Services on Demand
Journal
Article
Indicators
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de biodiversidad
On-line version ISSN 2007-8706Print version ISSN 1870-3453
Rev. Mex. Biodiv. vol.82 n.3 México Sep. 2011
Ecología
Diet of Rhinella scitula (Anura, Bufonidae) in the Cerrado, Brazil: the importance of seasons and body size
Dieta de Rhinella scitula (Anura, Bufonidae): la importancia de la variación estacional y la talla corporal
Franciéle P. Maragno1* and Franco L. Souza2
1 Programa de Pós–Graduação em Biodiversidade Animal, Universidade Federal de Santa Maria, Centro de Ciências Naturais e Exatas, 97105–900, Santa Maria, Brasil. * Correspondent: fmaragno@gmail.com
2 Universidade Federal de Mato Grosso do Sul, Centro de Ciências Biológicas e da Saúde, Departamento de Biologia, 79060–900 Campo Grande, Brasil.
Recibido: 28 abril 2010;
aceptado: 10 enero 2011
Abstract
The aims of this study were (1) to analyze the diet of Rhinella scitula in different seasons (dry and rainy), and (2) to examine resource partitioning among sexes and body–size categories. Individuals were collected during active searches along a riverbank in the Serra da Bodoquena National Park, Brazil. Formicidae, followed by Coleoptera and Isoptera, had the highest importance index values for males, females, and all individuals combined. Diet composition was similar between males and females. Larger individuals consumed larger prey, although they fed on small prey as well. Similar–sized individuals had high dietary overlap. Smaller individuals had a diet as broad as larger individuals, although composed of different items. Formicidae was the most common prey item for animals collected in both the dry and rainy seasons, but was more important in the rainy season. During the dry season, R. scitula remained closer to the edge of the water bodies and showed the widest dietary niche, represented by similar importance index values.
Keywords: amphibians, diet, seasonal.
Resumen
Los objetivos de este estudio fueron (1), analizar la dieta de Rhinella scitula en 2 estaciones del año y (2), examinar el reparto de recursos entre sexos y entre diferentes categorías de tamaño corporal. Los ejemplares fueron capturados mediante búsqueda visual a lo largo de las orillas de un riachuelo del Parque Nacional da Serra da Bodoquena. Los individuos pertenecientes a los grupos Formicidae, seguidos por Coleoptera e Isoptera fueron las presas con mayores valores de importancia para machos, hembras y para todos los individuos de ambos sexos combinados. No se registraron diferencias entre sexos en la composición de la dieta. Los individuos de mayor tamaño, consumieron presas de mayor volumen, si bien no dejaron de consumir presas pequeñas. La superposición de dieta fue mayor entre individuos pertenecientes a clases de talla próximas. Los sapos de menor tamaño presentaron una dieta tan amplia como los más grandes, si bien su dieta estaba compuesta por ítems diferentes. Aunque predominaron ejemplares del grupo Formicidae en los contenidos estomacales de organismos capturados tanto en el período seco como en el lluvioso, su importancia fue mayor en este último. Durante la estación seca, R. scitula permaneció más próximo al agua y presentó un nicho trófico más amplio.
Palabras clave: anfibios, dieta, estacionalidad.
Introduction
Information about food habits is important to identify habitat conditions and to determine the influence of prey availability on the distribution of a species (Parker and Goldstein, 2004). The feeding strategy of amphibians includes choice, location, capture, and ingestion of prey. For leaf–litter anurans, the mechanisms that determine differences in sizes and types of prey are related to the foraging mode (Toft, 1981; Lima and Magnusson, 1998).
Individuals of the same species can differ in types and quantities of ingested prey. Among populations, variation in diet may be caused by differences in habitat–prey composition or by different prey–selection behaviors (Bonansea and Vaira, 2007). In leaf–litter anurans, variation in diet during ontogeny can be as large as or larger than the differences in diet among species (Lima and Magnusson, 1998). Ontogenetic changes are a consequence of a predator’s capacity to subdue its prey, if predator size limits prey size. However, as well as prey size, variation in diet composition can be a result of different types of prey consumed (Lima and Moreira, 1993). Habitat use and sex can also result in differences in diet within a population (Wu et al., 2005). Furthermore, external factors such as seasonal prey availability may cause seasonal changes in predators’ diets, due to population dynamics of the prey (Hirai and Matsui, 2001; Maneyro et al., 2004; Santos et al., 2004).
Bufonids are usually considered generalists, and their diets reflect prey availability. However, studies that took prey availability into account have suggested that some species select their prey, consuming items in different proportions than would be expected considering their availability (Toft, 1981; Strüssmann et al., 1984; Hirai and Matsui, 2002; Isacch and Barg, 2002; Moseley et al., 2005).
Rhinella scitula Caramaschi and Niemeyer (2003) is a member of the Rhinella margaritifera group, and occurs at the type locality (Bonito County), Bodoquena County (Uetanabaro et al., 2007), and in the Piraputanga district in Aquidauana County (Maragno and Souza, 2007), all in the state of Mato Grosso do Sul, Brazil. It also occurs in Amambay and Concepción in Paraguay (Brusquetti and Lavilla, 2006). R. scitula inhabits Cerrado regions, and seems to be associated with leaf litter near creeks with well–preserved gallery vegetation (Caramaschi and Niemeyer, 2003; Maragno and Souza, 2007). The aims of the present study were to analyze the diet of R. scitula in the dry and rainy seasons, and resource partitioning among sexes and individuals of different body sizes.
Materials and methods
The study was carried out in the Serra da Bodoquena National Park, Mato Grosso do Sul, Brazil (Fig. 1). The Bodoquena Mountain Range is about 300 km long and 20 to 50 km wide. The National Park is located in the middle of the range, and covers a massive rocky area at altitudes from 450 to 650 m. The climate of the region is humid tropical, with maximum temperatures ranging from 35 – 40°C and the minimum temperature approaching 0°C during June and July (Alvarenga et al., 1982). The vegetation belongs to the Cerrado biome, with deciduous and semideciduous seasonal forest in the highest parts, and typical gallery forest along the rivers (Pott and Pott, 1994; Damasceno Jr. et al., 2000).

The sampling area was located on both banks of the Salobrinha River (around 20°40’49’’S and 56°53’17’’W), which flows into the Salobra River. The Serra da Bodoquena has streams with rocky bottoms, subterranean in some parts, and with marked seasonal changes in flow. During rainy periods, some streams receive large volumes of water and overflow their banks (Filho et al., 2004). The riparian forest is well preserved.
We collected data in 2 seven–day sampling periods, 1 in the dry season (July 2006) and the other in the rainy season (March 2007). Two people walked about 2 kilometers along the riverbanks in each field excursion, and a different part of the bank was observed each day. We walked randomly from close to the water’s edge to about 50 m distant from the water, searching for R. scitula. We found and collected individuals of different sizes. We measured the distance of each individual from the water’s edge. To preserve the stomach contents, we immediately killed the animals captured using 5% xylocain pomade on their belly, and after death, we fixed them with 10% formaldehyde solution. In the laboratory, we measured their snout–vent length (SVL) and determined the sex by direct observation of the gonads. Animals collected were deposited in the Zoological Collection of Mato Grosso do Sul Federal University.
We identified prey items to order level, under a stereoscopic microscope, using Borror and DeLong (1988) as reference. All larvae were considered a single resource; spiders and scorpions were grouped in Arachnidae. We calculated numeric and volumetric percentages of the food items. We estimated volume by the parallelepiped formula using a millimetered plaque (Hellawell and Abel, 1971). For each stomach sample, we grouped all items of the same category and used the total volume. For example, in each stomach, all the formicids were combined and the total volume was measured. We also measured de volume of the largest prey in the stomach. We used this value to evaluate the association between the body size (snout–vent length (SVL) of an individual and the prey with greater volume in its stomach (largest prey in the stomach). We used the Spearman correlation coefficient to answer this question. Each variable was transformed to its natural logarithm to correct for differences in measuring units (Zar, 1999).
For the next indices, we used the following classification of individuals: 1. all grouped together; 2. separated by sex; 3. by body size categories (SVL); and 4. by seasons (dry and rainy). The categories of body size were defined in 2 distinct forms. First, since individuals smaller than 20 mm did not have differentiated gonads, they were considered young and we compared their diet with individuals with SVL ≥ 20 mm. We were also interested in a more refined relation of amphibian body size and prey type and size. The diet of amphibians may be limited to their ability to subjugate prey, and since the largest R. scitula individual found in this study had 53.6 mm, we decide to create 5 body size categories: ≤9.9 mm, 10 to 19.9 mm, 20 to 29.9 mm, 30 to 39.9 mm and ≥ 40 mm.
We calculated prey indices of relative importance according to Pinkas et al. (1971):
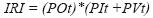
where IRI is the index of relative importance; POt is the percentage of occurrence (percentage of stomachs containing item t); PIt is the numerical percentage (percentage of item t in the total of items consumed in all stomachs); PVt is the volumetric percentage (volumetric percentage of item t in the total of items in all stomachs). The higher the index, the higher the importance of the item.
We calculated the dietary niche width in relation to food items according to Simpson (1949):

where pij is the probability of encountering item i in sample j. The higher the index value, indicates the lower probability of encountering each item in the sample, and the wider will be the dietary niche.

We calculated dietary overlap ccording to Morisita simplified indices (Krebs, 1989):
where CH is the Morisita simplified index between j and k classes; pij is the numerical proportion of resource i in the total of resources used by j; pik is the numerical proportion of resource i in the total of resources used by k. The index varies from 0 (no overlap) to 1 (complete overlap). We used the Mann–Whitney test if individuals were found at different distances from the water’s edge in each season (Zar, 1999).
Results
In the 2 seasons we collected 99 individuals, of which 88 had prey in their stomachs (Table 1). Of these, 29 were females, 30 males, and 29 were considered juveniles. In the dry season, 47 individuals had prey in the stomachs and during the rainy season we captures 41 individuals with prey in the stomach. Plant material was found in 9 samples (10.2%) and in small amounts, represented by flowers and leaf parts.
Ants, followed by coleopterans and termites, were the items with the highest importance index values for males, females, and all individuals combined. Collembolans, mites, and psocopterans were the main items for small individuals (SVL ≤ 9.9 mm), whereas ants and coleopterans had the highest importance indices for the larger individuals (> 10 mm). Among the young individual category (SVL < 20 mm), ants followed by mites and coleopterans showed the highest values. Ants were the most important item for individuals collected in the dry and rainy seasons, followed by coleopterans (mainly in the dry season) and termites (mainly in the rainy season) (Table 2).
The volume of the largest prey items in the stomachs varied from 0.02 mm3 (winged Hymenoptera) to 240 mm3 (Coleoptera). The largest individuals consumed the largest prey (rs = 0.673; p < 0.001; n = 88). Diet composition was similar between males and females, indicated by 94% diet overlap and similar diet width (Table 3, Fig. 2). Individuals with SVL ≤ 9.9 mm consumed very different prey items than those consumed by larger individuals, with the widest dietary niche. Larger individuals had the second widest dietary niche, with a minor consumption of ants (Tables 2 and 3, Fig. 2). For individuals with body lengths greater than 10 mm, the greatest dietary similarity was among closer body size categories. However, if individuals from this same body size category (SVL > 10 mm) was compared with individuals from the largest body size category (SVL > 40 mm), the diet is less than 50% similar (Table 4; Fig. 2). Considering only 2 body size categories (SVL < 20 mm and SVL ≥ 20 mm), we found individuals had similar diet width and overlap in dietary niche in almost 90% (Tables 3 and 4).


The dietary niche was twice the width in the dry season than in the rainy season (Table 3) and the 67% of the diet overlap among seasons was due mainly to ant consumption. Other prey items were consumed in distinct proportions (Table 2 and Fig. 2). There was a significant difference in distance of individuals from water’s edge between seasons (Mann–Whitney U = 153; p < 0.001). They were found closer to water’s edge in the dry (mean, interval = 1.81, 0.2 – 12.3 m; n = 30) than in the rainy season (mean, interval = 8.04 m, 0.5 – 26.5 m; n = 40).
Discussion
Ants, coleopterans, and termites are considered important in anuran feeding, particularly in the Bufonidae. These prey items have been recorded in many diet studies (Berry and Bullock, 1962; Toft, 1981; Strüssmann et al., 1984; Lajmanovich, 1994; Menéndez–Guerrero, 2001; Hirai and Matsui, 2002; Isacch and Barg, 2002; Nicoara et al., 2005; Moseley et al., 2005 and Bull, 2006). Ants and coleopterans were found in higher proportions than other prey items in 26 of 29 studies on 14 bufonid species in many regions of the world (Clarke, 1974a). According to the present study, the diet of R. scitula is similar to that of other bufonids, including species of the R. margaritifera group (Toft, 1981; Menéndez–Guerrero, 2001).
Amphibians do not manipulate their prey, so prey size limits consumption. For R. scitula, the volume of prey consumed increased according to body size, and the largest items consumed were diplopods, lepidopterans, and large coleopterans. Ants and coleopterans were the small items consumed by larger individuals. Whitefield and Donnelly (2006) observed that a shift in prey size during ontogeny is a common pattern in leaf–litter amphibians and lizards; and it is more intense in species which show a large variation in body size from metamorphose to adult length. However, a shift in prey type was observed only in species which have a small variation in body size during ontogeny, such as Rhaebo haematiticus (Whitefield and Donnelly, 2006).
There was a great difference in the diet composition between the smallest and largest body size category of R. scitulla. When we consider only 2 categories of body size (< 20 and ≥ 20mm), they overlap in almost 90% and have a similar dietary width. However, if a more refined classification is considered, the diet of the smallest individuals overlaps in less than 10% with the diet of the largest individuals. In these 2 body size extremes, we find the greatest diet width. Besides, utilizing this body size classification, it is possible to observe which dietary items were consumed along the growth stages. Among bufonids, small individuals prey mainly on collembolans and mites, whereas large individuals prey on ants, coleopterans, and termites (Clarke, 1974a; Strüssmann et al., 1984; Flowers and Graves, 1995), as was observed for R. scitula. Heterogeneous habitats, such as leaf litter, support a great diversity and richness of ants (Vargas et al., 2007). Rhinella scitula uses forest leaf litter and consumes large amounts of ants. The size of ants could be a limiting factor for small individuals, and this item was mainly consumed by medium and larger individuals.
Besides dietary differences related to morphological limitations on catching prey, dietary variation may be caused by different predatory behaviors among individuals of different body sizes. For Rhinella margaritifera, the total distance moved during prey search and movement frequency per minute were related to individual body size (Lima and Magnusson, 1998, 2000). The predation behavior of R. scitula was not evaluated in the present study, but it is possible that it is similar to that of R. margaritifera, since they belong to the same taxonomic complex, eat the same types of prey, and are found in similar habitats.
In this study we found that the diet was similar between the sexes of R. scitula. In other studies on bufonids A. fowleri (Clarke, 1974a), P. viridis (Nicoara et al., 2005), B. j. formosus (Hirai and Matsui, 2002), and A. boreas (Bull, 2006) no sexual dietary differences were found. Biavati et al. (2004) found that for Ameerenga flavopicta, a species from Cerrado, the diet was independent of sex, but related with the reproductive stage of females and the seasons of year (rainy or dry). In the present study, few reproductive females were found and this may be the reason of the similarity between the diet of males and females. However, the diet of R. scitula was distinct between the dry and rainy seasons.
In the rainy season, dietary niche was narrow as a consequence of high ant consumption, and the second most important item (termites) had a much lower importance index. Ant activity is related to rainfall (Levings, 1983) and in the Cerrado, ants are more active in the warm rainy months (Silvestre and Brandão, 2001). During the rainy season, individuals of R. scitulla were found dispersed through forest leaf–litter farther from the water’s edge than in the dry season. The greater availability of ants during the rainy season associated with higher individual mobility may be the cause of greater seasonal ant consumption.
During the dry season, individuals showed the widest dietary niche, represented by similar importance index values. Consumption of larvae was almost as important as termites during the dry season and mainly larvae of dipterans, coleopterans, and lepidopterans were consumed. Many coleopteran and lepidopteran species spend the winter in larval form (Borror and DeLong, 1988), and could therefore be found more frequently. Individuals of R. scitula remained closer to the water’s edge in the dry season than in the rainy season, and this is reflected on aquatic prey consumption (larvae of dipterans). Furthermore, food items that were consumed only in the dry season, such as psocopterans, are found under stones and in humid places (Borror and DeLong, 1988).
This was the first study concerning R. scitula. In the area surrounding the Serra da Bodoquena National Park, the natural vegetation is being replaced by agricultural crops and cattle, destroying natural habitats. This situation is a threat to this species, and the population may become restricted to the park area. Therefore, additional studies are important to evaluate the distribution and conservation status of the species.
Acknowledgements
We thank the Director of the Serra da Bodoquena National Park for permitting this research in the park. Thanks to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for supporting F. P. Maragno. F.L. Souza received a research grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq (306034/2008–5). All procedures were approved by the Federal University of Mato Grosso do Sul Animal Care and Use Committee (Protocol 148/ 2007).
Literature cited
Alvarenga, S. M., A. E. Brasil and D. M. Del'arco. 1982. Geomorfologia. IBGE–Levantamento de Recursos Naturais 28:125–184. [ Links ]
Berry, P. Y. and J. A. Bullock. 1962. The food of common Malayan toad, Bufo melanosticus Schneider. Copeia 4:736–741. [ Links ]
Biavati, G. M., H. C. Wiederhecker and G. Colli. 2004. Diet of Epipedobates flavopictus (Anura: Dendrobatidae) in a neotropical savanna. Journal of Herpetology 38:510–518. [ Links ]
Bonansea, M. I. and M. Vaira. 2007. Geographic variation on the diet of Melanophryniscus rubriventris (Anura: Bufonidae) in northwestern Argentina. Journal of Herpetology 41:231–236. [ Links ]
Borror, D. J. and D. M. DeLong. 1988. Introdução ao Estudo dos Insetos. Edgard Blücher. São Paulo. Brasil. 653 p. [ Links ]
Brusquetti, F. and E. O. Lavilla. 2006. Lista comentada de los anfibios de Paraguay. Cuadernos de Herpetología 20:3–79. [ Links ]
Bull, E. L. 2006. Sexual differences in the ecology and habitat selection of western toads (Bufo boreas) in northeastern Oregon. Herpetological Conservation and Biology 1:27–38. [ Links ]
Caramaschi, U. and H. Niemeyer. 2003. Nova espécie do complexo de Bufo margaritifer (Laurenti, 1768) do Estado do Mato Grosso do Sul, Brasil (Amphibia, Anura, Bufonidae). Boletim do Museu Nacional–Zoologia 501:1–16. [ Links ]
Clarke, R. D. 1974a. Food habits of toads, genus Bufo (Amphibia: Bufonidae). American Midland Naturalist 91:140–147. [ Links ]
Clarke, R. D. 1974b. Postmetamorphic growth rates in a natural population of Fowler`s toad, Bufo woodhousei fowleri. Canadian Journal of Zoology 52:489–1498. [ Links ]
Damasceno Jr., G. A., J. N. Nakajima and U. M. Rezende. 2000. Levantamento florístico das cabeceiras dos rios Negro, Aquidauana, Taquari e Miranda no Pantanal, Mato Grosso do Sul, Brasil. In RAP Bulletin of Biological Assesment, 18: Uma Avaliação Biológica dos Ecossistemas Aquáticos do Pantanal, Mato Grosso do Sul, Brasil, P. W. Willink, B. Chernoff, L. Alonso, J. R. Montambault and R. Lourival (eds.). Conservation International, Washington. p. 152–162. [ Links ]
Filho, W. S., I. Karmann and P. C. Boggiani. 2004. Paisagens cársticas da Serra da Bodoquena (MS). In Geologia do Continente Sul–Americano: evolução da obra de Fernando Flávio Marques de Almeida, V. Mantesso–Neto, A. Bartorelli, C. D. R. Carneiro and B. B. Brito–Neves (eds.). Beda, São Paulo. p. 423–433. [ Links ]
Flowers, M. A. and B. M. Graves. 1995. Prey selectivity and size–specific diet changes in Bufo cognatus and B. woodhousii during early post metamorphic ontogeny. Journal of Herpetology 29:608–612. [ Links ]
Hellawell, J. M. and R. Abel. 1971. A rapid volumetric method for analysis of the food of fishes. Journal of Fish Biology 3:29–37. [ Links ]
Hirai, T. and M. Matsui. 2001. Food habits of an endangered Japanese frog, Rana porosa brevipoda. Ecological Research 16:737–743. [ Links ]
Hirai, T. and M. Matsui. 2002. Feeding ecology of Bufo japonicus formosus from the montane region of Kyoto, Japan. Journal of Herpetology 36:719–723. [ Links ]
Isacch, J. P. and M. Barg. 2002. Are bufonid toads specialized ant–feeders? A case test from the Argentina flooding pampa. Journal of Natural History 36:2005–2012. [ Links ]
Krebs, C. J. 1989. Ecological Methodology. Harper and Row Pub., New York. 294 p. [ Links ]
Lajmanovich, R. C. 1994. Hábitos alimentários de Bufo paracnemis (Amphibia, Bufonidae) em el Paraná médio, Argentina. Revista de Hydrobiologia Tropical 27:107–112. [ Links ]
Levings, S. C. 1983. Seasonal, annual and among site variation in the ground ant community of a deciduous tropical forest: some causes of patch species distribution. Ecological Monographs 53:435–455. [ Links ]
Lima, A. L. and G. Moreira. 1993. Effect of prey size and foraging mode on the ontogenic change in feeding niche of Colostethus stepheni (Anura: Dendrobatidae). Oecologia 95:93–102. [ Links ]
Lima, A. L. and W. E. Magnusson. 1998. Partitioning seasonal time: interactions among size, foraging activity and diet in leaf–litter frogs. Oecologia 16:259–266. [ Links ]
Lima, A. L. and W. E. Magnusson. 2000. Does foraging activity change with ontogeny? An assessment for 6 sympatric species of postmetamorphic litter anurans in Central Amazonia. Journal of Herpetology 34:192–200. [ Links ]
Maneyro, R., D. E. Naya, I. Rosa, A. Canavero and A. Camargo. 2004. Diet of South American frog Leptodactylus ocellatus (Anura, Leptodactylidae) in Uruguay. Iheringia–Série Zoolgia 94:57–61. [ Links ]
Maragno, F. P. and F. L. Souza. 2007. Geographic distribution. Rhinella scitula. Herpetological Review 38:216–217. [ Links ]
Menéndez–Guerrero, P. A. 2001. Ecología Trófica de la Comunidad de Anuros del Parque Nacional Yasuní en la Amazonía Ecuatoriana. Mongraphie, Pontificia Universidad Catolica del Ecuador, Pichincha. Ecuador. 164 p. [ Links ]
Moseley, K., S. B. Castleberry, J. L. Hanula and W. M. Ford. 2005. Diet of Southern toads (Bufo terrestris) in loblolly pine (Pinus taeda) stands subject to coarse woody debris manipulations. American Midland Naturalist 153:327–337. [ Links ]
Nicoara, A., M. Nicoara and F. Bianchini. 2005. Diet composition during breeding period in populations of Bufo viridis, Pelobates fuscus, and Rana sculenta complex from ciric river's basin (Iasi, Romania). Analele Stiintifice ale Universitatii "Al.I. Cuza" lasi/Biologie animal 51:179–187. [ Links ]
Parker, M. L. and M. I. Goldstein. 2004. Diet of Rio Grande leopard frog (Rana berlandieri) in Texas. Journal of Herpetology 38:127–130. [ Links ]
Pinkas, I., M. S. Oliphant and Z. L. Iverson. 1971. Foods habits of albacore bluefin, tuna and bonito in California waters. California Department of Fish and Game Bulletin 152:1–350. [ Links ]
Pott, A. and V. J. Pott. 1994. Plantas do Pantanal. Centro de Pesquisas Agropecuárias do Pantanal. Serviço de Produção e Informação, Distrito Federal, Brasil. 320 p. [ Links ]
Santos E. M., A. V. Almeida and S. D. Vasconcelos. 2004. Feeding habits of six anuran (Amphibia: Anura) species in a rainforest fragment in Northeastern Brazil. Iheringia–Série Zoologia 94:433–438. [ Links ]
Silvestre, R. and C. R. F. Brandão. 2001. Formigas (Hymenoptera, Formicidae) atraídas a iscas em uma ilha de cerrado no município de Cajuru, Estado de São Paulo, Brasil. Revista Brasileira de Entomologia 44:71–77. [ Links ]
Simpson, E. H. 1949. Measurements of diversity. Nature 163:688. [ Links ]
Strüssmann, C., M. B. R. Vale, M. H. Meneguini and W. E. Magnusson.1984. Diet and foraging mode of Bufo marinus and Leptodactylus ocellatus. Journal of Herpetology 18:138–146. [ Links ]
Toft, C. A. 1981. Feeding ecology of Panamanian litter anurans: patterns in diet and foraging mode. Journal of Herpetology 15:139–144. [ Links ]
Uetanabaro, M., F. L. Souza, P. Landgref, A. F. Beda, and R. A. Brandão. 2007. Anfíbios e répteis do Parque Nacional da Serra da Bodoquena, Mato Grosso do Sul, Brasil. Biota Neotropica 7:279–289. [ Links ]
Vargas, A. B., A. J. Mayhé–Nunes, J. M. Queiroz, G. O. Souza and E. F. Ramos. 2007. Efeitos de fatores ambientais sobre a mirmecofauna em comunidade de restinga no Rio de Janeiro, RJ. Neotropical Entomology 36:28–37. [ Links ]
Whitfield, S. M. and M. A. Donnelly. 2006. Ontogenetic and seasonal variation in the diets of a Costa Rican leaf–litter herpetofauna. Journal of Tropical Ecology 22:409–417. [ Links ]
Wu, Z., Y. Li, Y. Wang and M. J. Adams. 2005. Diet of introduced bullfrogs (Rana catesbeiana): predation and diet overlap with native frogs on Daishan Island, China. Journal of Herpetology 39:668–674. [ Links ]
Zar, J. H. 1999. Biostatistical Analysis. Prentice Hall, New Jersey. 663 p. [ Links ]














