Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de biodiversidad
versión On-line ISSN 2007-8706versión impresa ISSN 1870-3453
Rev. Mex. Biodiv. vol.82 no.2 México jun. 2011
Ecología
Mixed bird flocks: patterns of activity and species composition in a region of the Central Andes of Colombia
Bandadas mixtas de aves: patrones de actividad y composición de especies en una región de la Cordillera Central de los Andes de Colombia
Enrique Arbeláez-Cortés1,2*, Hernando A. Rodríguez-Correa1,3 and Manuela Restrepo-Chica1
1 Semillero de Investigación en Aves. Programa de Licenciatura en Biología y Educación Ambiental. Facultad de Educación. Universidad del Quindío. Cra 15, Cll 12 N, Armenia, Quindío. Colombia. *Correspondent: enriquearbelaez@gmail.com
2 Museo de Zoología. Departamento de Biología Evolutiva. Facultad de Ciencias. Universidad Nacional Autónoma de México. Apartado postal 70-399, 04510, México D.F., México.
3 Centro de Investigaciones en Ecosistemas. Universidad Nacional Autónoma de México. Antigua carretera a Pátzcuaro Núm. 8701. Col. San José de la Huerta, 58190 Morelia, Michoacán, México.
Recibido: 15 abril 2010
Aceptado: 09 septiembre 2010
Abstract
Mixed bird flocks are groups of individuals from different species that travel and forage together. Such groups are common in several bird communities around the world. We present species composition and activity patterns of mixed bird flocks in a region of the Central Andes of Colombia. We compared the number of species per flock, as well as the number of flocks among 3 different habitats. We tested hypotheses concerning the flocks daily activity and the co-occurrences of species within them. We recorded 75 species, and the species number per flock varied from 4 to 21. Our data suggest that habitat affects the number of flocks but not their species number, and that the activity of flocks is similar throughout the day. In addition, the association of birds in flocks is affected by interspecific facilitation, with some species co-occurrences found more times than expected by chance. We hypothesize that some tanager species could have a role in flock cohesion. We witnessed 2 predator attacks upon flocks, a number of agonistic interactions among flock members, and squirrels following bird flocks. Our results meet some general patterns described for mixed bird flocks.
Key words: La Patasola Natural Reserve, montane forest, Quindío, species richness, South America.
Resumen
Las bandadas mixtas de aves son grupos de individuos de diferentes especies que viajan y forrajean juntos, y son comunes en varias comunidades de aves alrededor del mundo. Presentamos la composición de especies y los patrones de actividad de las bandadas mixtas de aves en una región de la Cordillera Central de los Andes Colombianos. Comparamos el número de especies por bandada y el número de bandadas en 3 hábitats distintos. Evaluamos hipótesis relacionadas con la actividad de las bandadas durante el día y la presencia simultánea de especies en estos grupos. Observamos 75 especies, y el número de especies por bandada varió entre 4 y 21. Nuestros datos indican que el hábitat parece afectar el número de bandadas pero no su número de especies y que la actividad de las bandadas es similar durante el día. La asociación de especies en bandadas parece estar influida por facilitación intraespecífica y algunas especies se presentan simultáneamente más de lo esperado por azar. Hipotetizamos que algunas tangaras pueden tener un papel en la cohesión de las bandadas. Observamos 2 ataques de depredadores, así como varias interacciones hostiles entre miembros de las bandadas y la participación de ardillas. Nuestros resultados se ajustan a ciertos patrones descritos para las bandadas mixtas.
Palabras clave: Reserva Natural La Patasola, bosque montano, Quindío, riqueza de especies, Sudamérica.
Introduction
Heterospecific groups, defined as wild groups of animals made up of individuals of different species traveling and feeding together, are a common phenomenon in different habitats around the world. These groups have been documented for birds, mammals, and fishes (Morse, 1977; Terborgh, 1990; Haugaasen and Peres, 2008; Goodale et al., 2010), and their occurrence in such diverse lineages suggests an adaptive value. In general, heterospecific groups of birds have been studied in more detail (Morse, 1977). Mixed bird flocks, also known as avian mixed-species flocks or mixed bird parties, are ubiquitous phenomena, and represent a conspicuous and prevalent characteristic of many habitats (e.g., Powell, 1985; Terborgh, 1990; Jullien and Clobert, 2000; Sridhar et al., 2009).
Mixed bird flocks (referred hereafter as MBFs) result from attractions among their participants and not from an external concentration of food resources (Morse, 1977; Powell, 1985; Sridhar et al., 2009). MBFs are different from bird feeding aggregations that form around clumped resources such as water, fruiting trees, or army ant swarms (Diamond and Terborgh, 1967; Morse, 1977; Powell, 1985; Sridhar et al., 2009). MBFs show wide variation in size, temporary cohesion, and strength of association (Terborgh, 1990; Sridhar et al., 2009). The species that join MBFs have been divided into groups according to their role in the cohesion of the flock (e.g., Moynihan, 1979; Munn and Terborgh, 1979; Powell, 1985; Sridhar et al., 2009). In general, these species groups are depicted as leaders/followers or nuclear/satellites, and their species seem to obtain different benefits in their association with MBFs (Sridhar et al., 2009).
Two major non-mutually exclusive hypotheses have been proposed to explain why and how birds might benefit from flocking behavior (e.g., Morse, 1977; Jullien and Clobert, 2000; Sridhar et al., 2009). The first suggests that birds can get feeding benefits from joining flocks by obtaining food more efficiently than when solitary (Morse, 1977; Munn and Terborgh, 1979; Powell, 1985; Jullien and Clobert, 2000; Sridhar et al., 2009). Alternatively, the second hypothesis considers the flock as a strategy for predator avoidance through several behavioral mechanisms (Morse, 1977; Powell, 1985; Jullien and Clobert, 2000; Sridhar et al., 2009). Further, MBFs may simultaneously confer more than one benefit upon their members, and it is possible that each species obtains different benefits while staying in MBFs (Morse, 1977; Moynihan, 1979). Nevertheless, the MBFs may also generate costs for their participants such as an increase in competition and aggressiveness, changes in foraging patterns, and increases in predation risk because these groups are more noisy and conspicuous than the solitary foragers (Short, 1961; Jones, 1977; Hutto, 1994; Jullien and Clobert, 2000). In fact, previous studies have tested these hypotheses, but no consensus has arisen on the primary cause involved in the formation of MBFs (e.g., Gaddis, 1980; Thiollay, 1999; Hino, 2000; Goodale and Kotagama, 2006; Sridhar et al., 2009).
Altogether, MBFs in different habitats and at various latitudinal and altitudinal sites appear to have some features in common, but others are highly distinct (Poulsen, 1996). Studies of MBFs have illustrated several patterns. First, the number of species in MBFs in 1 locality reflects the local bird richness (Hutto, 1994; Latta and Wunderle, 1996; Bohórquez, 2003; Brandt et al., 2009; Perón and Crochet, 2009). Second, forest fragmentation and habitat differences affect MBF activity and species composition (Croxall, 1974; Munn, 1985; Stouffer and Bierregaard, 1995; Maldonado-Coelho and Marini, 2000; Develey and Stouffer, 2001; Lee et al., 2005; Sridhar and Sankar, 2008). Third, species typically acting solitary in 1 type of habitat (e.g., forest) could be found as part of MBFs in adjacent habitats (e.g., open areas) (Tubelis et al., 2006; Tubelis, 2007; Péron and Crochet, 2009). Fourth, MBFs exhibit variation in their activity throughout the day (Eguchi et al., 1993; Poulsen, 1996; Machado, 1999; Farley et al., 2008), and changes in their frequency and composition throughout the year (Davis, 1946; Dean, 1990; Machado, 1999; Develey and Peres, 2000; Maldonado-Coelho and Marini, 2003; Tubelis, 2007). Fifth, species with cohesive roles seem to occur in mono-specific flocks and exhibit some special plumage color pattern or particular behavioral traits (Moynihan, 1979; Powell, 1985; Tubelis, 2007; Goodale and Beauchamp, 2010). Sixth, predation risk is a factor leading to the formation of MBFs (Morse, 1977; Tubelis, 2007; Sridhar et al., 2009). Finally, migrant species can seasonally influence the composition of MBFs (Hutto, 1994; Machado, 1999; Maldonado-Coelho and Marini, 2003).
To test the generality of these patterns, comparisons can be drawn with information gathered about MBFs in complex and highly diverse biological systems such as the Andean avifauna of northern South America, for which the documentation of this phenomenon is scarce (Moynihan, 1979; Poulsen, 1996; Montero, 1999; Bohórquez, 2003). Considering the latter, we focused our efforts in this study to document some of the mentioned patterns of MBFs in a region of the Central Andes of Colombia by (1) describing species composition of MBFs; (2) comparing the number of species per MBF among different habitats; (3) checking for variation in the number of MBFs among different habitats and through time; (4) evaluating if there is evidence of cohesion in these groups by testing the hypothesis that species join MBFs randomly; and (5) placing our data in the framework of other studies conducted around the world.
Materials and methods
Study area. We conducted field work at La Patasola natural reserve (4°41'N 75°33'W, 2 200–2 600 m asl); the site is considered an Important Bird Area (Bird Life International 2010). This natural reserve is located in the Salento Municipality of Quindío Department on the western slope of the Central Andes of Colombia. The avifauna of the area includes about 170 species, among which several are Colombian endemics or endangered species, such as Penelope perspicax, Odontophorus hyperythrus, Andigena nigrirostris, Leptosittaca branickii, Chlorochrysa nitidísima, and Saltator cinctus (Arbeláez-Cortés, 2007; Fundasilvestre, unpublished data). The reserve encompasses 150 ha, and is located within a zone of very humid sub-montane forest with steep topography. The landscape is composed of different habitats such as mature old growth forest, secondary old growth forest, early secondary forest, and commercial forestry plantations of Pinus sp. and Eucalyptus sp. Around 50% of the reserve's area was covered by livestock pastures and early secondary forest 50 years ago (Fundación Ecoandina/ WCS Colombia, unpublished document). In fact, some patches of such pastures still remain in the zone. Shrubs and trees in the study area belong to more than 59 species from 30 families, of which Asteraceae, Cunoniaceae, Melastomataceae, and Rubiaceae are the most common. Some genera such as Miconia (Melastomataceae), Guettarda (Rubiaceae), and Ocotea (Lauraceae) are also common. Species such as Cecropia telealba, Aegiphila bogotensis, Axinaea macrophylla, Wettinia kalbreyeri, and Guettarda chiriguensis are frequent in the area (Fundación Ecoandina/ WCS Colombia, unpublished document; authors' pers. obs.).
We conducted MBF observations along 3 sections ranging from 2 to 3 km in length. Each section was located in a different type of habitat and was separated from other sections by 0.5 to 1.5 km. Section A was located in 50-year-old second growth forest. This section is bordered by a road, and has a 10 to 15 m height canopy with some emerging trees that reach up to 21 m, and a dense understory of shrubs up to 4.5 m high. Section B was located in 15-year-old scrubby second growth. This section is crossed by a path, and harbors principally shrubs between 3.5 and 4.5 m in height. It also contains some scattered groves with trees up to 7.5 m and a narrow patch of old growth forest along one stream. Section C was located in a mature old growth forest with low human intervention on a steep slope near a river (Río Boquía). This section is crossed by a narrow path. The canopy of section C is 9–12 m in height, and the open understory includes growing trees up to 3.5 m. It is necessary to note that all these sections are intermixed in different degrees and do not represent a linear gradient. We chose these sections because they are representative of the habitats at La Patasola and reflect the condition of other montane forests along the Colombian Andes. We took precautions to ensure that the time of surveys in each section was the same. Despite the fact that the 3 sections were not separated by long distances or by some kind of barrier, we consider our observation scheme as useful for our aims related to describing patterns in MBFs among sections representing different habitats and through time, not centered on documenting differences related to spatial separation.
Data collection. Data collection was carried out by conducting 4 field trips between September of 2006 and February of 2007. Each field trip lasted 3 to 4 days, including observations for a total of 108 hours (36 hours in each section). We defined a MBF as 1 group of 2 or more individuals of different species moving around actively, showing evidence of cohesion (e.g., constant vocalizations and individuals moving in the same direction) without the presence of an external concentration of food resources. A similar definition for MBF was used in other studies (e.g., Morse, 1977; Develey and Stouffer, 1999; Maldonado-Coelho and Marini, 2000; Ippi and Trejo, 2003; Tubelis et al., 2006). The differentiation of the MBFs from another kind of birds groups, like frugivorous birds foraging in trees, is straightforward due to the conspicuousness of MBFs being enhanced by the fact that they tend to remain continually on the move (Morse, 1977).
MBFs in the Andes and other areas of South America seem to be loosely structured, presenting turnover of individuals and lacking territoriality (Poulsen, 1996; Aleixo, 1997; Herzog et al., 2002). A similar result was reported for MBFs in Asia (Kotagama and Goodale, 2004). It is suggestive that the same MBF is not maintained for a long time. However, to avoid taking data from the same MBF repeatedly we used 3 different strategies. First, we checked the direction of movement of each MBF and compared that with the next MBF detected, assuring that it was coming from a different direction. Second, we conducted observations in each section for only 3 hours daily, avoiding prolonged continuous observations in the same place. For this reason we divided each day into sampling periods as follows: (1) 06:00–09:00 hours; (2) 09:00–12:00 hours; and 3) 14:30–17:30 hours. Third, there were at least 10 days between each pair of field trips.
We carried out observations along the main trail present in each section (see above). We detected MBFs by observations through binoculars, since there was extreme multi species bird activity. Once a MBF was located, it was followed and observed as long as possible, noting the time of detection and the species composition. Other information on agonistic interactions among constituent members, predator attacks upon them, and the number of co-specific individuals was observed when the opportunity arose. For some MBFs we did not document the species composition; these cases were excluded from the analyses dealing with the number of species. For the species observed, we followed the taxonomic treatment of Remsen et al. (2010), and classified them according to their occurrence in MBFs as: regular (present in 25 % or more of the MBFs), common (10–24.9%), uncommon (3–9.9%), and rare (< 2.9 %) following Machado (1999).
Data analyses. To verify if the set of species recorded joining MBFs were well represented in our observations, we used EstimateS 7.5 (Colwell, 2005) to construct a species accumulation curve, considering each MBF as a sample unit. Additionally, we used the following analyses performed in PAST 3 (Hammer et al., 2001) and XLSTAT to test specific hypotheses. First, we checked for variation in the number of MBFs and for changes in the occurrence of frequent species throughout the study using a χ2 test comparing data from our 4 field trips. Second, we tested whether agonistic interactions among individuals in one flock were related with species number using a Fisher's exact test and grouping MBFs in 2 categories: <10 species and ≥10 species. Third, we evaluated differences in the number of MBFs and in the number of species per MBF among the 3 sections by using Kruskal–Wallis ANOVAs, considering observations from each 3 hour periods as a single sample. It was followed by a Mann Whitney pairwise comparison. We used these non-parametric tests because our data were not normally distributed (Shapiro-Wilk test W < 0.91 p < 0.003). Furthermore, to test for differences in the activity of MBFs during the day we used a χ2 test to compare the number of MBFs observed in each hour of the day. Finally, we used 2 different approaches to test if species joined MBFs on a random basis. On one hand, we tested the effect of facilitation among species in the cohesion of MBFs, as a whole using CoOccur version 1.0 (Ladau et al., 2008). This program uses null models to analyze a matrix of species presence/absence in different sites (MBFs in our case), and tests hypotheses about facilitation and competition among a set of given species. For this analysis we grouped the species by family following Remsen et al. (2010). On the other hand, we conducted multiple χ2 and Fisher's exact tests to detect which species pairs co-occurred in MBFs more than expected by chance. These comparisons were carried out only for species present in 4 or more MBFs. This last approximation and some variants have been used previously to test association among species in MBFs (e.g. Jones, 1977; Hutto, 1994; Latta and Wunderle, 1996). Although those 2 analyses test hypotheses related with the association of species in MBFs, they are conducted at 2 different, but complementary, levels: in a general level of the study zone MBFs as a whole in the case of CoOccur and at a particular level of species pairs in the case of χ2 and Fisher's tests.
Results
We detected a total of 104 MBFs. Of these, 71 were observed under conditions that allowed us to document their species composition, and were included in the analyses that consider the number of species. We recorded a total of 75 species joining MBFs (Appendix). The species accumulation curve did not reach an asymptote. However, the number of new species decreased with sample effort, and the singleton curve peaked and began to decline, indicating that our sample is representative of the species that join MBFs in this area (Figure 1). A great proportion of the recorded species did not join flocks regularly. According to the species occurrence in the MBFs we classified 12 as regular, 13 as common, 26 as uncommon, and 24 as rare (Appendix). A total of 4 Neartic-Neotropical migrant species were recorded in MBFs; 2 of which, Dendroica fusca and Mniotilta varia, were regular in the flocks.
In general MBFs were conspicuous and noisy. We followed each MBF for periods ranging from 5 to 45 min. The individuals in the MBF were moving principally up in the trees, although some species (e.g., Synallaxis azarae, Atlapetes albinucha, and Cinnycerthia unirufa) were seen in shrubs. The number of species per MBF varied from 4 to 21 (mean= 9.43, s.d.= 4.1, n= 71; Figure 2). We observed that some species like Aulacorhynchus prasinus, Andigena nigrirostris, and Turdus fuscater followed some MBFs, but did not join them in a consistent manner. These species were observed moving behind the MBF, exhibiting more pauses, and proceeding slower than the other species in the flock. On the other hand, we managed to observe monospecific pairs or groups of species (e.g., Anisognathus somptuosus, Tangara heinei, T. vassorii, T. nigroviridis, Chlorospingus ophthalmicus, Dendroica fusca, and Atlapetes schistaceus) in several MBFs. Likewise, we observed, on 8 occasions, squirrels (Sciurus sp.) joining MBFs. Among the regular species only the migrant Mniotilta varia presented variation in its number throughout the duration of this study (χ2= 13.3, 3 d.f. 3, p<0.01). We failed to observe this species on the first field trip, but it was a regular in the following 3 surveys. Although this study was conducted during 5 months, the number of MBFs did not change significantly through time (χ2= 2.3; 3 d.f.; p= 0.51, n= 104). In addition, around 13 % of the MBFs were observed during rainy periods in which constituent individuals did not decrease their activity. We witnessed 2 predator attacks upon MBFs and several agonistic interactions among members of MBFs. During the 2 predator attacks, we observed that different individuals in the MBF gave simultaneous alarm calls from different places followed by an evasive behavior. In the first case, a raptor (possibly Buteo magnirostris) was involved. In the second instance, a group of 5 Cyanocorax yncas approached a MBF, which elicited an evasive behavior by the members of the MBF after one of the jays vocalized. We detected 16 agonistic interactions among individuals in 10 MBFs, but we only identified the involved species on 9 occasions. Ten species were involved in agonistic behaviors, and 4 interactions were intraspecific among individuals of: Dendrocolaptes picumnus, Chlorospingus ophthalmicus, Dendroica fusca, and Atlapetes schistaceus. Although the majority of agonistic interactions were recorded within MBFs with 10 or more species, our analysis did not indicate an association between the number of species and the occurrence of agonistic events (Fisher's exact test, p= 0.12, n= 16).
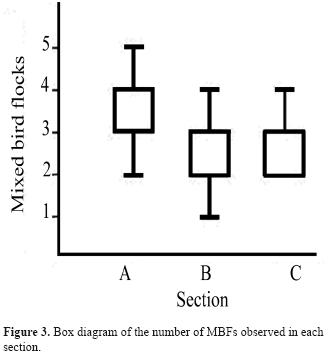
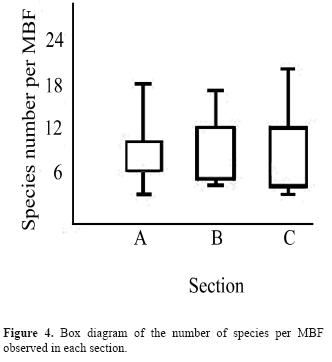
We detected significant differences in the number of MBFs among the 3 sections surveyed (Kruskal-Wallis ANOVA, H= 7.49, p= 0.024, n= 104; Figure 3). The Mann-Whitney pairwise comparisons indicated that the differences were significant among section A and B (p= 0.018) and section A and C (p= 0.024). However, the number of species per MBF did not show significant differences among sections (Kruskal-Wallis ANOVA, H= 0.15, p= 0.93, n= 71; Figure 4). In addition, the number of MBFs did not change along the hours of the day (χ2= 6.25, 8 f.d., p= 0.62, n= 104; Figure 5), but 2 activity peaks were observed: 1 in the middle of the morning (9-10 h) and another at the end of the afternoon (16:30–17:30 h). The results of the co-occurrence/facilitation test received a p-value of 0, with a maximum power of 1; suggesting that the co-occurrence pattern between MBFs species is affected by interspecific facilitation interactions. We obtained additional evidence for non-random co-occurrences of species pairs in MBFs using a set of χ2 and Fisher's exact tests. Of the 1275 species pair comparisons performed; 17 were significant (p < 0.01) (Table 1). And of these co-occurrences, 15 were positive and 2 negative. However, because of the multiple comparisons conducted and an α= 0.01, 12 associations could be deemed significant only by chance. Several tanager species (family Thraupidae) were found in the positive co-occurrences. The only 2 negative co-occurrences were between Myioborus miniatus and 2 Tangara species.

Discussion
Our data indicate that around 43% of the total species known from the studied region participated in MBFs. Similar proportions of species joining MBFs have been reported for other South American avian communities in the Bolivian Andes (Remsen, 1985), the Atlantic forest (Aleixo, 1997), and the Cerrado (Tubelis et al., 2006; Tubelis, 2007). Comparing our results with other studies from the Neotropics, which report the participation of 10 to 120 species (mean= 60 species) (Davis, 1946; Moynihan, 1979; Powell, 1979; Ewert and Askins, 1991; Hutto, 1994; Latta and Wunderle, 1996; Poulsen, 1996; Aleixo, 1997; Machado, 1999; Montero, 1999; Develey and Peres, 2000; Herzog et al., 2002; Bohórquez, 2003; Brandt et al., 2009), the number of species joining MBFs in our study area is among the highest. These results follow the general pattern that the number of species joining MBFs in one zone is a reflection of the local species pool (Hutto, 1994; Latta and Wunderle, 1996; Bohórquez, 2003; Brandt et al., 2009; Perón and Crochet, 2009). Additionally, the frequency of MBFs and the mean number of species per MBF in our study zone are among the highest documented for the Andes (Moynihan, 1979; Herzog et al, 2002; Montero, 1999; Bohórquez, 2003; Poulsen, 1996), a region that apparently contains MBFs composed of more species than other regions in South America (e.g. Cerrado, Tubelis, 2007). All these data lead us to conclude that MBFs represent a biologically important feature of the bird community in this region of the Colombian Andes.
Among the bird species recorded joining MBFs, we noticed a consistent participation of 2 migrants, a pattern documented by other studies in different Neotropical regions (Ewert and Askins, 1991; Hutto, 1994; Latta and Wunderle, 1996). This pattern could be explained as a result of migrant individuals joining MBFs in order to obtain local information about foraging resources or predators (Smith, 1975). We also detected variation in the association with MBFs over time only for the migrant Mniotilta varia. Variation in yearly species composition and richness of MBFs may be a result of seasonal presence or absence of these migrant species in the zone (e.g., Develey and Peres, 2000).
Concerning the singular joining of large birds and squirrels to MBFs, the inconsistent participation of T. fuscater and A. prasinus in MBFs could be caused by their abrupt movements and large body size, which promotes a break-up of small species groups (Moynihan, 1979). However, we do not exclude the possibility that the evasion of toucanets (Aulacorhynchus sp.) is because they are occasional predators of small birds (Moynihan, 1979; E. Arbeláez-Cortés, pers. obs.). Our observation of squirrels following MBFs contributes additional data to the documentation of this phenomenon around the world (Chapin, 1932; McClure, 1967; Partridge and Ashcroft, 1976; Moynihan, 1979; Paschoal and Galetti, 1995; Kotagama and Goodale, 2004; Buitrón-Jurado and Toba, 2007; Sridhar and Sankar, 2008). In the Neotropics some squirrel species such as Sciurus granatensis, S. ingrami, and Mycrosciurus flaviventer exhibit a tendency to develop different interspecific relationships (Moynihan, 1979; Paschoal and Galetti, 1995; Buitrón-Jurado and Toba, 2007). All this evidence together with some data on tree shrews, monkeys, and coatis joining MBFs (Stresemann, 1917; Kotagama and Goodale, 2004; Beisiegel, 2007), lead us to believe that heterospecific groups of birds and mammals are a general phenomenon.
Documented predator attacks on MBFs are rather scarce (e.g., Morse, 1970; Powell, 1985; Herzog et al., 2002; Ippi and Trejo, 2003; Tubelis, 2007), but the behavioral response that we witnessed of MBFs members to predators was similar to the one documented in many flocking species that call while sitting motionless subsequent to being alerted (Morse, 1970; Morse, 1977). These vocalizations are generally believed to confuse their predators by virtue of being given simultaneously from many locations (Morse, 1977). We consider it noteworthy that one predatory attack involved a jay and not a raptor. Although there exists at least one other report of a response of MBFs to jays (Cyanocorax cristatellus, Silva, 1980 cited in Tubelis, 2007), and some jays are predators of small birds (Moynihan, 1979), C. yncax has not been previously reported as a predator (H. Álvarez-López pers. com.). As such, this positive response of the flock members to the presence of C. yncax could be considered as derived from an "inappropriate stimulus" (Morse, 1970). However, our 2 observations support the idea that species joining MBFs have mechanisms of alert and reaction against potential predators.
Temporal variation in the activity of MBFs has been reported at different scales in many localities (e.g., Powell, 1979; Machado, 1999; Herzog et al., 2002; Farley et al., 2008). However, we found that the number of MBFs did not change during the 5 months of our study. Although a similar pattern has been found in other Neotropical sites (Munn, 1985; Bohórquez, 2003), many studies indicate variation in MBFs in relation to reproductive season or fluctuation in resources (Davis, 1946; Powell, 1979; Dean, 1990; Machado, 1999; Develey and Peres, 2000). Therefore, it is possible that some variation could occur in the frequency of MBFs in the region throughout the year, but our data were not suitable to detect this pattern. Additionally, our data indicate that the number of MBFs did not vary throughout the day. This same pattern has been documented in other studies (Partridge and Ashcroft, 1976; Dean, 1990), and could be regarded as evidence for the hypothesis that advantages obtained by species joining MBFs do not change during the day. We did not conduct systematic observations around noon, but occasional observations showed that MBFs were active at these hours too. In contrast with our data, many studies have detected variation in the number of MBFs throughout the day (Eguchi et al., 1993; Poulsen, 1996; Machado, 1999; Montero, 1999, Bohórquez, 2003; Farley et al., 2008; Herzog et al., 2002). This contrast suggests that in different regions a diverse number of issues are involved in the formation and maintenance of MBFs. Finally, we observed MBFs during the rain. Other authors also have recorded MBFs in rainy periods (Morse, 1970; Moynihan, 1979; Montero, 1999). This phenomenon has been explained as a strategy to offset the diminution of insect biomass (Montero, 1999; Bohórquez, 2003).
Although we did not detect variation in the number of species found per MBF among the 3 different sections we surveyed, habitat differences affect MBFs richness in other localities around the world (Maldonado-Coelho and Marini, 2000; Lee et al., 2005). For example, in the Amazonas region, MBF richness is lower in areas in early regeneration stages (Maldonado-Coelho and Marini, 2000). Also, significant changes in the number of species per flock among different habitats have been documented in Asia (Lee et al., 2005), but the latter study was conducted under different conditions (an escalating gradient of anthropogenic modification, including forest and urban areas) than those presented in our study area. The stability in the number of species per MBF in our study region could be due to the fact that scrubby second growth harbors some groves and is near to larger forest patches (see Methods). Likewise, MBFs could be promoting the use of open areas by forest birds (Dolby and Grubb, 2000; Tubelis et al., 2006; Péron and Crochet, 2009). These factors potentially are precluding the decrease in number of species per MBF in the scrubby second growth in our study area.
In contrast with our species richness results, the number of MBFs was affected by the vegetation stage in the sections studied. The number of MBFs was low in sections B and C, while it was significant in section A. It is known that in Neotropical lowlands, understory MBFs tend to avoid open areas with low vegetation (Develey and Stouffer, 2001); while the canopy MBFs prefer to stand in continuous canopy, thus avoiding dangers and energetic consumption associated with flying through discontinuous vegetation (Munn, 1985). In the scrubby second growth area (section B), we observed MBFs traveling principally in the tallest trees and bushes. Something similar has been observed in Brazil where the effect of secondary growth on MBFs seems to be related with the kind of vegetation present, and the MBFs have been observed using secondary areas near the forest fragments (Stouffer and Bierregaard, 1995; Borges and Stouffer, 1999). We consider this a plausible explanation for the slight decrease of MBFs documented between sections A and B, representing different habitats. However, our results are somewhat puzzling, as the differences were also between section A and C, both with forest habitats, and not between sections B and C, which represent different habitats.
We are aware that some concerns could arise because the study area was small (e.g., results did not show differences among habitats because MBFs can move through the entire zone). However as we have said in the methods this study is not centered on documenting differences related to spatial separation. To deal with this issue it is necessary to consider that the home range of MBFs in different localities has been reported to vary from 0.8 to 15.4 ha (mean= 7 ha, n= 12) (Morse, 1970; Buskirk et al, 1972; Munn and Terborgh, 1979; Poulsen, 1996), which could be used to indicate that in the study zone a single MBF does not move through the entire area. Finally, the study area is not isolated; it is connected with other larger continuous forests (e.g., Santuario de Fauna y Flora Otun-Quimbaya). As a result the MBFs could be moving in a greater area where they could be "choosing" the habitats.
In general, our results indicate that MBFs forage and travel through secondary growth habitats with bushes and scattered trees in a similar manner as they do in old growth forests. It seems to be evidence for another kind of advantage gained by individuals joining MBFs. which is the greater use of adjacent vegetation patches (Tubelis et al, 2006). This result could have implications for bird conservation in landscape mosaics like the ones found in the Colombian Andes. In these landscapes MBFs could cross areas with early vegetation stages, allowing several species, including those of special conservation concern, to reach other forests or forest patches. MBFs traveling through secondary forest or narrow forest corridors have been witnesses in other localities in this same region of the Colombian Andes (Arbeláez-Cortés obs. pers). However, specially designed studies would be necessary to test the relation of MBFs and conservation biology issues in the Andes, as has been studied in other regions of the world (e.g., Sridhar and Sankar, 2008).
Although the benefits that a species get from participating in MBFs could come from being with the flock as a whole, not being with a particular species in the flock (Goodale and Beauchamp, 2010), our results do not support this hypothesis. Conversely, this study supports the idea that the MBFs are cohesive groups where some species are present because others are present too. However, previous studies have reported higher numbers of positive associations between species pairs than the number found in our study (Jones, 1977; Eguchi et al., 1993; Hutto, 1994; Latta and Wunderle, 1996; Bohórquez, 2003; Péron and Crochet, 2009). Principally, species in the family Thraupidae, the tanagers, were found in the positive associations. This could have some kind of biological meaning such as a role of these species in the cohesion of the MBFs in the study area. We avoid characterizing the kind of role of any of these species (e.g., nuclear, core, leader) because we did not obtain data to that extent. However, we found that several tanagers were frequent in the MBFs, and on many occasions were observed in pairs or in small groups. Furthermore, other studies in the Colombian Andes (Moynihan, 1979; Bohórquez, 2003) and Brazilian Cerrado (Tubelis, 2007) indicated that Thraupidae species are characteristic of MBFs and could have a nuclear role. It is known that tanagers form groups synchronized in movement, speed, and direction around which other species can join, conforming a MBF (Moynihan, 1979; Munn, 1985; Machado, 1999). Likewise, Anisognathus somptuosus, Tangara nigroviridis, T. heineii, T. arthus, and T. ruficervix meet some characteristics of nuclear species (sensu Moynihan, 1979) such as colorful plumage and the formation of mono-specific groups. Some of these species have been reported also as nuclear in a different region of the Colombian Andes (Moynihan, 1979; Bohórquez, 2003). All this information indicates that tanagers have an important role in the formation and maintenance of MBFs in the Andes. Additionally, we consider that other species, such as Chlorospingus ophthalmicus, C. canigularis, and Atlapetes albinucha, could have similar roles in MBFs. These species were usually seen in pairs or groups in MBFs where tanagers were absent.
In conclusion, our study documents that MBFs of this part of the Andes meet some general patterns described in other regions. Examples of these are: (1) the number of species joining MBFs in the area reflects the local species pool; (2) MBFs are cohesive groups where some species pairs co-occurrences are not driven by chance; (3) squirrels occasionally follow MBFs; (4) MBFs have alert and reaction mechanisms targeted against potential predators. However, our results also differ from the general pattern reported in previous studies; for example, (1) different habitats do not affect the number of species per MBF; (2) MBFs can use early secondary growth in a similar manner as more mature forest habitats; (3) the number of MBFs did not show significant differences throughout the day. Additionally, a few aspects pertinent to the number and kind of species that make up MBFs in our study area differed from observations of previous studies. We hope that this study will enrich the understanding of this biological phenomenon and contribute to the knowledge of the overwhelming biodiversity of the Colombian Andes.
Acknowledgments
This work is a product of the semillero de investigación en aves de la Universidad del Quindío. We are grateful to the Universidad del Quindío and their Vicerrectoría de Investigaciones for providing financial support for our field work. Héctor F. Gómez and Fundasilvestre helped us with logistic support during field work. Adolfo Navarro-Sigüenza, Árpád S. Nyári, Morelia Camacho-Cervantes, Clara I. Bohorquéz, and Ralf Strewe made valuable comments and corrections on initial versions of this manuscript. We also thank to 2 anonymous referees for their valuable corrections that helped improve this paper. EAC and HRC also acknowledges CONACyT-Mexico for a graduate studies scholarship during the writing of this manuscript.
Literature cited
Aleixo, A. 1997. Composition of mixed-species bird flocks and abundance of flocking species in a semideciduous forest of southeastern Brazil. Ararajuba 5:11-18. [ Links ]
Amaral, P. P. and J. Ragusa-Netto. 2008. Bird mixed–flocks and nuclear species in a tecoma savanna in the Pantanal. Brazilian Journal of Biology 68:511-518. [ Links ]
Arbeláez-Cortés, E. 2007. Registro fotográfico de algunas especies de la avifauna de la Reserva Natural "La Patasola", Quindío, Colombia. Boletín SAO 1:149-163. [ Links ]
Beisiegel, B. d. M. 2007. Foraging association between coatis (Nasua nasua) and birds of the Atlantic Forest, Brazil. Biotropica 39:283-285. [ Links ]
Bird Life International, 2010. Data zone. Online URL: http://www.birdlife.org/datazone/index.html. Last access: 14.03.2010. [ Links ]
Bohórquez, C. I. 2003. Mixed-species bird flocks in a montane cloud forest of Colombia. Ornitologia Neotropical 14:67-78. [ Links ]
Borges, S. H. and P. C. Stouffer. 1999. Bird communities in two types of anthropogenic successional vegetation in central Amazonia. Condor 101:529-536. [ Links ]
Brandt, C. S., H. Hasenack, R. R. Laps and S. M. Hartz. 2009. Composition of mixed-species bird flocks in forest fragments of southern Brazil. Zoologia 26:488-498. [ Links ]
Buitrón-Jurado, G. and M. Tobar. 2007. Posible asociación de la ardilla enana Microsciurus flaviventer (Rodentia:Sciuridae) y bandadas mixtas de aves en la amazonía ecuatoriana. Mastozoología Neotropical 14:235-240. [ Links ]
Buskirk, W. H., G. V. N. Powell, J. F. Wittenberger, R. E. Buskirk and T. U. Powell. 1972. Interspecific bird flocks in tropical highland Panama. Auk 89:612-624. [ Links ]
Chapin, J. P. 1932. The birds of the Belgian Congo. Part 1. Bulletin of the American Museum of Natural History 65:1-736 [ Links ]
Colwell, R. K. 2005. EstimateS: Statistical estimation of species richness and shared species from samples. Version 7.5. Online URL: purl.oclc.org/estimates [ Links ]
Croxall, J. P. 1976. The composition and behaviour of some mixed-species bird flocks in sarawak. Ibis 118:333-343. [ Links ]
Davis, D. E. 1946. A seasonal analysis of mixed flocks of birds in Brazil. Ecology 27:168-181. [ Links ]
Dean, S. 1990. Composition and seasonality of mixed-species flocks of insectivorous birds. Notornis 37:27-36. [ Links ]
Develey, P. F. and C. A. Peres. 2000. Resource seasonality and the structure of mixed species bird flocks in a coastal Atlantic forest of southeastern Brazil. Journal of Tropical Ecology 16:33-53. [ Links ]
Develey, P. F. and P. C. Stouffer. 2001. Effects of roads on movements by understory birds in mixed-species flocks in central Amazonian Brazil. Conservation Biology 15:1416-1422. [ Links ]
Diamond, J. M. and J. W. Terborgh. 1967. Observations on bird distribution and feeding assemblages along the Rio Callaria, Deparment of Loreto, Peru. Wilson Bulletin 79:273-282. [ Links ]
Dolby, A. S. and T. G. Grubb Jr. 2000. Social context affect risk taking by satellite species in a mixed-species foraging group. Behavioural Ecology 11:110-114. [ Links ]
Eguchi, K., S. Yamagishi and V. Randrianasolo. 1993. The composition and foraging behaviour of mixed-species flocks of forest-living birds in Madagascar. Ibis 135:91-96. [ Links ]
Ewert, D. N. and R. A. Askins. 1991. Flocking behavior of migratory warblers in winter in the Virgin Islands. Condor 93:864-868. [ Links ]
Farley, E. A., K. E. Sieving and T. A. Contreras. 2008. Characterizing complex mixed-species bird flocks using an objective method for determining species participation. Journal of Ornithology 149:451-468. [ Links ]
Gaddis, P. 1980. Mixed flocks, accipiters, and antipredator behavior. Condor 82:348-349. [ Links ]
Goodale, E. and G. Beauchamp. 2010. The relationship between leadership and gregariousness in mixed-species bird flocks. Journal of Avian Biology 41:99-103. [ Links ]
Goodale, E. and S. W. Kotagama. 2006. Vocal mimicry by a passerine bird attracts other species involved in mixed-species flocks. Animal Behaviour 72:471-477. [ Links ]
Goodale, E., G. Beauchamp, R. D. Magrath, J. C. Nieh, and G. D. Ruxton. 2010. Interspecific information transfer influences animal community structure. Trends in Ecology and Evolution 25: 354-361. [ Links ]
Hammer, O., D. A. T. Harper and P. D. Ryan. 2001. PAST: Palaeontological Statistics software package for education and data analysis. Online URL: htt://folk.uio.no/ohammer/past/ [ Links ]
Haugaasen, T. and C. A. Peres. 2008. Associations between primates and other mammals in a central Amazonian forest landscape. Primates 49: 219-222. [ Links ]
Herzog, S. K., A. R. Soria A., Troncoso J. and E. Matthysen. 2002. Composition and spatial structure of avian mixed-species flocks in a high-Andean Polylepis forest in Bolivia. Ecotropica 8:133-143. [ Links ]
Hino, T. 2000. Intraspecific differences in benefits from feeding in mixed-species flocks. Journal of Avian Biology 31:441-446. [ Links ]
Hutto, R. L. 1994. The composition and social organization of mixed-species flocks in a tropical deciduous forest in western Mexico. Condor 96:105-118. [ Links ]
Ippi, S. and A. Trejo. 2003. Dinámica y estructura de bandadas mixtas de aves en un bosque de Lenga (Nothofagus pumilio) del Noroeste de la Patagonia Argentina. Ornitolologia Neotropical 14:353-362. [ Links ]
Jones, S. E. 1977. Coexistence in mixed species antwren flocks. Oikos 29:366-375. [ Links ]
Jullien, M. and J. Clobert. 2000. The survival value of flocking in Neotropical birds: reality or fiction? Ecology 81:3416-3430. [ Links ]
Kotagama, S. and E. Goodale. 2004. The composition and spatial organization of mixed-species flocks in a Sri Lankan rainforest. Forktail 20:63-70. [ Links ]
Ladau, J., S. J. Ryan and S. J. Schwager. 2008. CoOccur: Software for implementing robust null model tests in ecology. Online URL: www.santafe.edu/~jladau/CoOccur. [ Links ]
Latta, S. C. and J. M. Wunderle Jr. 1996. The composition and foraging ecology of mixed-species flocks in pine forests of Hispaniola. Condor 98:595-607. [ Links ]
Lee, T. M., M. C. K. Soh, N. Sodhi, L. P. Koh and S. L. H. Lim. 2005. Effects of habitat disturbance on mixed species bird flocks in a tropical sub-montane rainforest. Biological Conservation 122:193-204. [ Links ]
Machado, C. G. 1999. A composição dos bandos mistos de aves na Mata Atlântica da Serra de Paranapicaba, no sudeste Brasileiro. Revista Brasileira de Biologia 59:75-85. [ Links ]
Maldonado-Coelho, M. and M. A. Marini. 2000. Effects of forest fragment size and successional stage on mixed-species bird flocks in Southeastern Brazil. Condor 102:585-592. [ Links ]
Maldonado-Coelho, M. and M. A. Marini, 2003. Composição de bandos mistos de aves em fragmentos de mata atlântica no Sudeste do Brasil. Papéis Avulsos de Zoologia, Museu de Zoologia da Universidade de São Paulo 43:31-54. [ Links ]
McClure, H. E. 1967. The composition of mixed species flocks in lowland and sub-montane forest of Malaya. Wilson Bulletin 79:131-154. [ Links ]
Montero, H. A. 1999. Composición, organización y comportamiento de bandadas mixtas en un bosque de niebla en los Farallones de Cali. Tesis de Grado Biología. Universidad del Valle. Cali. Colombia. 70 p. [ Links ]
Morse, D. H. 1970. Ecological aspects of some mixed-species foraging flocks of birds. Ecological Monographs 40:119-168. [ Links ]
Morse, D. H. 1977. Feeding behaviour and predator avoidance in heterospecific groups. BioScience 27:332-339. [ Links ]
Moynihan, M. 1979. Geographic variation in social behavior and in adaptations to competition among Andean birds. Publications of the Nuttall Ornithological Club 18:89-147. [ Links ]
Munn, C. A. 1985. Permanent canopy and understory flocks in Amazonia: species composition and population density. Neotropical Ornithology. Ornitological Monographs 36:683-711. [ Links ]
Munn, C. A. and J. W. Terborgh. 1979. Multi-species territoriality in Neotropical foraging flocks. Condor 81:338-347. [ Links ]
Partridge, L. and R. Ashcroft. 1976. Mixed-species flocks of birds in hill forest in Ceylon. Condor 78:449-453. [ Links ]
Paschoal, M. and M. Galetti. 1995. Seasonal food use by neotropical squirrel Sciurus ingrami in Southeastern Brazil. Biotropica 27:268-273. [ Links ]
Péron, G. and P. A. Crochet. 2009. Edge effect and structure of mixed-species bird flocks in an Afrotropical lowland forest. Journal of Ornithology 150:585-599. [ Links ]
Poulsen, B. O. 1996. Structure, dynamics, home range and activity pattern of mixed-species bird flocks in a montane alder-dominated secondary forest in Ecuador. Journal of Tropical Ecology 12:333-343. [ Links ]
Powell, G. V. N. 1979. Structure and dynamics of interspecific flocks in a mid-elevation neotropical forest. Auk 96:375-390. [ Links ]
Powell, G. V. N. 1985. Sociobiology and adaptive significance of interspecific foraging flocks in the Neotropics. Neotropical Ornithology. Ornitological Monographs 36:713-732. [ Links ]
Remsen, J. V. 1985. Community organization and ecology of birds of high elevation humid forest of the Bolivian Andes. Ornithological Monographs 36:733-756. [ Links ]
Remsen, J. V. Jr., A. Jaramillo, M. Nores, J. F. Pacheco, M. B. Robbins, T. S. Schulenberg, F. G. Stiles, J. M. C. da Silva, D. F. Stotz and K. J. Zimmer. 2010. A classification of the bird species of South America. American Ornithologists' Union. Online URL http://www.museum.lsu.edu/~Remsen/SACCBaseline.html. last access: 15.02.2010. [ Links ]
Short, L. L. Jr. 1961. Interspecies flocking of birds of montane forest in Oaxaca, Mexico. Wilson Bulletin 73:341-347. [ Links ]
Smith, G. N. 1975. "Spshing noise": biological significance of its attraction and nonattraction by birds. Proceedings of Natural Academy of Science USA 72:1411-1414. [ Links ]
Sridhar, H., G. Beauchamp and K. Shanker. 2009. Why do birds participate in mixed-species foraging flocks? A large-scale synthesis. Animal Behaviour 78:337-347. [ Links ]
Sridhar, H. and K. Sankar. 2008. Effects of habitat degradation on mixed-species bird flocks in Indian rain forest. Journal of Tropical Ecology 24:135-147. [ Links ]
Stouffer, P. C. and J. R. O. Bierregaard.1995. Use of Amazonian forest fragments by understory insectivorous birds. Ecology 76:2429-2445. [ Links ]
Stresemann, E. 1917. Uber gemischte Vogel-schwarme. Verhandlungen der Ornithologischen Gesellschaft in Bayern 13:127-151. [ Links ]
Terborgh, J. 1990. Mixed flocks and polyspecific associations: costs and benefits of mixed groups to birds and monkeys. American Journal of Primatology 21:87-100. [ Links ]
Thiollay, J-M. 1999. Frequency of mixed species flocking in tropical forest birds and correlates of predation risk: an intertropical comparison. Journal of Avian Biology 30:282-294. [ Links ]
Tubelis, D. P., A. Cowling and C. Donnelly. 2006. The role of mixed-species flocks in the use of adjacent savannas by forest birds in central Cerrado, Brazil. Austral Ecology 31:38-45. [ Links ]
Tubelis, D. P. 2007. Mixed-species flocks of birds in the Cerrado, South America: a review. Ornitología Neotropical 18:75-97. [ Links ]














