Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de biodiversidad
versión On-line ISSN 2007-8706versión impresa ISSN 1870-3453
Rev. Mex. Biodiv. vol.82 no.1 México mar. 2011
Ecología
Tail loss incidence in the Chihuahuan fringe toed–lizard Uma paraphygas (Squamata: Phrynosomatidae)
Incidencia de autotomía caudal en la lagartija de arena de Chihuahua Uma paraphygas (Sauria: Phrynosomatidae)
Gamaliel Castañeda1*, Cristina García–De la Peña1, Héctor Gadsden2, Armando J. Contreras–Balderas3 and William E. Cooper Jr.4
1 Escuela Superior de Biología. Universidad Juárez del Estado de Durango. Av. Universidad s/n. Fracc. Filadelfia. 35070 Gómez Palacio, Durango, México.
2 Instituto de Ecología, A. C., Centro Regional Chihuahua. Apartado Postal 28. Carretera Chihuahua–Ojinaga, 32900 Ciudad Aldama, Chihuahua, México.
3 Laboratorio de Ornitología. Facultad de Ciencias Biológicas. Universidad Autónoma de Nuevo León. Apartado postal 425. 66450 San Nicolás de los Garza, Nuevo León, México.
4 Department of Biology. Indiana University–Purdue University. Fort Wayne, 46805, Indiana. USA.
*Correspondent:
gamaliel.cg@gmail.com
Recibido: 18 mayo 2009
Aceptado: 22 abril 2010
Abstract
We analyzed lizard population density and tail loss frequency in 2 populations of Uma paraphygas to determine if the proportion of lizards with tail loss was different between populations, sexes, and age classes. Also, we estimated unbroken and regenerated tail growth rates for adult males and females, and juveniles. Data were collected between fall 1997 and summer 1999. Tail loss incidence was relatively low overall, but was significantly higher at the site (Dune 1) with lower vegetation cover. Adult lizards showed a higher tail loss frequency than juveniles at the site with higher vegetation density (Dune 2); there was no difference between adult males and females in both dunes. Observations on 33 lizards with bite marks suggest that intraspecific encounters are not a direct cause of caudal autotomy in this species. The caudal growth rates of lizards were similar for unbroken and regenerated tails between sexes and among age classes.
Key words: tail loss, Uma paraphygas, agonistic behavior.
Resumen
Analizamos la densidad poblacional y la frecuencia de autotomía caudal en 2 poblaciones de Uma paraphygas para determinar si la proporción de lagartijas con cola regenerada fue diferente entre poblaciones, sexos y clases de edad. También estimamos las tasas de crecimiento de la cola en regeneración y la original para machos y hembras adultas y juveniles. Los datos se tomaron estacionalmente desde el otoño de 1997 hasta el verano de 1999. La incidencia de pérdida de cola fue relativamente baja en proporción con la densidad poblacional y fue mayor en la población con menor cobertura vegetal (Duna 1). Las lagartijas adultas presentaron mayor autotomía caudal sólo en la población con mayor vegetación (Duna 2); sin embargo, no hubo diferencia entre machos y hembras adultos en ambas poblaciones. Observaciones en 33 lagartijas con marcas de mordidas sugieren que los encuentros intraespecíficos no causan directamente la autotomía caudal en esta especie. Las tasas de crecimiento de la cola original y regenerada de las lagartijas fueron similares entre sexos y entre clases de edad.
Palabras clave: autotomía caudal, Uma paraphygas, comportamiento agonístico.
Introduction
Tail loss (caudal autotomy), is an important escape strategy for lizards that may be the result of failed predation attempts or intraspecific encounters (Vitt et al., 1974). Caudal autotomy incidence and tail regeneration are usually considered as indicators of predation pressure (Rand, 1954; Turner et al., 1982). Nevertheless, Jaksic and Busack (1984), and Jaksic and Greene (1984) argue that there is an inverse relationship between tail loss frequency and occurrence of lizards in the diets of sympatric predators. Caudal autotomy produces an immediate benefit in survival, but also produces some costs during tail regeneration (Arnold, 1988), including the reduction in equilibrium capacities (Ballinger, 1973), decrease in somatic growth rates (Ballinger and Tinkle, 1979), reduced energetic reserves (Daniels, 1984), reduced locomotor speed (Punzo, 1982), decreased reproductive success (Dial and Fitzpatrick, 1981), microhabitat restrictions (Martín, 1992), loss of social status (Fox et al., 1990), and lowered survival rates (Wilson, 1992). Differences in caudal autotomy frequency between male and female lizards has been associated with a behavioral disparity in which male lizards have a higher exposure to predation than females as a result of territorial behavior over a greater activity area (Tinkle and Ballinger, 1972; Parker and Pianka, 1973).
Usually, low survival rates correspond to high predator pressure (Tinkle, 1969, Pianka 1970); however, tail loss is not easily attributed to predators. During intraspecific encounters, bite marks, injuries, or tail loss can result (Vitt et al., 1974). If a lizard population has low survival rates, high tail loss frequencies, and intraspecific bite marks, some or all tail loss may be caused by intraspecific encounters. Alternatively, if a lizard population has low survival rates and high tail loss frequencies unrelated to bite marks or aggressive encounters, then, intraspecific competition may not cause tail loss directly, and tail loss can be attributed to unsuccessful predator attacks (Jaksic and Greene, 1984).
Restriction to insular dune systems within the southeastern Chihuahuan desert coupled with isolation and low genetic variability have endangered the endemic Chihuahuan fringe–toed lizard, Uma paraphygas (Adest, 1977; Gadsden et al., 1993; SEMARNAT, 2001; Castañeda et al., 2003). Due to the endangered status of this species, it is important to know sources of stress to its populations, including whether tail loss could be caused by predators or by intraspecific encounters, and if any sex or age class is more vulnerable to predators or to competition. Indirectly, knowledge of the low survivorship registered for this species (Castañeda et al., 2003) in addition to tail loss frequencies and bite marks, might suggest if this lizard has a high or low escape success rate. This study increases the knowledge of the ecology of this species by comparing individuals with caudal autotomy in different sex and age classes (adults and juveniles) in 2 lizard populations.
Materials and Methods
Field work was carried out on 2 adjacent dunes, each approximately 2 hectares in size (Dune 1: 26°29'N, 103°58'W and Dune 2: 26°52'N, 103°32'W) and 250–m apart from each other within the Reserva de la Biosfera de Mapimí, Mexico. The dunes and their lizard populations were considered separate because Dune 1 is bound on 2 of its sides by compact and rocky soil (without sand), whereas Dune 2 is found within a larger dune system. Previously, Gadsden et al. (1995, unpub. report, Consejo Nacional de Ciencia y Tecnología, México) studied these populations over a 6 year period (1989–1994); during that period only 1 adult male lizard crossed from Dune 2 to Dune 1, supporting our hypothesis that the 2 dunes provide habitat for separate fringe–toed lizard populations.
The dominant vegetation type on the studied areas is desert scrub, composed of species including Whitehorn acacia (Acacia constricta), Cat claw acacia (A. greggii), Senna casia (Cassia covesii), Goatbush (Castela texana), Ocotillo (Fouquieria splendens), Creosote bush (Larrea tridentata), Berlandier wolfberry (Lycium berlandieri), Narrowleaf pectis (Pectis angustifolia), American Threefold (Trixis californica), and the Soaptree (Yucca elata). Vegetation cover (m2) was measured for both dunes using a digital map and considering the whole surface covered by each plant or by any plant asociation within both studied areas.
Data were collected during a demographic study conducted from the fall of 1997 through the summer of 1999 (Castañeda et al., 2003). Field work was carried out during 12–day periods each season during our study. Each lizard was captured and marked permanently by toe–clipping (Gadsden et al., 2001). Data collected on each lizard included snout–vent length (SVL), sex (males identified by the presence of large postanal scales, well developed femoral pores, or everted hemipenes), tail condition (unbroken and regenerated tail lengths measured to the nearest mm), and age class. Age classes were determined according to Gadsden et al. (1993): adult males (SVL ≥ 70 mm), adult females (SVL ≥ 46 mm), and juveniles (males: SVL ≤ 69 mm, females: SVL ≤ 45 mm). After data collection, lizards were released at the point of capture. Autotomized tails were recorded only in the first season in which they were observed, and were not considered in analyses of other seasons to prevent pseudo replication.
We applied Kolmogorov–Smirnov tests to establish the normality of the population densities (number of lizards caught and marked in 2 ha. by season) in both locations. Student's t tests were applied to compare density means between populations and between adults and juveniles within each location. Chi–square tests (χ2) were used to assess differences in frequency of tail loss in marked lizards of both populations (adults–juveniles and male–female adults). To explore the relationship between numbers of captured adults on each season of the year (considering each seasonal sample for both dunes) with those that had a regenerated tail, a regression analysis was performed after assuring that both data groups were normally distributed with a Kolmogorov–Smirnov test. We considered the numbers of captured lizards (during each season) as densities because it previously was demonstrated that these values were close to estimated densities according the Schumacher–Eschmeyer method (Castañeda et al., 2003).
Tail growth rates (unbroken and regenerated) were calculated as follows. The difference between the tail lengths at first capture and recapture were determined. This difference was divided by the number of days since recapture to determine daily caudal growth rate (mm/day). Tail growth rate was estimated for both populations using pooled data due to the small number of records. Due to non–normality, Mann–Whitney (U) tests were used to compare the unbroken/regenerated tail growth rates within each defined category. Finally, Kruskal–Wallis (H) tests were performed to determine whether the unbroken/regenerated tail growth rates were similar between the different categories. We assumed all tests to be significant at α ≤ 0.05.
Results
Vegetation cover was 2 643.0 m2 for Dune 1 and 4 293.1 m2 for Dune 2.
Two hundred and eightysix lizards were captured at Dune 1, of which only 15.7% showed tail loss with regenerated tails (Table 1). At Dune 2, 304 lizards were caught and only 6.9% showed tail loss. Although the total number of lizards captured (density) was similar (Dune 1 = 23.8 ± 1.7 ind/ha; Dune 2 = 25.3 ± 2.9 ind/ha; t = 0.437, df = 10, P > 0.05), lizards at Dune 1 had a higher frequency of individuals with tail loss (χ2 = 9.22, P < 0.05).
Adults showed a higher frequency of tail loss than juveniles only at Dune 2 (Dune 1: χ2 = 2.59, P > 0.05; Dune 2: χ2 = 8.53, P < 0.05); however, there was no difference in the proportion of adult males and females with regenerated tails (Dune 1: χ2 = 0.81, P > 0.05; Dune 2: χ2 = 0.77, P > 0.05). Additionally, we observed that out of 33 individuals (22 adult males, 10 adult females, and 1 juvenile male) with bite marks on the base of the tail and on the sides of the body that were similar to the shape and size of the snout of U. paraphygas, only one of them showed caudal autotomy. Two individuals with scars (on the back and on a hind leg, respectively) that were unlikely to be the result of intraspecific bites had a regenerated tail. It is not likely that bite marks in the female bodies were caused by males during courtship because these marks are commonly seen in the neck region and not in the tail or in the lower portion of the belly as were observed in this study (Carpenter, 1967).
Regression analysis applied to the adult lizard population showed that the proportion of individuals with caudal autotomy is positively correlated with population density (r = 0.44, F = 5.56, df = 1,22, P < 0.05).
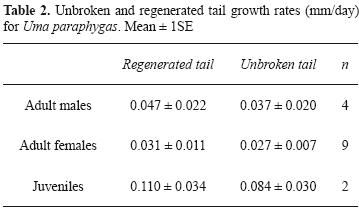
There was no difference in growth rates of unbroken and regenerated tails (growth rates of adult males: U = 7, P > 0.05; growth rate of adult females: U = 35.5, P > 0.05; and growth rate of juveniles: U = 1, P > 0.05). Tail growth rates of the 3 categories (adult males, adult females, and juveniles) were not significantly different for unbroken (H = 3.49, df = 2, P > 0.05) or regenerated tails (H = 2.96, df = 2, P > 0.05) Table 2.
Discussion
Potential predators of U. paraphygas observed in both study areas include: leopard lizards (Gambelia wislizenii), diamondback rattlesnakes (Crotalus atrox), burrowing owls (Athene cunicularia), crows (Corvux corax), roadrunners (Geococcyx californianus), red–tailed hawks (Buteo jamaiciencis), and coyotes (Canis latrans). These predators may produce tail loss and contribute to the low survival rates recorded for U. paraphygas (Castañeda et al., 2003).
Uma paraphygas is a sit and wait forager that avoids predation by relying on crypsis (dorsal coloration resembles sand), escape velocity, capacity to bury itself in the sand, burrowing within vegetation, and, as a last resort, caudal autotomy (Vitt, 1983; Gadsden et al., 1993). The higher incidence of lizards with regenerated tails in Dune 1 than in Dune 2 despite similar lizard densities might be influenced by differences in the availability of vegetation cover. This habitat condition tends to affect escape frequency as has been demonstrated by Congdon et al. (1984) with geckos. Dune 1 was bordered on 2 sides by an open area (compact soil) with less vegetation (26.4% of cover), whereas Dune 2 was surrounded by a larger dune area containing less open sand and more vegetation (42.9% of vegetation cover). Thus vegetation cover and substrate type may be 2 important components determining movement and habitat preferences of some predators and preys. Vegetation may play an important role due to difference in availability of shelters with variable visibility, which modifies movement abilities of both lizards and predators (Hendricks and Dixon, 1988; Eason and Stamps, 1992; Warrick et al., 1998).
Adults had a higher proportion of tail loss than juveniles only on Dune 2. Though still losing tails in close encounters with predators, adults have more experience with predators, and have a greater knowledge of effective escape microhabitats (rodent burrows, dense vegetation, and loose sand to bury into) and so likely escape predators more efficiently than do juveniles. Juvenile encounters with predators are more likely to result in their being preyed upon. This may explain the reduced survival rates found in juveniles lizards (Gadsden et al., 2001; Castañeda et al., 2003).
Because the snout morphology of U. paraphygas is substantially different from other syntopic lizards (Uta stejnegeri, Aspidoscelis marmorata, and Gambelia wislizenii) the bite marks of U. paraphygas were easily recognizable. Although Carpenter (1967) suggested that intraspecific male–male encounters can result in tail loss, the low proportion of lizards with bite marks that showed tail loss indicates that caudal autotomy is not caused directly by such encounters (as has been suggested with Sceloporus magister Vitt et al., 1974). However, while lizard density was greater, tail loss incidence was greater too. All lizards with bite marks were captured during the breeding season, indicating intraspecific aggression is intensified during this period (Gadsden et al., 1993; Guerra–Mayaudón, 1995). Increased intraspecific aggression and reproductive behavior likely result in increased activity and reduced vigilance, making the lizards more vulnerable to predators (Parker and Pianka, 1973; Guerra–Mayaudón, 1995).
Tinkle and Ballinger (1972) suggested that low frequency of caudal autotomy may be either an indicator of rare predator encounters or of high probability of escape without tail loss. The number of individuals of U. paraphygas with regenerated tails was relatively low in proportion to population density. The lowest survival rate (in different sex and age classes) of the genus Uma has been reported in the Chihuahuan fringe–toed lizard (Muth and Fisher, 1991, unpub. report, California Department of Fish and Game, California; Gadsden et al., 2001; Castañeda et al., 2003). Therefore, it is possible that potential predators are more successful in the the Reserva de la Biosfera de Mapimí (Jaksic and Busack, 1984; Jaksic and Greene, 1984). As has been observed in Sceloporus undulatus (Tinkle and Ballinger, 1972), as U. paraphygas population density rises, the presence of individuals with caudal autotomy tends to be higher and the lizards' survival rates are lower (Castañeda et al., 2003). Based on these assumptions, if intraspecific activity increases due to higher population density, the presence of individuals with caudal autotomy will also increase and the survival rates of the individuals will decrease.
Finally, even though the estimated caudal growth rates in this study may be inaccurate due to the sample size, a similar tendency can be appreciated in the somatic growth rates for this species (Castañeda et al., 2003), juveniles having slightly higher growth rates than adults (Gadsden et al., 2001). Moreover, it is possible that the regenerated tail growth rates are higher than that of the original tail segment as in the case of juveniles of Uma exsul (García–De la Peña et al., 2004). However, to ascertain this, it is necessary to increase the sample of lizards with regenerated tails and estimate the caudal growth rates under controlled conditions.
Acknowledgements
We thank Octavio Hinojosa, A. Orona, J. Estrada, and U. Romero for their help in the field; Daniel Diaz, Robert Aldridge, and F. Zaidan III for their valuable help to improve this manuscript; and Cameron W. Barrows for improving the grammar and the manuscript. This study was supported by Comisión Nacional para la Biodiversidad (L173).
Literature cited
Adest, G. A. 1977. Genetic relationships in the genus Uma (Iguanidae). Copeia 1977:47–52. [ Links ]
Arnold, E. N. 1988. Caudal autotomy as a defense. In Biology of the Reptilia, 16 Vol., C. Gans and R. B. Huey (eds.). Alan R. Liss, New York. p. 235–273. [ Links ]
Ballinger, R. E. 1973. Experimental evidence of the tail as a balancing organ in the lizard Anolis carolinensis. Herpetologica 29:65–66. [ Links ]
Ballinger, R. E. and D. W. Tinkle. 1979. On the cost of tail regeneration to body growth in lizards. Journal of Herpetology 13:374–374. [ Links ]
Carpenter, C. C. 1967. Display patterns of the Mexican iguanid lizards of the genus Uma. Herpetologica 23:285–293. [ Links ]
Castañeda, G., H. Gadsden, H. Lopéz–Corrujedo and J. L. Estrada–Rodríguez. 2003. Historia de vida de Uma paraphygas (Sauria: Phrynosomatidae) en dunas de la Reserva de la Biosfera de Mapimí, Durango. Acta Zoológica Mexicana (nueva serie) 89:169–184. [ Links ]
Congdon, J. D., L. J. Vitt and W. W. King. 1974. Geckos: adaptive significance and energetics of tail autotomy. Science 184:1379–1380. [ Links ]
Daniels, C. B. 1984. The importance of caudal lipid in the gecko Phyllodactylus marmoratus. Herpetologica 40:337–344. [ Links ]
Dial, B. E. and L. C. Fitzpatrick. 1981. The energetic cost of tail autotomy to reproduction in the lizard Coleonyx brevis (Sauria: Gekkonidae). Oecologia 51:310–317. [ Links ]
Eason, P. K. and J. A. Stamps. 1992. The effect of visibility on territory size and shape. Behavioral Ecology 3:166–172. [ Links ]
Fox, S. F., N. A. Heger and L. S. Delay. 1990. Social cost of tail loss in Uta stansburiana: lizard tail as status signaling badges. Animal Behavior 39:549–554. [ Links ]
Gadsden, H., F. Méndez–de la Cruz, R. Gil–Martínez and G. Casas–Andreu. 1993. Patrón reproductivo de una lagartija (Uma paraphygas) en peligro de extinción. Boletín de la Sociedad Herpetológica Mexicana 5:42–50. [ Links ]
Gadsden, H., H. López–Corrujedo, J. L. Estrada–Rodríguez and U. Romero–Méndez. 2001. Biología poblacional de la lagartija de arena de Coahuila Uma exsul (Sauria: Phrynosomatidae): implicaciones para su conservación. Boletín de la Sociedad Herpetológica Mexicana 9:51–66. [ Links ]
García–De la Peña, C., G. Castañeda, H. Gadsden, A. J. Contreras–Balderas and D. Lazcano. 2004. Autotomía caudal de Uma exsul (Sauria: Phrynosomatidae). Boletín de la Sociedad Herpetológica Mexicana 12:43–48. [ Links ]
Guerra–Mayaudón, G. 1995. Ámbito hogareño de un gremio de lagartijas en las dunas de la reserva de la Biosfera de Mapimí, Durango. Tesis doctorado, Facultad de Ciencias, Universidad Nacional Autónoma de México. México, D.F. 66 p. [ Links ]
Hendricks, F. S. and J. Dixon. 1988. Regenerated tail frequencies in populations of Cnemidophorus marmoratus (Reptilia:Teiidae). Southwestern Naturalist 33:21–24. [ Links ]
Jaksic, F. M. and S. D. Busack. 1984. Apparent inadequacy of tail loss figures as estimates of predation upon lizards. Amphibia–Reptilia 5:177–179. [ Links ]
Jaksic, F. M. and H. W. Greene. 1984. Empirical evidence of non–correlation between tail loss frequency and predation intensity on lizards. Oikos 42:407–411. [ Links ]
Martín, J. S. 1992. Tail loss consequences on habitat use by the Iberian rock–lizard, Lacerta monticola. Oikos 65:328–333. [ Links ]
SEMARNAT (SecretarÍa del Medio Ambiente y Recursos Naturales). 2001. Norma Oficial Mexicana (NOM–059–ECOL–2001). Protección ambiental–Especies nativas de México de flora y fauna silvestres–Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio–Lista de especies en riesgo. Diario Oficial de la Federación (6 de marzo del 2002), México, D. F. [ Links ]
Parker, W. S. and E. R. Pianka. 1973. Notes on the ecology of the iguanid lizard, Sceloporus magister. Herpetologica 29:143–152. [ Links ]
Pianka, E. R. 1970. Comparative autoecology of the lizard Cnemidophorus tigris in different parts of its geographic range. Ecology 51:703–720. [ Links ]
Punzo, C. M. 1982. Tail autotomy and running speed in the lizards Cophosaurus texanus and Uma notata. Journal of Herpetology 16:331–332. [ Links ]
Rand, A. S. 1954. Variation and predation pressure in an island and a mainland population of lizards. Copeia 1954:260–262. [ Links ]
Schoener, T. W. 1979. Inferring the properties of predation and other injury–producing agents from injury frequencies. Ecology 60:1110–1115. [ Links ]
Tinkle, D. W. 1969. The concept of reproductive effort and its relation the evolution of life history in lizards. American Naturalist 103:501–516. [ Links ]
Tinkle, D. W. and R. E. Ballinger. 1972. Sceloporus undulatus: a study of intraspecific comparative demography of a lizard. Ecology 53:570–584. [ Links ]
Turner, F. B., P. A. Medica, R. I. Jennrich and B. G. Maza. 1982. Frequency of broken tails among Uta stansburiana in southern Nevada and a test of the predation hypothesis. Copeia 1982:835–840. [ Links ]
Vitt, L. J. 1983. Tail loss in lizards: the significance of foraging and predator escape modes. Herpetologica 39:151–162. [ Links ]
Vitt, L. J., J. Congdon, A. C. Hulse and J. E. Platz. 1974. Territorial aggressive encounters and tail breaks in the lizard Sceloporus magister. Copeia 1974:990–993. [ Links ]
Warrick, G. D., T. T. Kato and B. R. Rose. 1998. Microhabitat use and home range characteristics of Blunt–nosed leopard lizards. Journal of Herpetology 32:183–191. [ Links ]
Wilson, B. S. 1992. Tail injuries increase the risk of mortality in free–living lizards (Uta stansburiana). Oecologia 92:145–152. [ Links ]














