Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de biodiversidad
versión On-line ISSN 2007-8706versión impresa ISSN 1870-3453
Rev. Mex. Biodiv. vol.83 no.3 México sep. 2012
https://doi.org/10.7550/rmb.33595
Evolución
Egg retention and intrauterine embryonic development in Sceloporus aeneus (Reptilia: Phrynosomatidae): implications for the evolution of viviparity
Retención de huevos y avance embrionario intrauterino en Sceloporus aeneus (Reptilia: Phrynosomatidae): implicaciones para la evolución de la viviparidad
Rodolfo García–Collazo1, Maricela Villagrán–Santa Cruz2, Eduardo Morales–Guillaumin3, Rubi Nelsi Meza Lázaro4,5, and Fausto R. Méndez–de la Cruz4*
1 Laboratorio de Zoología, FES–Iztacala, Universidad Nacional Autónoma de México. Av. de los Barrios No. 1 Los Reyes Iztacala, 54090 Tlalnepantla, Estado de México, México.
2 Laboratorio de Biología de la Reproducción Animal, Departamento de Biología Comparada, Facultad de Ciencias, Universidad Nacional Autónoma de México, 04510 México, D. F., México.
3 Comisión Nacional para el Conocimiento y uso de la Biodiversidad. Liga Periférico – Insurgentes Sur, Núm. 4903, Col. Parques del Pedregal, Delegación Tlalpan, 14010 México, D. F., México.
4 Departamento de Zoología, Instituto de Biología, Universidad Nacional Autónoma de México. Apartado postal 70–153, 04510 México, D. F., México. *faustor@ibiologia.unam.mx
5 Present address: Laboratorio de Herpetología, Facultad de Ciencias, Universidad Nacional Autónoma de México. Apartado postal 70–399, 04510 México, D. F., México.
Recibido: 12 agosto 2010.
Aceptado: 17 febrero 2012.
Abstract
Egg retention (ER) and intrauterine embryonic development (IED) have been considered intermediate steps in the evolution from oviparity to viviparity. Sceloporus aeneus is an oviparous lizard that is closely related to the viviparous species (Sceloporus bicanthalis). The present study addresses the following 2 questions: 1) Are intermediate stages of egg retention (ER and IED) present in populations of Sceloporus aeneus? and 2) Are ER and/or IED explained by elevation, precipitation or phylogenetic effects? Results indicate that in S. aeneus, ER differs among populations. ER was negatively related to altitude and pluvial precipitation, whereas average environmental temperature had no effect on ER or IER. In contrast to previous observations of oviparous species related to viviparous species, populations of S. aeneus with advanced IED are associated with environmental factors such as low elevation and aridity instead of high elevation and cold climate, whereas the comparative analysis shows that there are no significative evolutionary changes throughout the phylogeny, which means that the altitude has no effect on the transition to the evolution of viviparity in S. aeneus–S bicanthalis.
Key words: reproductive mode, oviparity–viviparity evolution, environmental factors.
Resumen
La retención de huevos (ER) y el desarrollo embrionario intrauterino (IED) han sido considerados pasos intermedios hacia la viviparidad. Sceloporus aeneus es un lacertilio ovíparo estrechamente relacionado a una especie vivípara (Sceloporus bicanthalis). El presente estudio derivó de las preguntas: ¿El ER y el IED presentes en las poblaciones de S. aeneus son estadios intermedios a la viviparidad? ¿ER y/o IED son explicados por la temperatura, altitud, precipitación pluvial o son un efecto filogenético? En S. aeneus, el ER y el IED fueron diferentes entre poblaciones. Los resultados indicaron que la ER se relacionó negativamente con la altitud y la precipitación pluvial, mientras que no se encontró efecto con la temperatura ambiental. En contraste con observaciones previas en especies emparentadas con las especies vivíparas, los resultados de la presente investigación indican que en las poblaciones de S. aeneus estudiadas, el mayor avance en el IED lo presentaron los organismos que habitan en baja elevación y áreas secas en lugar de alta elevación y climas fríos. Por otro lado, el análisis comparativo mostró que no existen cambios evolutivos significativos en los valores a lo largo de la filogenia, lo que significa que la altitud no tiene un efecto significativo en la transición hacia la viviparidad en Sceloporus aeneus–S. bicanthalis.
Palabras clave: modo reproductor, evolución oviparidad–viviparidad, factores ambientales.
Introduction
Squamata represents a good model to understand the evolution of viviparity as it has evolved more than 100 times independently within this group (Blackburn, 2000). In general, viviparity is considered an adaptation to cold climates (Shine, 1985a, 2004). In the genus Sceloporus, most of viviparous lizards inhabit elevations greater than 1 500 m (Guillette et al., 1980). The generally cooler temperatures at high elevations reduce the probability of successful egg incubation (Andrews, 1997). Viviparous species have been considered to evolve in cold climates, because retaining the eggs in the oviduct offers better opportunities to incubate eggs at higher or at least more constant temperatures than in the nest (Andrews, 2000; Andrews and Mathies, 2000). Therefore, the cold climate model for the evolution of viviparity was supported by evidence of prolonged egg retention in oviparous reptiles (Qualls, 1996).
The advantages of egg retention (ER) and intrauterine embryonic development (IED) may result from eggs remaining less time in the nest, which has been considered the most vulnerable stage of the organism. Although prolonged egg retention is costly for the mother, because the female is exposed for a longer time to predators while she is burdened with eggs (Shine, 1985b).
In the genus Sceloporus, viviparity has evolved at intermediate latitudes, because oviparous species have morphological and physiological features that facilitate the prolonged retaining of the egg in the oviduct (Shine, 1985a; Shine and Guillette, 1988; Guillette, 1993; Andrews, 1997; Méndez–de la Cruz et al., 1998). Viviparity, and extended ER–IED requires morphological and physiological features that facilitate the prolonged retaining of the egg in the oviduct, such as thin eggshell, increases of the oviduct vascularity, placentation, and modification in the hormonal system (Shine, 1985a; Shine and Guillette, 1988; Guillette, 1993; Andrews, 1997; Méndez–de la Cruz et al., 1998). In fact ER and IED have been poorly studied within squamata and this offers a different scenario for the evolution of viviparity.
Oviparous species closely related to the viviparous ones can offer insight into the evolution of viviparity, as in Sceloporus spinosus, which is related to the viviparous S. formosus group (Wiens and Reder, 1997). In S. spinosus maximum stage at oviposition varied within clutches from the same locality, as well as among localities. Also, the variation in maximum embryonic stage at oviposition among different study populations suggested that the invasion of high elevations was associated with an enhanced potential for longer periods of egg retention (Calderón–Espinosa et al., 2006).
Another species that may offer information related to the evolution of viviparity is Sceloporus aeneus, considered a sibling species of the viviparous S. bicanthalis (Benabib et al., 1997). Sceloporus aeneus is distributed throughout the mountainous region and exhibits both ER and IED (Andrews, 2000), a trait similar to that observed in S. spinosus. Besides being oviparous, S. aeneus inhabits a wide altitudinal gradient that ranges from 2 400 to 3 400 m, with wide ranges of yearly pluvial precipitation and environmental temperatures, which allows to test if the ER and IED is present and what environmental or physical conditions promote more advanced stages of IED.
The present study of ER in S. aeneus documents the variability of IED among 6 populations and tests the influence of the environment (altitude, precipitation, and temperature) and phylogenetic effects that may drive the maximum stage of intrauterine embryonic development as an intermediate step toward viviparity.
Materials and methods
To evaluate the capability of females to extended ER and IED during June 1998, we obtained a total of 57 gravid females of the oviparous lizard S. aeneus from 6 locations in the Transversal Neo–Volcanic mountain range in Mexico: 14 females from Milpa Alta, D. F. (MA; 19°11' N, 99°01' W, 2 400 m); 9 females from Ajusco, D. F. (Aj; 19°13' N, 99°17' W, 2 900 m); 14 females from Caimacán, Mexico (Ca; 19°37' N, 99°25' W, 2 750 m); 11 females from Autódromo, Hidalgo (Au; 20°05' N, 99°25' W, 2 450 m); 9 females from Españita, Tlaxcala (Es; 19°35' N, 98°33' W, 2 450 m) and 7 from Nevado deToluca, México (Nv; 19°11' N, 99°50' W, 3 200 m ). As an external group to root the tree, we used S. scalaris (Mathies and Andrews, 1996) and S. graciosus (Shine, 1983).
Both analyzed parameters (ER and IED) were considered capable of evolving toward viviparity. To evaluate the ER and IED from each collected female, the females were maintained on a dry substrate to promote egg retention (Andrews and Mathies, 2000), with relative moisture of 12% ± 2.0 inside a terrarium consisting of a plastic container (30 x 18.5 x 15 cm) with 3 cm of soil. We kept the environmental temperature between 22 to 24° C with an incandescent lamp with a photoperiod of 12L: 12D. We fed the lizards daily with crickets, and water was supplied every day in the morning by wetting the wall of the terrarium.
Each terrarium was checked daily to detect oviposition, as evidenced by the flaccidity of the female's abdomen or by observation of the eggs in the terrarium. To establish the degree of retention, 1 egg from each nest was fixed in 10% formaldehyde to determine the development stage according to Dufaure and Hubert (1961). Embryonic development of lizards has been divided into 40 stages, each characterized by anatomical features (Durfaure and Hubert, 1961). Squamate species laid eggs within a wide range of development stages, from gastrula (8/40) to almost complete or full development (39/40), but most of them laid at stages 25–33/40 (Blackburn, 1995).
We evaluated the effects of environmental factors using correlations between the maximum stage of development in every population and the altitude, average environmental temperature, and precipitation from each collected site. We reconstructed ancestral states of the characters (maximum stage of development from each population) using maximum parsimony unordered after Mesquite (Madisson and Madisson, 2008) and a comparative analysis with Compare 4.6b (Martins, 2004) according to phylogeny described by Meza–Lázaro (2008) and using S. scalaris and S. graciosus as the outgroups.
Results
Considerable intrapopulation variation in the maximum stage of oviposition was observed among study populations (Fig. 1). Wide variation in the stage of oviposition was observed in the Nevado de Toluca, Milpa Alta and Cahuacán populations (32–38, 33–39, 31–37, respectively), narrow variation in Autódromo and Españita (34–39, 35–39, respectively) and the narrowest variation was observed in the Ajusco population (36–38, Fig. 1). In all 6 localities, some embryos reached stages close to full development, but some lizards from Autódromo, Españita and Milpa Alta reached the most advanced developmental stages (39 of 40). However, there was no significant difference in the average stage of embryonic development among localities (F 5, 39= 1.72, p= 0.152).
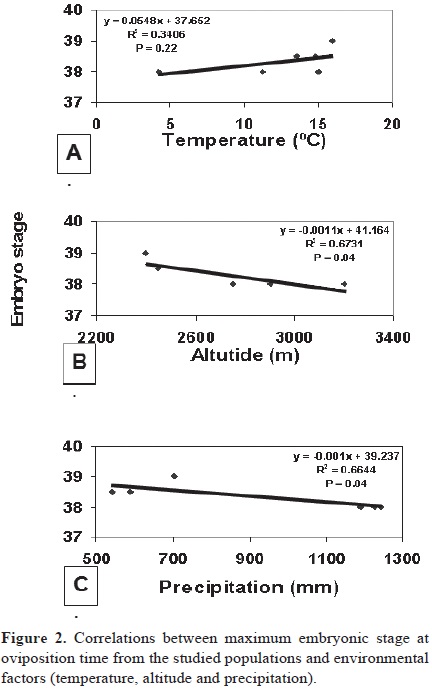
Analyses of association between environmental factors and maximum embryonic development showed no correlation between embryo stage and temperature, but a negative correlation was found with altitude and precipitation in the populations studied (Figs. 2A, B, C). Also, minimum stages of development (Fig. 1) were found to be more advanced (Au, MA, and Es) in low and arid habitats (50.1, 72.2 and 10.2 mm of pluvial precipitation) than in high elevations, as well as mesic ones (Ca and NT with 88.7 and 111 mm of pluvial precipitation respectively).
The reconstruction of ancestral states of maximum embryonic development in the studied populations (Fig. 3) shows that the ability to develop embryos in the uterus also occurs in the species used as the outgroups: S. graciosus (30/40) and S. scalaris (39.5/40). Also, advanced stages appear throughout the remaining populations of S. aeneus: the Cahuacán population had the lowest average stage (37/40), Ajusco and Nevado de Toluca (38/40), Autódromo and Españita (38.5/40), and Milpa Alta with (39/40), whereas the viviparous species S. bicanthalis shows the maximum stage (40/40). According to COMPARE (Martins, 2004), the estimated ancestral state analysis shows that there are no significant evolutionary changes throughout the phylogeny (Fig. 4)
Discussion
According to the cold climate hypothesis, viviparity has evolved in high elevations or latitudes (Tinkle and Gibbons, 1977; Shine, 1985a, 2004) in response to cold environmental temperatures. In the genus Sceloporus, this idea was supported by the evidence that most viviparous species occur at elevations higher than 1 500 m (Guillette et al., 1980). Several physiological or morphological modifications have been considered as intermediate steps toward viviparity (Blackburn, 2006). For example, some of the traits that are considered intermediate steps toward viviparity are diminished egg shell thickness, prolonged presence of the corpus luteum, increased uterine vascularization, ER and IED (Guillette, 1993; Shine, 2004, and Blackburn, 2006). A recent study has shown that populations of the oviparous lizard S. spinosus from low elevations oviposit at relatively early embryonic stages (Chamela, population: 31–32.5), whereas populations from high elevations oviposit at more advanced stages (30–35). Also, when clades containing species capable of prolonged egg retention and reinvade low elevations, they conserve the maximum stages reached by their high–elevation ancestors. Also, high levels of intrapopulation variation were observed in the maximum stage of oviposition (Calderon–Epinosa et al., 2006).
In S. scalaris, evidence has shown that populations from high elevations retain intrauterine embryos to more advanced stages than those from low elevations (33–38 vs 31–33.5, respectively (Mathies and Andrews, 1995). The same phenomenon occurs in Lerista bouganvillii, where altitude is correlated with maximum stage of embryonic development (Qualls, 1997), and high–elevation populations are viviparous. These studies support the idea that high altitudes promote intermediate stages toward viviparity (Blackburn, 1995). Contrasting with the previous evidence, S. aeneus does not show the same pattern, as the low–elevation populations retain the eggs in the oviduct to more advanced stages than high elevation populations. The 3 populations with most advanced stages of development were Españita (2 450 m) and Autódromo (2 450 m), (embryo stage: 38.5/40), whereas lizards from Milpa Alta (2 400 m) retained embryos until stage 39/40. These populations also present low precipitation values (Españita: 589 mm, Autódromo: 543 mm and Milpa Alta: 705 mm). On the other hand, in the high–elevation mesic populations such as Nevado de Toluca (with 3 200 m altitude and 1 243 mm precipitation) and Ajusco (2 900 m altitude and 1 226 mm precipitation) the maximum stage was 38, whereas lizards from Cahuacán (2 750 m elevation and 1 192 mm precipitation) retain eggs until stage 37/40. In contrast, evidence from S. spinosus and S. scalaris supports the cold climate hypothesis. In S. spinosus, low–elevation populations oviposit at relatively early stages of development (31–32.5), whereas high elevation populations oviposit at relatively advanced stages (30–35). After high elevation populations reinvade low–elevation environments, they maintain the same capacity to retain eggs to advanced stages as high–elevation populations (Calderón–Espinosa et al., 2006). On the other hand, according to Mathies and Andrews (1995), S. scalaris showed that altitude determines the maximum stage of embryonic development attained in utero, as high altitude populations oviposit embryos at more advanced stages of development than those from a lower altitude (35.5–37 vs 31.0–33.5, respectively). Nevertheless, in S. aeneus, the populations that inhabit low elevations are associated with low precipitation and also oviposit at advanced stages of embryonic development. Populations from high elevations laid eggs at earlier stages of embryonic development, contrary to the cold climate hypothesis. Even though no significant differences in maximum stages of embryonic development were found among the studied populations, more experiments should be conducted to determine which factors promote maximum stages of ER.
In oviparous species, retaining the egg demands 2 fundamental things from the mother: oxygen and water (Andrews, 1997). In S. scalaris, the oxygen required for the adequate development of the embryo in the oviduct does not seem to be limiting (Parker and Andrews, 2006). Also, the early development of the chorio–alantois may enhance oxygen delivery to the embryo during egg retention (Andrews, 1997). Considering that S. scalaris and S. aeneus have a close phylogenetic relationship, the same mechanism may facilitate intra–uterine development in S. aeneus.
The capability to retain the eggs and develop embryos until stage 39 (stage 40 is total development according to Dufaure and Hubert, 1961) inside the oviduct, in response to hydric stress, is a feature of S. scalaris (Mathies and Andrews, 1996). This capability was retained in the derived species S. aeneus (Wiens and Reeder, 1997), as observed in the population of Milpa Alta (39), and nearly observed in Españita and Autódromo, with stage 38.5. According to our results the stage of retention cannot be explained by a phylogenetic effect (Figs. 3, 4). Even though lower states were present in populations with greater amounts of precipitation and higher elevations, in contrast, in the dry, low elevation environments the females oviposit at more advanced embryo stages compared to moister, high elevation environments. Also, shorter periods of retention seem to be determined by the amount of precipitation during the month of oviposition. Populations with low precipitation values oviposit at more advanced embryo stages: in Autódromo (34), Milpa Alta (33.5) and Españita (35) during the month of oviposition (May) precipitation levels were 50.1, 73.2 and 10.2 mm respectively, whereas populations with lower minimum stages were Cahuacán (31), Nevado de Toluca (32) and Ajusco (36), which experience more rainfall (88.7, 111, 116 mm, respectively). Results indicate that higher habitats also receive more precipitation during oviposition months, causing females to exhibit low minimum retention stages, whereas in lower–altitude populations, the females acquire the capability to develop eggs to more advanced stages. In S. aeneus, retention capabilities appear different in populations that occur in higher and more mesic areas, even though this is considered a young species (3.2–4.9 mya according to Benabib et al., 1997) in contrast to the older species S. spinosus (4.2–6.5 mya), in which low–elevation clades retain the capability to advance embryos that was acquired by high–elevation clades (Calderón–Espinosa et al., 2006). The comparative analysis (Martins, 2004) determines that there are no significant evolutionary changes throughout the phylogeny. This implies also that the elevation seems to have no effect on the evolution of viviparity in the S. aeneus–S. bicanthalis.
In general, considering that prolonged egg retention is an intermediate step toward the evolution of viviparity, S. aeneus appears to be a key species to understand the environmental factors that promote advanced intrauterine embryo stages. Nevertheless, the present study shows that more advanced embryonic stages were not found in the populations that occur in high–elevation habitats with cold climate, but rather in populations that occur in lower elevations and dryer habitats.
Acknowledgements
To projects PAPIIT–UNAM 213405 and 224208 for financial support; to Elizabeth Bastiaans for the English translation of this text. Lizards were collected under permit SEMARNAT–FAUT 0074.
Literature cited
Andrews, R. M. 1997. Evolution of viviparity: Variation between 2 sceloporine lizards in the ability to extend egg retention. Journal of Zoology (London) 243:579–595. [ Links ]
Andrews, R. M. 2000. Evolution of viviparity in squamata reptiles (Sceloporus spp.) a variation of the cold–climate model. Journal of Zoology (London) 250:243–253. [ Links ]
Andrews, R. M. and T. Mathies. 2000. Natural History of Reptilian Development Constraints on the Evolution of viviparity. BioScience 50:227–238. [ Links ]
Benabib, M., K. M. Kjer and J. W. Sites. 1997. Mitocondrial DNA sequence based phylogeny and the evolution of viviparity in the Sceloporus scalaris group (Reptilia, Squamata). Evolution 51:1262–1275. [ Links ]
Blackburn, D. G. 1995. Saltationist and punctuated equilibrium models for the evolution of viviparity and placentation. Journal of Theoretical Biology 174:199–216. [ Links ]
Blackburn, D. G. 2000. Reptilian viviparity: past research, future directions, and appropriate models. Comparative Biochemistry and Physiology A: Physiology 127:391–401. [ Links ]
Blackburn, D. G. 2006. Squamate reptiles as model organism for the evolution of viviparity. Herpetological Monographs 20:131–146. [ Links ]
Calderón–Espinosa, M. L., R. M. Andrews and F. R. Méndez–de la Cruz. 2006. Evolution of egg retention in the Sceloporus spinosus group: exploring the role of physiological, environmental, and phylogenetic factors. Herpetological Monographs 20:147–158. [ Links ]
Dufaure, J. P. and J. Hubert. 1961. Table de development du lézard vivipare. Lacerta (Zoóteca) vivípara Jacquin. Archives Anatomie Microscopie Morphologie Experimental 50:309–328. [ Links ]
Guillette, L. J., Jr. 1993. The evolution of viviparity in lizards. BioScience 43:742–751. [ Links ]
Guillette, L. J., Jr., R. E. Jones, K. T. Fitzgerald and H. M. Smith. 1980. Evolution of viviparity in the lizard genus Sceloporus. Herpetologica 36:201–215. [ Links ]
Maddison, W. P. and D. R. Maddison. 2009. Mesquite: A modular system for evolutionary analysis. Version 2.72. http://mesquiteproject.org; last access: 16.V.2010. [ Links ]
Martins, E. P. 2004. COMPARE, version 4.6b. Computer programs for the statistical analysis of comparative data. Distributed by the author at http://compare.bio.indiana.edu/. Department of Biology, Indiana University, Bloomington IN. [ Links ]
Mathies, T. and R. M. Andrews. 1995. Thermal and reproductive biology og high and low elevation populations of the lizard Sceloporus scalaris: implications for the evolution of vivivarity. Oecologia 104:101–111 [ Links ]
Mathies, T. and R. M. Andrews. 1996. Extended Egg Retention and its influence on Embryonic Development and Eggs Water Balance: Implications for the Evolution of Viviparity. Physiological Zoology 69:1021–1035. [ Links ]
Méndez–de la Cruz F. R., M. Villagrán–Santa Cruz and R. M. Andrews. 1998. Evolution of viviparity in the lizard genus Sceloporus. Herpetologica 54:521–532. [ Links ]
Meza–Lázaro, R. N. 2008. Filogenia de Scelopororus aeneus y Sceloporus bicanthalis (Reptilia: Phrynosomatidae). Tesis de Maestría. Posgrado en Ciencias Biológicas, Universidad Nacional Autónoma de México, México D. F. 44 p. [ Links ]
Qualls, C. P. 1996. Influence of the evolution of viviparity on eggshell morphology in the lizard, Lerista bougainvillii. Journal of Morphology 228:119–125. [ Links ]
Qualls, C. P. 1997. The effects of reproductive mode and climate on reproductive success in the Australian lizard, Lerista bougainvillii. Journal of Herpetology 31:60–65. [ Links ]
Shine, R. 1983. Reptilian reproductive modes: the oviparity–viviparity continuum. Herpetologica 39:1–8. [ Links ]
Shine, R. 1985a. The evolution of viviparity in reptiles: an ecological analysis. In Biology of the Reptilia, C. Gans and F. Billett (eds.). Vol. 15. Academyc Press, New York. p. 605-694. [ Links ]
Shine, R. 1985b. The reproductive biology of Australian reptiles: A search for general patterns. In Biology of Australasian Frogs and Reptiles, G. Grigg, R. Shine and H. Ehmann (eds.). Royal Zoological Society of New South Wales. p. 297–303. [ Links ]
Shine, R. 2004. Does viviparity evolve in cold climate reptiles because pregnant females maintain stable (not high) body temperatures? Evolution 58:1809–1811. [ Links ]
Shine, R. and L. J. Guillette. 1988. The evolution of viviparity in Reptiles; a physiologycal model and its ecological consequences. Journal of Theoretical Biology 132:43–50. [ Links ]
Tinkle, D. W. and J. W. Gibbons. 1977. The distribution and evolution of viviparity in reptiles. Miscellaneous Publish Museum of Zoology. University of Michigan 154:1–55. [ Links ]
Wiens, J. J. and T. W. Reeder. 1997. Phylogeny of the spiny lizards (Sceloporus) based on molecular and morphological evidence. Herpetological Monographs 11:1–101. [ Links ]














