Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de biodiversidad
On-line version ISSN 2007-8706Print version ISSN 1870-3453
Rev. Mex. Biodiv. vol.83 n.4 México Dec. 2012
https://doi.org/10.7550/rmb.30966
Conservación
Phylogenetic measures applied to the conservation of Mexican marsupials
Medidas filogenéticas aplicadas para la conservación de los marsupiales mexicanos
Margarita Medina-Romero1, Irene Goyenechea2* and Jesús Castillo-Cerón1
1 Museo de Paleontología, Centro de Investigaciones Biológicas, Universidad Autónoma del Estado de Hidalgo. Apartado postal 1-397, 42001 Pachuca, Hidalgo, México.
2 Laboratorio de Sistemática Molecular, Centro de Investigaciones Biológicas, Universidad Autónoma del Estado de Hidalgo. Apartado postal 1-69, 42001 Pachuca, Hidalgo, México. *ireneg28@gmail.com
Recibido: 30 marzo 2012
Aceptado: 02 julio 2012
Abstract
The didelphimorphs in Mexico are found all over the country except for the Baja California Peninsula. The aim of this study was to use 3 methods to assess the phylogenetic diversity of the species Marmosa mexicana, Tlacuatzin canescens, Caluromys derbianus, Chironectes minimus, Didelphis marsupialis, Didelphis virginiana, Metachirus nudicaudatus, and Philander opossum, and to determine the potential conservation areas for these mammals. Phylogenetic information was included to measure the taxonomic weighting, taxonomic dispersion, and taxonomic distinctness within the Mexican biogeographic provinces. In addition, a gap analysis was performed to show which protected areas contain the didelphimorphs listed under a conservation category. Considering phylogenetic diversity with the former analysis, results indicate that the biogeographic provinces most important for conservation of didelphimorphs are the Gulf of Mexico, the Pacific Coast, and Oaxaca, although Soconusco and Sierra Madre del Sur also have to be considered. We also observed that not all of the richest sites corresponded with current protected areas. This study is important because it employed different conservation approaches based on phylogenetic measures and was focused on Mexican marsupials, of which 1 species is endemic and 2 are of conservation concern.
Key words: didelphimorphs, gap analysis, phylogenetic diversity, taxonomic dispersion, taxonomic distinctness, taxonomic weight.
Resumen
El orden Didelphimorphia se encuentra distribuido en todo México excepto en la península de Baja California. En este trabajo se evaluó la diversidad filogenética para las especies Marmosa mexicana, Tlacuatzin canescens, Caluromys derbianus, Chironectes minimus, Didelphis marsupialis, Didelphis virginiana, Metachirus nudicaudatus y Philander opossum, y se determinaron las áreas potenciales de conservación para estos organismos. Para realizar los análisis de peso taxonómico, dispersión taxonómica y diferenciación taxonómica se incluyó información filogenética. También se realizó un análisis de vacíos y omisiones (gap analysis) para evaluar si las áreas protegidas contienen a los didelfimorfos bajo alguna categoría de protección. Al combinar los resultados de todos los análisis se concluyó que las provincias biogeográficas más importantes en la conservación de los marsupiales son la del Golfo de México, Costa del Pacífico y Oaxaca, pero también son importantes Soconusco y la Sierra Madre del Sur. Se identificaron sitios de gran riqueza específica que no corresponden con la ubicación de áreas naturales protegidas. Por último este estudio es importante por emplear diferentes medidas filogenéticas que pueden ser usadas en conservación de los marsupiales mexicanos, de los cuales dos especies se encuentran bajo alguna categoría de conservación y una es endémica.
Palabras clave: didelfimorfos, análisis de vacíos, diversidad filogenética, dispersión taxonómica, diferenciación taxonómica, peso taxonómico.
Introduction
Over the last 30 years, conservation biology has improved the quality of information used in diversity studies. When the species is considered the unit of analysis, results can provide important information on conservation decision-making if they include phylogenetic diversity and complementarity (Eguiarte et al., 1999). Traditionally, species richness in areas was analyzed giving the same value to all the taxa. In recent times, distinct taxa have different conservation priorities when threatened; also, different conservation values are given to species when they are not part of the same phylogenetic group (Atkinson, 1989).
Didelphimorphs are American marsupials distributed along the entire American Continent. They are an ancient group present since the Cretaceous. South America has a vast number of endemic species and the highest diversity. In Mexico, marsupials are distributed throughout the country except Baja California Peninsula, but their highest richness and diversity is in the southern part of the country (Ceballos y Oliva, 2005). Species under protection are Caluromys derbianus and Metachirus nudicaudatus listed as threatened, and Chironectes minimus listed as in danger of extinction (SEMARNAT, 2010).
Conserving American marsupials is important from an evolutionary, biogeographical, ecological, and morphological point of view due to the features kept from the Australasian radiations. Different methods used to analyze taxonomic distinctness depend on spatial and taxonomic scale and the quality of data. It is important to consider that to make the best conservation decisions, priority of areas is not based only on possession of a high number of species, but also on the evolutionary history of the species (Eguiarte et al., 1999; Martin-Piera, 1999). In contrast, the method of gap analysis compares places with high biological richness with protected areas to propose new places for conservation (Scott et al., 1987; Bojórquez and Flores-Villela, 1991). The aim of this work was to determine which of the Mexican biogeographic provinces (sensu Arriaga et al., 1997) have the highest phylogenetic diversity of didelphimorph mammals and which of these provinces are most relevant for conservation considering different approaches. We used the taxonomic weight measure by Vane-Wright et al. (1991), the taxonomic dispersion measure proposed by Williams et al. (1991), and the taxonomic distinctness measure of Warwick and Clarke (1995). Then we used gap analysis to identify which protected areas include the highest richness of didelphimorphs, to verify whether the species are protected in any of these areas, and to identify places that should be protected.
Materials and methods
Data. A phylogenetic analysis was performed with PAUP* 4.0 b10 (Swofford, 1999). For maximum-parsimony analysis, all the characters were equally weighted based on the phylogenetic relationships inferred by Voss and Jansa (2003). Heuristic searches were performed with 1 000 random additions of taxa and a tree-bisection reconnection (TBR) algorithm was used for the branch swapping with nodal support assessed by 1 000 bootstrap replicates. Caluromys derbianus was added to the data matrix and the codification of characters was based on previous published descriptions (Voss and Jansa, 2003; Bucher and Hoffman, 1980).
The data matrix of 45 taxa and 71 morphological-karyological characters (Voss and Jansa, 2003; Bucher and Hoffman, 1980) is shown in Appendix. The cladogram obtained was the result of a bootstrap analysis, which was used for the taxonomic weight method.
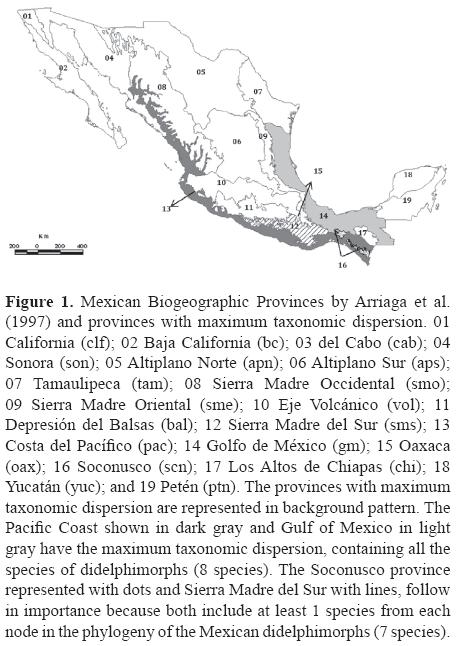
Taxonomic dispersion, taxonomic distinctness, and gap analysis were performed based on georreferenced data from 5 electronic databases of zoological collections for the 8 species of didelphimorphs present in Mexico: Marmosa mexicana Merriam, 1897; Tlacuatzin canescens Allen, 1893, Voss and Jansa, 2003; Caluromys derbianus Waterhouse, 1841; Chironectes minimus Zimmerman, 1780; Didelphis marsupialis Linnaeus, 1758; Didelphis virginiana Kerr, 1792; Metachirus nudicaudatus Desmarest, 1817; and Philander opossum Linnaeus, 1758. Data were retrieved from SNIB (Sistema Nacional de Información sobre Biodiversidad) of CONABIO, AMNH (American Museum of Natural History), MaNis (Mammal Network Information System), GBIF (Global Biodiversity Information), and REMIB (Red Mundial de Información sobre Biodiversidad). The final data set included 1 828 records taxonomically validated by experts from each database and a georeferenced site or locality where the organism was collected. Species collection records were assigned to 16 of the19 biogeographic provinces of Mexico (Fig. 1) defined by Arriaga et al. (1997); the remaining 3 correspond to California, Baja California, and el Cabo provinces, where didelphimorphs are absent.
Phylogenetic methods. a) Taxonomic weight and complementarity. Proposed by Vane-Wright et al. (1991), the taxonomic weight and complementarity method identifies diversity critical points. Like the taxonomic dispersion method (below), it takes into account phylogenetic and biogeographic information. This method does not consider whether species are plesiomorphic or apomorphic before the analysis is performed (Vane-Wright et al., 1991), but it considers monophyletic groups within taxa. The difference between taxonomic weight and taxonomic dispersion is that the former includes the taxonomic weight for each species and uses complementarity to detect and propose possible protected areas. Complementarity identifies the minimum number of protected areas needed to ensure the maximum number of species (Williams, 2001).
The analysis of taxonomic weight included the 45 species of didelphimorphs in order to avoid sampling error from including only the Mexican species. In this work we only presented the results for Mexican opossums. We used 4 taxonomic measures (I), (Q), (W), and (P), to evaluate the information on the cladogram. The first index (I) corresponds to the number of monophyletic groups to which each species belongs; this value reflects the number of nodes from the root of the tree to the node subtending the terminal taxon. The basal phylogenetic weight (Q) is the sum of I divided by the smallest value of I. The number of taxa that contribute to the total diversity of the group (W), is the total sum of Q divided by the minimum value of Q. The measure (P) is the percentage of the W value for each taxon, and is the result of the multiplication of each value of W times one hundred and divided by the total of W (Vane-Wright et al., 1991). The taxonomic singularity percentage (P%) was calculated to identify areas of highest priority in didelphimorphs conservation. Areas with 100 (P%) were the best option for conservation, but also the sets of areas that summed to 100.
Once the value of W was obtained, the distributions of the didelphimorphs in the biogeographic provinces and the phylogenetic information were included to prioritize using complementarity.
b) Taxonomic dispersion (Williams et al., 1991). We constructed a data matrix of taxa vs. biogeographical provinces (8x16) and coded the presence or absence of taxa to identify areas with the highest number of taxa in regard to the phylogeny of didelphimorphs (Voss and Jansa, 2003; modified by Medina-Romero, 2007). Those areas that include all taxa possess the maximum dispersion. Areas of interest are those with the highest representation of species in the subclades when it is not possible to protect all species (Williams et al., 1991).
c) Taxonomic distinctness. Warwick and Clarke (1995) proposed this method to synthesize the path of relationships in a sample. They described the mean taxonomic distance between 2 organisms selected randomly in the phylogeny or in the Linnaean taxonomy for the entire species community. This method avoids the requirement of having resolved phylogenies, which are unknown for many taxonomic groups, but it is essential to have a Linnaean classification that reflects the different relationships of similarity between species (Clarke and Warwick, 1998a, b, 2001; Warwick and Clarke, 1995, 1998). We only used the average taxonomic distinctnes proposed by Warwick and Clarke (1995), because the order Didelphimorphia comprises 8 species in Mexico and no other taxa were included to strengthen the analysis.
To obtain this value, 2 matrices with the taxonomic classification of didelphimorphs were prepared in Excel. The first matrix included different Linnaean categories (species, genus, family, order, superorder, subclass, and class); the second matrix was scored for presence or absence of taxa for each of the biogeographic provinces. Fourteen provinces were included under the criteria of having 3 or more taxa in each one. The provinces of Sonora (son) and the Altiplano del Norte (apn) were not included because they contained only 2 and 1 species, respectively. The average taxonomic distinctness values and a funnel graph were obtained with the program Primer 5 (PRIMER-E, 2001; Clarke and Warwick, 2001).
d) Gap analysis. Gap analysis is a cartographic technique proposed by Scott et al. (1987) to compare areas with high species richness and location of protected areas (Miller, 1994). There are 163 protected areas in Mexico (CONANP, 2007); the map used for the analysis included only 35 areas where the highest species richness for didelphimorphs was found based on results from taxonomic dispersion, taxonomic weight, and taxonomic distinctness (Fig. 2).

Results
We obtained a total of 100 trees and one strict consensus cladogram for the order Didelphimorphia (Fig. 3).The resulting values for the most parsimonious trees were length= 189 steps, consistency index= 0.481 and retention index= 0.830. The hypothesis shown was strongly supported by the bootstrap analysis.
a) Taxonomic weight and complementarity. The analysis of taxonomic weight showed C. derbianus, T. canescens, M. mexicana, and M. nudicaudatus as the most plesiomorphic taxa with the highest taxonomic weight of 3.00 (Fig. 3), followed by C. minimus with a value of 2.00, D. marsupialis and Philander opossum with a value of 1.20, and the lowest taxonomic weight was for D. virginiana with a value of 1.00. Based on the taxonomic singularity percentage, the provinces with the highest priority were the Gulf of Mexico (gm) and the Pacific Coast (pac) with 100% of phylogenetic information (20.40), followed by Soconusco (scn) and the Sierra Madre del Sur (sms) with 85.3%. The percentages of phylogenetic information for each biogeographic province are shown in Fig. 4. The taxonomic weight index is used along with complementarity to prioritize crucial areas for conservation. Applying this, the counterpart area for the Sierra Madre del Sur (sms) province is Soconusco (scn); the former province preserves all taxa except Metachirus nudicaudatus with 85.3% of phylogenetic information, but this species is included in the latter province with 14.7%. In other words, these 2 provinces include all 8 species of didelphimorphs in Mexico. Another possible complementarity hypothesis is the province of Soconusco with 85.3% of phylogenetic information, which is complemented by the 14.7% of Chiapas province.
b) Taxonomic dispersion. The Gulf of Mexico (gm) and Pacific Coast (pac) provinces had the highest taxonomic dispersion (Wtotal= 20.40 equivalent to 100%; Fig. 4). All species of didelphimorphs occur in both areas (Fig. 1). Assuming that it is not always possible to protect all species, the area with the highest taxonomic dispersion is the Soconusco province (scn; Fig. 4), because it includes at least 1 species from each node in the phylogeny of the didelphimorphs and 7 of the 8 species distributed in Mexico (C. derbianus, C. minimus, D. marsupialis, D. virginiana, P. opossum, M. nudicaudatus, and M. mexicana). Another important province is the Sierra Madre del Sur (sms), because it includes the same species as Soconusco except the narrow endemic, T. canescens, and it also includes M. nudicaudatus (Fig. 1); that is why it is preferred over the rest of the biogeographic provinces.
c) Taxonomic distinctness. The area with the highest taxonomic distinctness was the Oaxaca (oax) province with a value of 38.1. This province includes C. derbianus and T. canescens (endemic to Mexico), but 2 species are absent, M. nudicaudatus and C. minimus, both listed as protected (SEMARNAT, 2010). The provinces of Sonora (son) and the Altiplano del Norte (apn) were not included because they contained only 2 and 1 species, respectively.
d) Gap analysis. A total of 35 protected areas were identified within the biogeographic provinces of Pacific Coast, Gulf of Mexico, Soconusco, Sierra Madre del Sur, and Oaxaca (Table 1). The gap analysis comparing the species' shared in the protected areas (Table 2) indicates that Palenque has 5 species, and Los Tuxtlas and Cañón del Río Blanco 4 species each (Fig. 6). None of the protected areas contained all species of marsupials. Palenque had the highest number of didelphimorph species. Moreover, it included C. minimus and C. derbianus, which are listed as "protected" and "threatened" respectively (SEMARNAT, 2010).
Discussion
The application of biogeographic methods for conservation has led to a different perspective for making better proposals in this topic (Whittaker et al., 2005). Under this assumption we used different biogeographic methods to analyze and to assess priorities in conservation status for the didelphimorphs species in the natural protected areas of Mexico by combining taxonomic distinctness, taxonomic weight, taxonomic dispersion, complementarity, and gap analysis, although we are aware that there are other biogeographic and ecological methods available.
Previous studies on Mexican marsupials using taxonomic dispersion were not found even though this method integrates distribution and taxonomic relationships of didelphimorphs to establish possible areas for conservation. Taxonomic dispersion and taxonomic weight gave us similar results and indicate that the southern portions of the Gulf of Mexico and the Pacific Coast, followed by the Soconusco and the Sierra Madre del Sur, are important for didelphimorphs conservation. The taxonomic distinctness analysis shows that the province of Oaxaca stands out as the most important area for didelphimorphs conservation. Areas with maximum taxonomic dispersion were the Gulf of Mexico and the Pacific Coast. These provinces have a large territorial extension, and the 8 didelphimorphs species were located only in the southern portion of these areas. If it is not always possible to protect all species, the Soconusco and the Sierra Madre del Sur provinces should be the subsequent conservation areas considered in order of importance, where at least 1 taxon from each node of the cladogram is found, even though M. nudicaudatus is not included within the Sierra Madre del Sur. Gap analysis showed that most of the didelphimorphs richness is found in protected areas, including C. derbianus and C. minimus, which are in risk of extinction. Nevertheless we can propose to establish a biological corridor connecting the protected areas, focusing on those of the most southern part of the country, to strengthen dispersal among these mammals and to guarantee the protection of M. nudicaudatus and C. minimus, both with distributions restricted to the area mentioned above.
Taxonomic distinctness measures have been applied to conservation biology in different ways. Bhat and Magurran (2006), working with fish, argued that these measures have a vast potential for environmental evaluation and conservation biology. Garcia-Marmolejo et al. (2008) employed these measures to establish conservation priorities for Neotropical mammals. They determined that Oaxaca and Chiapas were the areas with the highest specific diversity. Our results show that Oaxaca is also an area with high taxonomic distinctness for marsupials. Garcia-Marmolejo et al. (2008) evaluated the overlapping of panbiogeographic nodes with protected areas, and found that 1 site of highest diversity richness for mammals was found in Los Altos de Chiapas, which included 2 didelphimorphs, M. mexicana and C. derbianus. This information partially coincides with our results where Palenque is considered the richest area for didelphimorphs. These results from Mexican marsupials represent another example of the usage of these measures to assess terrestrial mammal conservation biology.
Using gap analysis and herpetofauna data, Ochoa-Ochoa and Flores-Villela (2006) found that approximately 40% of the protected areas of Mexico coincided with places of high richness for these organisms. Additionally, Urbina-Cardona and Flores-Villela (2010) found that the hotspot areas were concentrated in the southeast part of Mexico, which is congruent with our results for marsupials. The above results also agree with the latitudinal and richness patterns proposed by Ceballos and Oliva (2005) and Ceballos (2007), who established that the highest richness of Mexican marsupials occurs in southern Mexico, and that the reserves with the highest species number are located in tropical forest areas.
Based on the results of our work, we noticed that it is better to combine different methods to make decisions, because a better conservation proposal can be obtained. This approach has been applied in different works; for example, Torres-Miranda et al. (2011) used complementarity, richness, and endemism patterns of red oaks in Mexico and Central America, and Vázquez and Valenzuela-Galván (2009) studied mammals with the same methods. Both studies coincide with our results in locating the highest richness area for plants and mammals in southern Mexico. Also the results of Vázquez et al. (2009) employing complementarity with mammals are congruent with our results in that the Pacific Coast, Sierra Madre del Sur, and Soconusco are the most species rich areas in the country. Finally, our results partially coincide with Escalante et al. (2009), who used optimality criterion, parsimony analysis of endemicity, and niche modelling methods and concluded that the regions of the central Pacific Coast, Chiapas, Transmexican volcanic belt, and the Yucatán Peninsula should be considered as endemism areas which partially correspond to similar areas in our study.
Acknowledgements
We thank D. Gernandt and A. Contreras for comments and corrections to an earlier version of the manuscript. This work was partially funded by Projects FOMIX-CONACyT Hidalgo 43761 "Diversidad Biológica del Estado de Hidalgo", and 95828 "Diversidad Biológica del Estado de Hidalgo (segunda fase)".
Literature cited
Arriaga, L. C., C. Aguilar, D. Espinosa-Organista and R. Jiménez. 1997. Regionalización ecológica y biogeográfica de México. Taller de la Comisión Nacional para el Conocimiento y Uso de la Biodiversidad CONABIO. México, D. F. http://www.biodiversidad.gob.mx/pais/pdf/CapNatMex/Vol%20I/I01_Elconocimientobiog.pdf; last access: 29.II.2012. [ Links ]
Atkinson, I. 1989. Introduced animals and extinctions. In Conservation for the twenty first century, D. Western and M. Pearl (eds.). Oxford University Press, New York. p. 54-69. [ Links ]
Bhat, A. and A. E. Magurran. 2006. Taxonomic distinctness in a linear system: a test using a tropical freshwater fish assemblage. Ecography 29:104-110. [ Links ]
Bojorquez-Tapia, L. A. and O. Flores-Villela. 1991. Aspectos Legales y Metodológicos de la Bioconservación en México. In Memorias del Seminario sobre conservación de la Diversidad Biológica en México, J. Llorente-Bousquets, H. E. Ponce, O. Flores-Villela. (eds.). México, D. F. No. 2. p.1-23. [ Links ]
Bucher, J. and R. Hoffmann. 1980. Caluromys derbianus. American Society of Mammalogists 140:1-4. [ Links ]
Ceballos G. and G. Oliva. 2005. Los mamíferos silvestres de México. 1a edición CONABIO y Fondo de Cultura Económica. México, D. F. 986 p. [ Links ]
Ceballos, G. 2007. Conservation priorities for mammals in megadiverse Mexico: the efficiency of reserve networks. Ecological Applications 17:569-578. [ Links ]
Clarke, K. R. and R. M. Warwick.1998a. Quantifying structural redundancy in ecological communities. Oecologia 113:278-289. [ Links ]
Clarke, K. R. and R. M. Warwick. 1998b. A taxonomic distinctness index and its statistical properties. Journal of Applied Ecology 35:523-53. [ Links ]
Clarke, K. R. and R. M. Warwick. 2001. Change in marine communities: an approach to statistical analysis and interpretation, 2nd ed. PRIMER-E, Plymouth. p. 172. [ Links ]
CONANP (Comisión Nacional de Áreas Naturales Protegidas). 2007. Mapa de áreas naturales protegidas de México. http://www.conanp.gob.mx/regionales/; last access: 29.II.2012. [ Links ]
Eguiarte, L. E., J. Larson-Guerra, J. Nuñez-Farfán, J. Martínez-Palacios, A. Santos-del Prado and H. T. Arita. 1999. Diversidad Filogenética y conservación: ejemplos a diferentes escalas y una propuesta a nivel poblacional para Agave victoriae-reginae en el desierto de Chihuahua, México. Revista Chilena de Historia Natural 72:475-492. [ Links ]
Escalante, T., C. Szumik and J. J. Morrone. 2009. Areas of endemism of Mexican mammals reanalysis applying the optimality criterion. Biological Journal of the Linnean Society 98:468-478. [ Links ]
García-Marmolejo, G., T. Escalante and J. J. Morrone. 2008. Establecimiento de prioridades para la conservación de mamíferos terrestres neotropicales de México. Mastozoología Neotropical 15:41-65. [ Links ]
Martín-Piera, F. 1999. Apuntes sobre biodiversidad y conservación de insectos: dilemas, ficciones y ¿soluciones? Versión electrónica del artículo publicado. Boletín de la Sociedad Entomológica Aragonesa 20:25-55. [ Links ]
Medina-Romero, M. 2007. Biogeografía del Orden Didelphimorphia en México. Thesis. Universidad Autónoma del Estado de Hidalgo (UAEH). México. 88 p. [ Links ]
Miller, R. I. 1994. Setting the scene. In Mapping the Diversity of Nature. R. I. Miller (ed.). Chapman and Hall, London. p. 3-17. [ Links ]
Ochoa-Ochoa, L. M. and O. Flores-Villela. 2006. Áreas de Diversidad de Endemismo de la Herpetofauna Mexicana. UNAM, CONABIO. México, D. F. p. 211. [ Links ]
PRIMER LTD. 2001. Plymouth Routines In Multivariate Ecological Research PRIMER ver 5.2.4. Plymouth United Kingdom. p. 243-256. [ Links ]
Scott, J. M., B. Csuti, J. D. Jacobi and J. E. Estes. 1987. Species Richness. Bioscience 37:782-788. [ Links ]
SEMARNAT (Secretaría de Medio Ambiente y Recursos Naturales). 2010. Norma Oficial Mexicana NOM-059-ECOL-2010. Protección ambiental-especies nativas de México de Flora y fauna silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-lista de especies en riesgo. Diario oficial de la federación, 30 de diciembre de 2010. p. 1-78. [ Links ]
Swofford, D. L. 1999. PAUP*. Phylogenetic analysis using parsimony (and other methods). Ver 4. Sinauer Associated. Sunderland. [ Links ]
Torres-Miranda, A., I. Luna-Vega and K. Oyama. 2011. Conservation Biogeography of red oaks (Quercus section Lobatae) in Mexico and Central America. American Journal of Botany 98:290-305. [ Links ]
Urbina-Cardona, J. N. and O. Flores-Villela. 2010. Ecological-Niche Modeling and Prioritization of Conservation-Area Networks for Mexican Herpetofauna. Conservation Biology 24:1031-1041. [ Links ]
Vane-Wright, R. I., C. J. Humphries and P. H. Williams. 1991. "What to protect? Systematics and the agony of a choice. Biological Conservation 55:235-254. [ Links ]
Vázquez, L. and D. Valenzuela-Galván. 2009. Que tan bien representados están los mamíferos mexicanos en la red federal de áreas naturales protegidas del país? Revista Mexicana de Biodiversidad 80:249-285. [ Links ]
Vázquez, L., Bustamante C. G. and D. G. Bahena-Arce. 2009. Area Selection for conservation of Mexican mammals. Animal Biodiversity and Conservation 32:29-39. [ Links ]
Voss S. R. and S. H. Jansa. 2003. Phylogenetic studies on didelphid marsupials II. Nonmolecular data and new IRBP sequences: separate and combined analyses of Didelphine relationships with denser taxon sampling. Bulletin of the American Museum of Natural History 276:1-82. [ Links ]
Warwick, R. M. and K. R. Clarke. 1995. New 'biodiversity' measures reveal a decrease in taxonomic distinctness with increasing stress. Marine Ecology and Progress Series 129:301-305. [ Links ]
Warwick, R. M. and K. R. Clarke. 1998. Taxonomic distinctness and environmental assessment. Journal of Applied Ecology 35:532-543. [ Links ]
Williams, P. H. 2001. Complementarity. In Encyclopedia of biodiversity, vol. I. S. A. Levin (ed.). Academic Press. p. 813-829. [ Links ]
Williams, P. H., C. H. Humphries and R. I. Vane-Wright. 1991. Measuring biodiversity for choosing conservation areas: taxonomic relatedness for conservation priorities. Australian Systematic Botany 4:665-679. [ Links ]
Whittaker, R. J., M. B. Araújo, P. Jepson, R. J. Ladle, J. E. M. Watson and K. J. Willis. 2005. Conservation biogeography: assessment and prospect. Diversity and Distributions 11:3-23. [ Links ]














