Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de biodiversidad
versión On-line ISSN 2007-8706versión impresa ISSN 1870-3453
Rev. Mex. Biodiv. vol.82 no.3 México sep. 2011
Biogeografía
Phytogeographic analysis of the genus Datura (Solanaceae) in continental Mexico
Análisis fitogeográfico del género Datura (Solanaceae) en México continental
Mario Luna–Cavazos1 and Robert Bye2*
1 Botánica, Colegio de Postgraduados, Campus Montecillo, Carretera México– Texcoco km 35.5, Montecillo, 56230 Texcoco, Estado de México, México.
2 Jardín Botánico, Instituto de Biología, Universidad Nacional Autónoma de México, Apartado postal 70–226, 04510 México, D.F., México. * Correspondent: rbyeunam@ibunam2.ibiologia.unam.mx
Recibido: 19 junio 2008;
aceptado: 15 noviembre 2010
Abstract
The geographic distribution of the species of Datura in Mexico was analyzed using numerical analysis of natural populations documented by herbarium specimens. A map of Mexico was divided into 239, 1° x 1° squares (latitude and longitude) which were used as sampling geographical units and in which the presence or absence of each species of Datura was recorded. Multivariate procedures were applied: a), TWINSPAN classification to define Datura´s main distribution areas; b), Detrended Correspondence Analysis (DCA) to define Datura´s main distribution gradients, and c), Canonical Correspondence Analysis (CCA) to relate distribution patterns with geographical and climatic factors. Species of Datura were found in 69% (165) of the squares. TWINSPAN defined 14 groups which, when associated with Mexico’s biogeographic provinces, were concentrated in the northwestern Mexican provinces as well as in the Altiplano Norte and Altiplano Sur and the Sierra Madre Occidental. DCA indicated that Datura’s main distribution patterns are explained by 3 principal gradients: altitude, humidity, and latitude. The CCA identified longitude, precipitation of the driest quarter, altitude, and average temperature of the warmest quarter as the most important variables affecting Datura´s distribution patterns. The Depresión del Balsas region of central Mexico is the area with greatest species richness of Datura.
Keywords: clustering, Datura, geographic distribution, Mexico, ordination, biogeographic provinces.
Resumen
Se analizó la distribución geográfica de las especies de Datura en México mediante análisis numérico de poblaciones naturales documentadas con ejemplares de herbario. Un mapa de México fue dividido en 239 cuadros de 1° x 1° (latitud y longitud), los cuales se usaron como unidades geográficas de muestreo, y en ellos se registró la presencia o ausencia de cada especie de Datura. Se aplicaron procedimientos estadísticos multivariados: a), clasificación por TWINSPAN para definir las principales áreas de distribución de Datura; b), análisis de correspondencia rectificado (ACR) para definir los principales gradientes en la distribución de Datura y c), análisis de correspondencia canónica (ACC) para relacionar los patrones de distribución con factores geográficos y climáticos. Las especies de Datura se localizaron en el 69 % (165) de los cuadros. TWINSPAN definió 14 grupos, los cuales, cuando se relacionaron con las provincias biogeográficas mexicanas, estuvieron concentrados en provincias del noroeste de México así como en el Altiplano Norte, Altiplano Sur y la sierra Madre Occidental. El ACR indicó que los patrones de distribución de Datura son explicados por 3 gradientes principales: altitud, humedad y latitud. El ACC definió a la longitud, precipitación del trimestre más seco, altitud y temperatura media del trimestre más cálido como las variables más importantes relacionadas con los patrones de distribución de Datura. La región de la depresión del Balsas es el área con la mayor riqueza de especies de Datura.
Palabras clave: agrupamiento, Datura, distribución geográfica, México, ordenación, provincias fisiográficas.
Introduction
The genus Datura L. includes 14 species (Hammer et al., 1983; Jiao et al., 2002): D. stramonium L., D. quercifolia Kunth and D. ferox L. of section Datura; D. discolor Bernhardi, D. inoxia Miller, D. kymatocarpa A.S. Barclay, D. lanosa A.S. Barclay ex Bye, D. metel L., D. pruinosa Greenman, D. reburra A.S. Barclay, D. wrightii Regel, D. leichhardtii F. Mueller ex Bentham, and D. velutinosa Fuentes of section Dutra Bernhardi; and D. ceratocaula Ortega of section Ceratocaulis Bernhardi. Only D. ferox, D. leichhardtii, and D. velutinosa are unknown in Mexico under natural conditions. Based upon phytogeographic evidence and historical documents, Symon and Haegi (1991) consider all the species of Datura to be native to America where Mexico is an important center of diversity of the genus. Europeans introduced such now cosmopolitan taxa as D. stramonium, D. inoxia, and D. metel to other parts of the world (Symon and Haegi, 1991). The natural (non–anthropogenic) geographic distribution of species of Datura suggests that most of the species are endemic to MegaMexico 3 as defined by Rzedowski (1993) which includes the Mexican territory plus adjacent areas in southwestern USA and northern Central America. Given that the majority of species of Datura are native to Mexico and southwestern USA, Barclay (1959) indicated that Mexico is the center of diversity and distribution of the genus and, consequently, the center of evolution and radiation. The extra–Mexican outliers have less diverse morphologies and are similar to some of the species found in Mexico. The majority of the species are adapted to xeric conditions of Mexico to which the species evolved in the region. The other taxa are thought to have migrated to (and diversified in) similar zones in southwestern South America, Australia and China. Within its natural geographic range, the Rio Balsas Depression has been considered the actual center of diversity of the genus.
Phytogeographic studies are important in the analysis and explanation of the distribution patterns of the species because they help understand past and present distribution changes (Cox and Moore, 1993) and can identify floristic areas (McLaughlin, 1994). The only study on Datura´s geography was conducted by Ewan (1944) who using leaf shapes of "Datura meteloides DC. ex Dunal" attempted a phytogeographic analysis to identify the original center of distribution of this complex in Mexico and southwestern USA. Although the variants in the leaf shapes at the periphery of distribution represent different taxa than are recognized today, Ewan considered the central populations in northern Mexico to coincide with the center of origin of this complex of related species. There are, however, no studies of Datura that include a complete phytogeographic analysis of the species found in Mexico and their relationships to environmental factors.
Many phytogeographic analyses include descriptive methods along with distribution maps, but quantitative methods that start with a record of taxa in geographic units have also been used (McLaughlin, 1994). Thus, Jardine (1972) emphasized the importance in phytogeography of using numerical methods that objectively define the distribution of taxa, adding that ordination methods indicate the distribution tendencies of the species with respect to range and direction. Examples of phytogeographic studies with multivariate methods are those by Kohlman and Sánchez (1984) for Bursera Jacq. ex L., Martínez (1995) for wild bean (Phaseolus L.) taxa, Myklestad and Birks (1993) for Salix L., Francisco–Ortega et al. (1994) for Chamaecytisus proliferus (L.Fil.) Link, and Gómez–González et al. (2004) for the tribe Cytiseae (Fabaceae).
No quantitative studies of geographic distribution for taxa of Datura have been published to date, and the phytogeographic references available for this genus consist of generalized maps in taxonomic and floristic treatments (Satina and Avery, 1959; Bye, 1996; Luna–Cavazos et al., 2000). In this study we assess the distribution of the species of Datura in continental Mexico by means of clustering and ordination multivariate analyses.
Materials and methods
Data gathering. The analysis of the geographic distribution of Datura in Mexico was based on information obtained from plant specimens deposited in the following herbaria: ASU, CHAPA, CHP, CICY, D, ENCB, FCME, K, MEXU, MICH, MO, NMC, NY, POM, TEX UC, UNM, US, and XAL (acronyms after Holmgren et al., 1990). Additional Datura collections that were made by R. Bye and collaborators during their field studies which transected Mexico from Baja California to Yucatán were also included. From the 1 260 samples of Datura, 1 035 unique geographic coordinates were obtained; specimens without precise locality data or those from previously registered sites were not included in the study.
The quantitative analysis was based on procedures suggested by Birks (1987) and McLaughlin (1994). Mexico was divided into 239 1° x 1° sampling geographical units (or squares) based upon the maps (1:1 000 000 scale) in Cuanalo et al. (1989); over two–thirds of the country had at least ones species registered employing this sampling unit size. Using the locality data from the herbarium specimens, the presence or absence of each species of Datura was recorded in each square. Afterwards, a database that noted the presence/ absence of species of Datura in squares was developed. In order to illustrate the geographic distribution of taxa and describe the regional diversity of the species of Datura, we generated maps using the program DIVA–GIS 5.2 (Hijmans et al., 2005). This same program was used to overlay these georeferenced points that were converted to decimals onto a map of the biogeographic regions as defined by Mexico’s biodiversity agency (Comisión Nacional para el Conocimiento y Uso de la Biodiversidad; CONABIO, 1997).
Climatic, topographic and geographic data. Seven climatic variables and altitude derived from WorldClim Global Climate GIS database (30–s resolution) were analyzed using the program DIVA–GIS 5.2 (Hijmans et al., 2005). Within each geographic sampling unit with a register of Datura, the climatic data for all included georeferenced points were averaged in order to produce a value for each climatic variable of each square. A similar average of latitude and longitude was calculated in the case of the sample points within each square. The topographic and climatic data from WorldClim Global Climate GIS database included: altitude (m.a.s.l.), annual mean temperature (°C), mean temperature of the wettest quarter (°C), mean temperature of the driest quarter (°C), mean temperature of the warmest quarter (°C), annual precipitation (mm), precipitation of the driest quarter (mm), and precipitation of the warmest quarter (mm). Using the variables of mean annual temperature and annual precipitation, the Aridity Index was calculated for each square (de Martonne,1926).
Analysis of the geographic distribution of Datura. One of the purposes of community analysis is to discover the similarities in biological diversity between sampling areas or sites (McCoy and Heck, 1987; McLaughlin and Bowers, 1990). Based on the above, the distribution data of 11 Mexican species of Datura were classified and arranged in order to answer 2 main questions: a), Are there similarities in the geographic distribution of the Mexican species of Datura? and b), Which environmental variables determine the similarities or differences in the geographic distribution of Mexican species of Datura?
Two complementary methods are used for the analysis of data of sites and species. Their purpose is to establish the similarities between plant communities in their response to environmental factors (Palmer, 1993): a) clustering, in which sites are grouped depending on their floristic similarity; and b) ordination, in which sites and/or species are placed along axes representing environmental gradients, that is an ordination through indirect gradient analysis (Palmer, 1993) and an analytical phase in order to explain the relationship between sites and species with environmental variables. To try to explain the geographic distribution of Datura, we used TWINSPAN (Two Way Indicators Species Analysis) as a clustering method, and Detrended Correspondence Analysis (DCA) and Canonical Correspondence Analysis (CCA) as ordination methods through indirect and direct gradient analyses, respectively.
Data Analysis
Clustering. The distribution areas of the species of Datura were classified with the use of TWINSPAN (Hill, 1979a) in order to detect general patterns in the biological data that will subsequently be related to differences in the environment (Gauch and Whittaker, 1981). Before assigning each division, TWINSPAN uses an ordination obtained with Correspondence Analysis (CA), which results in a hierarchy of the division levels; major differences would be expected in the higher hierarchy divisions, possibly revealing greater environmental gradients.
Ordination
Detrended Correspondence Analysis (DCA). The Detrended Correspondence Analysis (Hill, 1979b; Hill and Gauch, 1980), devoid of tendency, was used to detect the main patterns of floristic variation in the distribution data. The optimum for each species was estimated using reciprocal weighted averaging. The resulting eigenvalues for each axis were used as a measure of the relative importance of the ordination axes (ter Braak, 1987).
Canonical Correspondence Analysis (CCA). Repeatable general patterns of species’ distribution may be the result of a few environmental variables that affect simultaneously all species (Gauch, 1982); therefore, one of the main objectives of this sort of biogeographical studies is to detect such environmental factors (Myklestad and Birks, 1993). Thus, a Canonical Correspondence Analysis was used to correlate geographical and climatic variables with the distribution patterns of species of Datura. Mathematical models and procedures supporting the CCA are described by ter Braak (1986, 1987). We used CCA to order the squares and species related to the environment where a matrix of squares and species was related to a matrix of squares and environmental variables (McCune and Grace, 2002). The purpose of this was to test if the species are related to the measured environmental variables and to indicate the relative importance of those variables in explaining distribution patterns of species of Datura. The environmental data matrix included the 165 squares and 11 environmental characters (table not shown, but available upon request from R. Bye), which were standardized by means of a logarithmic transformation (McCune and Mefford, 1999). From the correlation matrix we obtained the eigenvalues from the first 3 ordination axes which were in turn estimated with a regression of the canonic coefficients for the standardized values. The correlation species–environment was also obtained, as well as the percentage of accumulated variance for the relation species–environment. With CCA we intend to answer the following questions: 1) Which environmental variables are more important to explain the observed distribution patterns of Datura? and 2) Are the environmental variables significantly correlated to the species’ data? Two kinds of analysis were used to answer this question: a) a Monte Carlo permutation test (99 permutations) was applied to each individual variable during the FORWARD selection of environmental variables (ter Braak, 1990) where the variables contributing the most to variance are probably the most important ones; b) a Monte Carlo permutation test from the individual axes was applied to determine if the canonical axes were greater than that at random, under the null hypothesis that there is no relationship between the floristic gradients detected for the species and the environmental variables measured.
The TWINSPAN, DCA, and CCA analyses were made with the PC–ORD Program, Version 4, MjM Software Design, Oregon, USA (McCune and Mefford, 1999).
Results
General distribution and species richness. Species of Datura are distributed over a wide area of Mexico; 165 of the 239 squares (69%) had at least 1 species of Datura. There is no 1° x 1° area that includes the 11 taxa studied. The highest number of species recorded in a single geographic sampling unit is 7; this square is located at 18°–19° N and 99°–100° W, in the state of Guerrero in the Depresión del Balsas (DB) province. Two squares containing 6 species are located at coordinates 19°–20° N and 98°–99° W, on the border of the State of Mexico and Hidalgo in the Eje Volcánico (EV) province, and at 27°–28° N and 107°–108° W, in western Chihuahua in the Sierra Madre Occidental (SMOC) province. Five taxa were found at coordinates 18°–19° N and 98°–99° W, on the border of the states of Morelos and Puebla in the DB province, and at 24°–25° N and 107°–108° W, within the state of Sinaloa in the Sonorense province (SON) (Fig. 1). The most widely distributed species are D. discolor, D. inoxia and D. stramonium, which were recorded in 87, 69, and 55 squares, respectively. The most restricted taxa are D. kymatocarpa and D. reburra, located in 7 and 4 squares, respectively.

Numerical Analysis of Datura´s distribution
Clustering. The 165 sampling geographical units with Datura formed 14 groups according to TWINSPAN (Fig. 2) that were associated with 19 biogeographic provinces. The first of the 2 TWINSPAN branches at the first level (Fig. 2) separates the groups G1 to G7 (91 squares) from G8 to G14 (74 squares). The difference is related to altitude. In the first division, the average altitude ranges from 52 m a.s.l in G7 to 1 882.3 m a.s.l in G3 with an average of 1 218.4 m a.s.l., while the altitude in the other division fluctuates between 191.5 m a.s.l. in G9 and 1 222.9 m a.s.l. in G13 with an average of 615.1 m a.s.l. (Table 1). Also, the aridity index influences the separation of these divisions where the set G1 to G7 has an average of 26.5, while the set G8 to G14 averages 16.7.
Within the first branch, G1 and G2 are characterized by D. inoxia and D. quercifolia, respectively, 2 taxa whose main distributions include central–northern Mexico (Fig. 3) and cover principally the Altiplano Norte (AN), the Altiplano Sur (AS), and the Sierra Madre Occidental (SMOC). G3 and G4 include D. ceratocaula, with the notable presence of D. stramonium (Fig. 4), elements located principally south of AS, the eastern part of SMOC, EV, and the extreme eastern DB. G3 is also represented by D. inoxia and D. quercifolia at the border of AS with EV. In the case of Groups 5, 6, and 7, the first 2 include squares mainly from central–southern Mexico, including southern Costa del Pacífico (CP), EV, DB, Sierra Madre del Sur (SMS), and isolated squares in other provinces such as extreme southern Golfo de México (GM), Yucatán (YUC), Petén (PET), and Los Altos de Chiapas (LACH) (Fig. 5). The most frequent species in the central and southern area is D. stramonium along with D. pruinosa and D. kymatocarpa to the south and D. metel further south in YUC and PET.



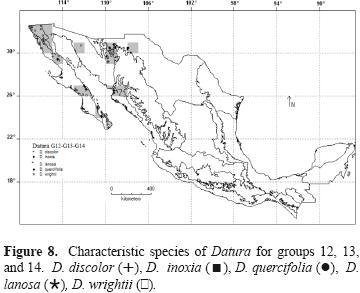
Within the second branch, groups G8, G9, and G10 include squares of northwestern Mexico, with the provinces of SON, north to CP, BC, and Del Cabo (DC), as well as AN and SMOC, and SON, where D. discolor is the characteristic species and also D. lanosa and D. reburra stand out as 2 important elements. In addition, D. discolor is found in other provinces such as CP, Tamaulipas (TAM) and YUC (Fig. 6). G11 is a set of non–contiguous squares located south of latitude 22° on both coasts of Mexico, except for 1 isolated element in BC; this group is characterized by D. stramonium and D. discolor (Fig. 7). Geographic sampling units in groups G12, G13, and G14 are located in the provinces of BC, California (CA), and DC, as well as SON, where D.wrightii and D. discolor are the characteristic species (Fig. 8).


Shifting to the sub–branches at level 2, the set G1 to G4 is characterized by altitudes that average 1 630.4 m a.s.l., range from 1 263.3 to 1 882.3 m a.s.l., and differ from all the other groups that average from 52 to 1 222.9 m a.s.l. (Fig. 2). Another important factor in these regions is the annual mean temperature, 18.2°C on average, compared to the remainder which average 21.7 °C (Table 1). The set G1 to G4 is characterized by species typical to the Altiplano Mexicano (AN and AS) as well as taxa from valleys and lower mountain areas of central Mexico, that is D. inoxia, D. quercifolia, and D. ceratocaula. Another characteristic set is that formed by G12 to G14, which includes geographic units basically from the Baja California Peninsula characterized by low precipitation levels, between 247 and 411.3 mm with an average of 306.9 mm, which are much less than those of the remaining sets with averages between 473.9 and 1335.4 mm annually. This precipitation pattern is further expressed in the low levels of the aridity index (8.3 to 14.2), typical of arid climates (de Martonne, 1926). The characteristic species of these regions are D. wrightii and D. discolor. The set G5 to G7 is differentiated by its geographic latitude, marked approximately on 20° N, and characterized by having the highest precipitation levels (1 134.5 mm on average). These levels result in higher aridity index values (33.1 on average and up to 50.5), typical of warm–sub–humid climates (de Martonne, 1926). The characteristic species are D. stramonium in central–southern Mexico (G5 and G6), and D. metel in the Yucatán Peninsula (G7). In regard to the set G8 to G11, the species are located on the Pacific coast and the Baja California Peninsula, with some squares on the coasts of the Gulf of Mexico and the Yucatán Peninsula. Altitude is the factor that differentiates these groups; the average altitude of this set is about 518.7 m a.s.l. while the others average 1 076 m a.s.l. The species appearing in all of these regions is D. discolor which, together with D. lanosa, characterizes G9, although its occurrence is very notable in G10 and G11.
Detrended Correspondence Analysis (DCA). Table 2 shows the eigenvalues of DCA’s first 3 axes which represent important biological information. These axes account for 65% of the total variation. Given that axis 3 represents a small fraction of the accumulated variance (10.5%), only the first 2 axes will be considered in the interpretation of the data. The scores of the species and the squares in the first 2 axes of DCA are found in Figure 9. The geographic tendency of change of Datura’s species along the first DCA axis (variance of 28.5%), indicates that the species composition changes with longitude and humidity from northwest Mexico (right side on the axis) to central–northern and southern Mexico (middle and left side on the axis), which shows a tendency towards a northwestern–southeastern direction. On the second axis (variance of 26.2% ), the tendency in species distribution follows a latitudinal gradient north to south in which the species whose primary distribution occurs in northern Mexico (i.e., D. quercifolia, D. lanosa, D. reburra, and D. wrightii) are located at the upper side of that axis, followed by D. stramonium. D. pruinosa and D. ceratocaula, taxa typical of central Mexico and, finally, D. kymatocarpa and D. metel, whose distribution area occurs in southern and south–southeastern Mexico. Datura inoxia and D. discolor are located in the central part of the gradient because of their wide distribution throughout Mexico. The differentiation tendencies are also evident in the squares where Datura is found (Fig. 9); axis 1 follows the northwest–southeast pattern mentioned above, which runs from the driest and lowest regions and includes mainly the squares from SON, BC, and the north of CP (right side on axis 1), and continues to the east, south and southeast of the country, which are higher and wetter areas (left side on axis 1). Axis 2 is influenced by a latitudinal factor associated with humidity; thus, the north and northwestern areas of Mexico, with less precipitation (upper side on axis 2), typical of the AN and along with the already mentioned northwestern provinces of Mexico, differ from those areas of central Mexico (middle part on axis 2) where moister areas from EV and the southern part of SMOC predominate. This tendency continues southward, to regions with a higher precipitation (approx. 1 000 mm) towards the south of the EV and SMS, ending on the far southeastern point of the Yucatán Peninsula (lower side in axis 2).

Canonical Correspondence Analysis (CCA). The first 3 eigenvalues and the percentage of accumulated variance of the data explained by the first 3 CCA axes are shown in Table 3. The first 2 axes (λ1= 0.47, λ2= 0.31) contain most of the biologically relevant information; therefore, the third axis will not be considered further. Table 4 depicts the intraset correlations of the environmental variables with axes 1 and 2, the t–values of canonical coefficients, and the variance explained by the corresponding variable.
The relative importance of environmental variables is shown on the biplot of Figure 10. The first axis is a gradient related to geographic longitude, precipitation of the driest quarter, altitude, mean temperature of the warmest quarter and aridity index. The second axis is related to mean temperature of the driest quarter, annual mean temperature, and latitude as the most important variables. The correlation species–environment on axes 1 and 2 is of the order of 0.847 and 0.809, respectively, and is significant (p < 0.01).
The geographic patterns of species of Datura in relation to environmental variables can be inferred from the biplot of Figure 10. The positions of the species on the biplot closely resemble their locations in the DCA graph (Fig. 9). The species range from the distribution areas with the higher geographic longitude, lower altitudes, and low aridity index values (right side on first axis) to lower longitudes, higher altitude, and higher aridity indices (left side on the first axis). The pattern continues from areas with high mean temperatures, high precipitation, and lower latitude (upper side on second axis) to areas with low mean temperatures, low precipitation, and higher latitude (lower side on second axis).
Discussion
General Distribution. In order to establish the origin of plant lineages, as well as their migration in space and time, we require fossil records. But when these resources are scarce, related research can include biosystematics and biogeography (Rzedowski, 1993). The descriptive analysis of the geographical distribution of species of Datura in Mexico permitted the visualization of the range of distribution of the studied species. The distribution maps allowed us: a), to identify the zones with the highest number of records of Datura species; b), to learn about the areas where there are no records of such species and which would be convenient to explore, and c), the areas of sympatry/allopatry of species. The extensive field work along with the consultation of herbarium specimens permitted the enrichment of the distributional information of the species in Mexico. In some cases, the presence of certain species in some localities was corroborated while in others, they were absent. In other cases, the records of species such as D. reburra with restricted distribution were enhanced and the inventories of those such as D. discolor, D. stramonium, and D. inoxia, which have wide ranges but an uneven coverage, were complemented.
Relationship between areas. A higher degree of similarity between sites would imply that 1 or more factors allow the exchange of taxa between them (McCoy and Heck, 1987). The similarity between the AN and AS with SMOC (G1 and G2, Fig. 2), which is confirmed by the ordination (Figs. 9, 10), suggest that shared climatic conditions favor the exchange and dispersion of taxa (e.g., D. inoxia and D. quercifolia, species typical of those provinces). This relationship between the Sierra Madre Occidental and the Mexican Plateau was also found in the geographic distribution of Phaseolus (Martínez, 1995). Another phytogeographic affinity is that of northwestern CP, SON, and BC provinces which share such species as D. discolor, D. lanosa, and D. wrightii. This distribution pattern between BC and SON provinces has also been noted for species of Bursera (Kohlmann and Sánchez, 1984) and Phaseolus (Martínez, 1995). According to Rzedowski (1978), similar climatic conditions between the physiographic region of Baja California and the Sonoran Plain – which have prevailed for approximately 10 000 years – contributed to the floristic affinity between both regions. A third example is that of SMOC, EV, and SMS as made evident by groups 4, 5, and 6 (Fig. 2). This pattern agrees with that found for Phaseolus, a genus for which this grouping of the SMOC, the western of EV, and the northwest section of SMS constitute a biological corridor between western and southern Mexico (Martínez, 1995).
Mexico’s Pacific coast, with a predominance of tropical deciduous forests, is considered an important phytogeographical region with many endemic species, and a high species richness (Lott, 1993; Ceballos and García, 1995; Gentry, 1995). In the case of Datura, this diversity is reflected in the number of species found along this coast; some of them, such as D. lanosa and D. reburra, are native to the northern portion while others are distributed mainly in the southern portion, such as D. metel and D. stramonium. In the case of the widespread D. discolor, it is very common all along Mexico’s Pacific coast from Baja California to Oaxaca.
Many species of Datura are found in Mexico´s northwestern provinces, the most frequent being D. discolor, D. lanosa, D. reburra, and D. wrightii, and, to a lesser degree, D. stramonium and D. quercifolia. This area is an important center of diversity as well as an area of endemism due to the presence of geographically restricted species D. lanosa and D. reburra (Barclay, 1959; Bye et al., 1991). This region was considered to be important in the evolution of Datura by Lockwood (1973), who designated northwestern Mexico and the adjacent area of southwestern United States as the center of origin and evolution of Datura.
Relationship between species. Species assemblages are important in relation to their presence in the geographic units because, according to Jardine (1972), the level of correspondence between the groups obtained from the clustering in the geographic distribution allows us to propose the speciation and/or dispersion centers of the species. In the case of Datura, the ordination analysis showed a distribution pattern with a northwest–southeast direction. Similar patterns have also been found in other plants, such as the wild species of Phaseolus in Mexico (Martínez, 1995).
The species’ phytogeographic patterns provide indications of their biological relationships because morphologically and genetically related taxa tend to have similar distributions. This indicates that the intrinsic characteristics of the organisms, inherited from their ancestors, determine the ecological interactions that limit their geographical distribution (Brown et al., 1996). This is evident in the relation between D. lanosa and D. wrightii; these 2 taxa are morphologically similar (Luna et al., 2000), genetically related (Palomino et al., 1988; Jiao et al., 2002) and both are distributed in arid northwestern Mexico, even though D. wrightii is more common in the southwestern United States. On the other hand, comparable distribution patterns of taxonomically unrelated species may indicate the occupation of microhabitats within a broad ecological region suggesting their tolerance of certain climatic parameters that results in sympatry of species from different sections (Gómez–González et al., 2004); such as the case of D. inoxia, D. quercifolia, and D. ceratocaula, of sections Dutra, Datura, and Ceratocaulis, respectively (Satina and Avery, 1959; Hammer et al., 1983) that grow together on the Mexican Plateau.
Endemic species are the result of isolation due to geographical and ecological barriers (Gómez–González et al., 2004); this explanation is appropriate in the case of D. kymatocarpa, a species endemic to the DB province in Guerrero, an inland basin surrounded by the EV and SMS, a site where numerous endemic species from different taxonomic groups have been recorded (Kohlmann and Sánchez, 1984). Also, many endemic taxa in Mexico are concentrated mainly in regions with dry climatic conditions (Rzedowski, 1993); in the case of Datura, this applies to D. lanosa and D. reburra, native to the dry northwestern areas of Mexico. Datura wrightii is a similar case because, although it is not endemic to geopolitical Mexico, it falls within the northern portion of MegaMexico (Rzedowski, 1993) which includes areas with similar arid climate in adjacent southwestern United States.
Probably the wide distribution of some species of Datura, such as D. stramonium, is related to human migrations (Satina and Avery, 1959) and to environmental alterations associated with human activity. The same could be true for D. inoxia, a species frequently grown in home gardens for medicinal and ornamental purposes (Satina and Avery, 1959; Luna–Cavazos, 2001). Datura metel is cultivated as an ornamental plant, which would imply a wider distribution in Mexico, but its adaptation to regions with a warm–humid climate, such as the one prevailing in southeastern Mexico, limits its distribution in other regions of the country.
Finally, the assemblages of groups based on floristic elements and floristic regions must be considered as reference points and not as definitive entities (Myklestad and Birks, 1993). With this research we propose that the distribution patterns uncovered in this study are a significant step forward in understanding the phytogeography of Datura.
Acknowledgments
We extend our gratitude to curators and staff of the herbaria that loaned the specimens and, in particular, to colleagues at COLO and MEXU. We acknowledge the bibliographic, field, and technical assistance of José Arellano, Jennifer Bain, Bruce Bartholomew, Francisco Basurto, Germán Bojórquez, Rafael Corral, Carlos Díaz, Francisco Félix, Oscar Ferrera, Raymundo García, Elia Herrera, Martín Hilerio, María Antonieta Isidro, Edelmira Linares, Rigoberto López, Gilberto Márquez, Miguel Ángel Martínez, Myrna Mendoza, Gustavo Morales, Eduardo Palacios, Guillermo Pichardo, Isaac Reyes, Lourdes Rico, Joel Rodríguez, Richard Spellenberg, Miguel Trejo, William A. Weber, and Hugh Wilson. Partial financial support for this work was provided by Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (Project 088), International Cooperative Biodiversity Groups ("Bioactive agents from dryland biodiversity of Latin America" grant U01 TW 00316 from the National Institutes of Health, National Science Foundation, and USAID), and Universidad Nacional Autónoma de México. Consejo Nacional de Ciencia y Tecnología supported this work by providing a doctoral scholarship to ML.
Literature cited
Barclay, A. S. 1959. New considerations in an old genus: Datura. Botanical Museum Leaflets (Harvard University) 18:245–272. [ Links ]
Birks, H. J. B. 1987. Recent methodological developments in quantitative descriptive biogeography. Annals of Zoologici Fennici 24:165–178 [ Links ]
Brown, J. H., G. C. Stevens and D. W. Kaufman. 1996. The geographic range: size, shape, boundaries, and internal structure. Annual Review of Ecology and Systematics 27:597–623. [ Links ]
Bye, R. 1996. Biodiversidad de Datura (Solanaceae) en México. Reporte final del proyecto PO88 a la Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO). México, D.F., 13 p. 10 tables and 4 maps. [ Links ]
Bye, R. A., R. Mata and J. Pimentel. 1991. Botany, ethnobotany and chemistry of Datura lanosa (Solanaceae) in Mexico. Anales del Instituto de Biología de la Universidad Nacional Autónoma de México, Serie Botánica 61:21–42. [ Links ]
Ceballos, G. and A. García. 1995. Conserving neotropical biodiversity: the role of dry forest in western Mexico. Conservation Biology 9:1349–1356. [ Links ]
Comisión Nacional para el Conocimiento y Uso de la Biodiversidad–CONABIO. 1997. Provincias biogeográficas de México. Escala 1: 4,000,000. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, México, D.F. [ Links ]
Cox, C. B. and P. D. Moore. 1993. Biogeography: an ecological and evolutionary approach. Blackwell, Oxford. 234 p. [ Links ]
Cuanalo, H., E. Ojeda, A. Santos and C. A. Ortiz. 1989. Provincias, regiones y subregiones terrestres de México. Centro de Edafología, Colegio de Postgraduados, Chapingo, México. 624 p. [ Links ]
de Martonne, E. 1926. Aréisme et indices d'aridité. Académie des Sciences, Paris, Comptes Rendus 182 :1395–1398. [ Links ]
Hijmans, R. J., L. Guarino, A. Jarvis, R. O'Brien, P. Mathur, C. Bussink, M. Cruz, I. Barrantes and E. Rojas. 2005. DIVA–GIS Version 5.2. A geographic information system for the management and analysis of genetic resources data. International Potato Center and International Plant Genetic Resources Research Institute, Lima, Peru. [ Links ]
Ewan, J. 1944. The perennial southwestern Datura and the validity of Matthew's hypothesis in plant geography. Proceedings of the California Academy of Sciences 25:235–244. [ Links ]
Francisco–Ortega, J., M. T. Jackson, A. Santos–Guerra, M. Fernández–Galván and B. V. Ford–Lloyd. 1994. The phytogeography of the Chamaecytisus proliferus (L. fil.) Link (Fabaceae: Genistae) complex in the Canary Islands: a multivariate analysis. Vegetatio 110:1–17. [ Links ]
Gauch, H. G. and R. H. Whittaker. 1981. Hierarchical classification of community data. Journal of Ecology 69:537–557. [ Links ]
Gauch, H. G. 1982. Noise reduction by eigenvector ordinations. Ecology 63:1643–1649. [ Links ]
Gentry, A. H. 1995. Diversity and floristic composition on Neotropical dry forest. In Seasonally dry forest, S. H. Bullock, H.A. Mooney and E. Medina (eds.). Cambridge University Press, Cambridge. p. 146–194. [ Links ]
Gómez–González, S., L. A. Cavieres, E. A. Teneb and J. Arroyo. 2004. Biogeographical analysis of species of the tribe Cytiseae (Fabaceae) in the Iberian Peninsula and Balearic Islands. Journal of Biogeography 31:1659–1671. [ Links ]
Hammer, K., A. Romeike and C. Tittel. 1983. Vorarbeiten zur monographischen Darstellung von Wildpflanzensortimenten: Datura L. sections Dutra Bernh., Ceratocaulis Bernh. et Datura. Kulturpflanze 31:13–75. [ Links ]
Hill, M. O. 1979 a. TWINSPAN. A fortran program for arranging multivariate data in an ordered two–way table by classification of the individuals and attributes. Cornell University, Ithaca, NY. [ Links ]
Hill, M. O. 1979 b. DECORANA– A FORTRAN program for detrended correspondence analysis and reciprocal averaging. Cornell University, Ithaca, NY. [ Links ]
Hill, M. O. and H. G. Gauch. 1980. Detrended correspondance analysis: an improved ordination technique. Vegetatio 42:47–58. [ Links ]
Holmgren, P. K., N. H. Holmgren and L. C. Barnett. 1990. Index Herbariorum, Part I: The Herbaria of the world. New York Botanical Garden, New York. 693 p. [ Links ]
Jardine, N. 1972. Computational methods in the study of plant distributions. In Taxonomy, phytogeography and evolution, D. H. Valentine (ed.). Academic Press, London. p. 381–393. [ Links ]
Jiao, M., M. Luna–Cavazos and R. Bye. 2002 Allozyme variation in Mexican species and classification of Datura (Solanaceae). Plant Systematics and Evolution 232:155–166. [ Links ]
Kohlmann, B. and S. Sánchez. 1984. Estudio aerográfico del género Bursera Jacq. ex L. (Burseraceae) en México: una síntesis de métodos. In Métodos cuantitativos en la biogeografía, Publicación No. 12, E. Ezcurra, M. Equihua, B. Kolman and S. Sánchez–Colón (eds.). Instituto de Ecología, A.C., México. p. 41–120. [ Links ]
Lockwood, T. E. 1973. Generic recognition of Brugmansia. Botanical Museum Leaflets (Harvard University) 23:273–284. [ Links ]
Lott, E. J. 1993. Annotated checklist of the vascular flora of the Chamela Bay region, Jalisco, México. Occasional Papers of the California Academy of Sciences 148:1–60. [ Links ]
Luna–Cavazos, M. 2001. Variación y domesticación de Datura metel L. (Solanaceae). Thesis. Facultad de Ciencias, Universidad Nacional Autónoma de México, México, D.F. 190 p. [ Links ]
Luna–Cavazos, M., M. Jiao and R. Bye. 2000. Phenetic analysis of Datura section Dutra (Solanaceae) in Mexico. Botanical Journal of the Linnean Society 133:493–507. [ Links ]
Martínez, J. V. 1995. Fitogeografía de los taxones silvestres de Phaseolus en México y Guatemala. Tesis. Colegio de Postgraduados, Montecillo, México. 226 p. [ Links ]
McLaughlin, S. P. 1994. Floristic plant geography: the classification of floristic areas and floristic elements. Progress in Physical Geography 18:185–208. [ Links ]
McLaughlin, S. P and J. E. Bowers. 1990. A floristic analysis and checklist for the northern Santa Rita Mountains, Pima Co., Arizona. The Southwestern Naturalist 35:61–75. [ Links ]
McCoy, E. D. and K. L. Heck. 1987. Some observations on the use of taxonomic similarity in large–scale biogeography. Journal of Biogeography 14:79–87. [ Links ]
McCune, B. and M. J. Mefford. 1999. PC–ORD.Multivariate analysis of ecological data, version 4. MjM software design, Gleneden Beach, OR. 237 p. [ Links ]
McCune, B. and J. B. Grace. 2002. Analysis of ecological communities. MjM Software Design, Gleneden Beach, OR. 300 p. [ Links ]
Myklestad, A. and H. J. B. Birks. 1993. A numerical analysis of the distribution patterns of Salix L. species in Europe. Journal of Biogeography 20:1–32. [ Links ]
Palomino, G., R. Viveros and R. A. Bye. 1988. Cytology of five Mexicam species of Datura L. (Solanaceae). The Southwestern Naturalist 33:85–90. [ Links ]
Palmer, M. W. 1993. Putting things in even better order: the advantages of canonical correspondence analysis. Ecology 74:2215–2230. [ Links ]
Rzedowski, J. 1978. Vegetación de México. Limusa, México, D.F. 432 p. [ Links ]
Rzedowski, J. 1993. Diversity and origins of the phanerogamic flora of Mexico. In Biological diversity of Mexico, origins and distribution. T. P. Ramamoorthy, R. Bye, A. Lot and J. Fa (eds.). Oxford University Press, New York. p. 129–144. [ Links ]
Satina, S., and A. G. Avery. 1959. A review of the taxonomy history of Datura. In Blakeslee: The genus Datura, A.G. Avery, S. Satina and J. Rietsema (eds.). The Ronald Press, New York. p. 16–47. [ Links ]
Symon, D. and L. A. R. Haegi. 1991. Datura (Solanaceae) is a new world genus. In Solanaceae III: taxonomy, chemistry, evolution, J. G. Hawkes, R. N. Lester, M. Nee and N. Estrada (eds.). Academic Press, London. p. 197–210. [ Links ]
ter Braak, C. J. F. 1986. Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67:1167–1179. [ Links ]
ter Braak, C. J. F. 1987. The analysis of vegetation–environment relationships by canonical correspondence analysis. Vegetatio 69:69–77. [ Links ]
ter Braak, C. J. F. 1990. Update notes: CANOCO version 3.10. Agricultural Mathematics Group, Wageningen. [ Links ]














