [ Links ]
Vila-Gispert, A., R. Moreno-Amich and E. García-Berthou. 2002. Gradients of life-history variation: an intercontinental comparison of fishes. Reviews in Fish Biology and Fisheries 12:417-427. [ Links ]
Waltz, R. D. and S. K. Burian. 2008. Ephemeroptera. In An introduction to the aquatic insects of North America, R. W. Merritt, K. W. Cummins and M. B. Berg (eds.). Kendall/ Hunt Publishing Company, Dubuque, Iowa. p. 181-236. [ Links ]
Wallace, J. L. and E. D. Walker. 2008. Culicidae. In An introduction to the aquatic insects of North America, R. W. Merritt, K. W. Cummins and M. B. Berg (eds.). Kendall/ Hunt Publishing Company, Dubuque, Iowa. p. 801-823. [ Links ]
White, D. S. and R. E. Roughley. 2008. Aquatic coleoptera. In An introduction to the aquatic insects of North America, R. W. Merritt, K. W. Cummins and M. B. Berg (eds.). Kendall/ Hunt Publishing Company, Dubuque, Iowa. p. 571-671. [ Links ]
Willis, S. C., K. O. Winemiller and H. López-Fernández. 2005. Habitat structural complexity and morphological diversity of fish assemblages in a Neotropical floodplain river. Oecologia 142:284-295. [ Links ]
Zambrano, L., E. Vázquez-Domínguez, D. García-Bedoya, W. F. Loftus and J. C. Trexler. 2006. Fish community structure in freshwater karstic waterbodies of the Sian Ka'an Reserve in Yucatán peninsula, Mexico. Ichthyological Exploration of Freshwaters 17:193-20. [ Links ]
Zar, J. H. 1999. Biostatistical Analysis, 5th edition. Upper Saddle River, Pearson Education, Inc. New Jersey. 944 p. [ Links ]














