Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de biodiversidad
On-line version ISSN 2007-8706Print version ISSN 1870-3453
Rev. Mex. Biodiv. vol.81 n.3 México Dec. 2010
Biogeografía
Predicting the distribution of a parasite using the ecological niche model, GARP
Predicción de la distribución de un parásito usando el modelo de nicho ecológico, GARP
Terry R. Haverkost1, Scott L. Gardner1* and A. Townsend Peterson2
1 Harold W. Manter Laboratory of Parasitology, University of Nebraska–Lincoln, W529 Nebraska Hall, Lincoln, NE 68588–0514, USA. *Correspondent: slg@unl.edu
2 Natural History Museum and Biodiversity Research Center, The University of Kansas, Lawrence, KS 66045, USA.
Recibido: 09 abril 2009
Aceptado: 22 marzo 2010
Abstract
The ecological niche of a parasite exists only at the nexus of certain abiotic and biotic conditions suitable for both the definitive and intermediate hosts. However, the life cycles of most parasites are not known, or are poorly known, and using known ranges of hosts to find endemic parasitic infections has been difficult. However, with ecological niche modeling, we can create potential range maps using known localities of infection. Testing the validity of such maps requires knowledge of the localities of other parasites with common history. Here, we find that the ecological niche of a tapeworm parasite of voles, Paranoplocephala macrocephala (Cestoda: Anoplocephalidae), allows prediction of the presence (in ecological and geographic space) of 19 related parasite species from 3 genera in 23 different hosts throughout the Nearctic. These results give credence to the idea that this group shares similar life cycle requirements despite phylogenetic distance. This work further validates ecological niche modeling as a means by which to predict occurrence of parasites when not all facets of the life cycle are confirmed. Such inductive methods create the opportunity for deducing potential reservoir or intermediate hosts, and complementing studies of parasite biodiversity and community ecology.
Key words: phylogenetics, ecology, geographical distribution, cestodes, Paranoplocephala, biodiversity.
Resumen
El nicho ecológico de un parásito existe sólo cuando coinciden condiciones abióticas y bióticas necesarias para los hospederos definitivos e intermediarios. No obstante, los ciclos de vida de la mayoría de los parásitos son poco conocidos; el usar áreas de distribución de hospederos para encontrar áreas endémicas de parasitismo ha resultado difícil. Con el modelado de nicho, se pueden producir mapas del área de distribución potencial con base en sitios conocidos de presencia. Para probar la validez de estos mapas, se requiere el conocimiento de sitios de presencia de otros parásitos relacionados. En este estudio, encontramos que el nicho ecológico de un gusano parásito de ratones, Paranoplocephala macrocephala (Cestoda: Anoplocephalidae) permite predecir la presencia de 19 especies relacionadas de parásitos de 3 géneros en 23 diferentes hospederos a través del Neártico. Estos resultados apoyan la idea de que este grupo comparte una historia filogenética común que se refleja en nichos compartidos y que el modelado de nichos ofrece una manera de predecir la presencia de parásitos aunque no se conozcan todos los detalles de su ciclo de vida. Estos métodos permiten deducir reservorios u hospederos para estos parásitos.
Palabras clave: filogenia, ecología, distribución, cestodos, Paranoplocephala, biodiversidad.
Introduction
The most thorough procedure for accurately defining the geographic range of a parasite would require 2 main pieces of information. Researchers would first have to know the realized and potential intermediate and definitive hosts used by the parasite. Researchers would also need a phylogeny including the parasite of interest and its relatives. The phylogeny would be used to identify closely–related congeners and compare the known distribution and host usage of those congeners to get a better picture of the occurrence and potential host usage of the original target species. While logical and intuitive, these pieces of information are rarely available for any given parasite. Studies aimed at elucidating intermediate hosts of parasites can take decades (e.g. Zelmer and Esch, 1998), and studies show that parasites utilize hosts with similar trophic tendencies despite their phylogeny (Hoberg, 1996) leading to incongruent host vs. parasite phylogenies (Kimura et al., 2006).
Ecological Niche Models (ENMs) have become a powerful tool that can be used to understand the potential distribution of parasites and diseases when not all facets of that disease are known (Peterson et al., 2002; Peterson et al., 2004). This approach is enticing to parasitologists since researchers can get a broad generalization of a parasite's geographic distribution without knowing the complexities of the parasite's transmission dynamics. However, a map of the parasite's distribution would allow researchers to make more accurate predictions of potential intermediate or reservoir hosts (Peterson et al., 2002; Peterson et al., 2007), if one of these hosts is not known.
The purpose of the present study was to create a potential distribution of Paranoplocephala macrocephala, (Douthitt, 1915) an anoplocephalid cestode, and use the resulting map to answer 2 separate questions. We were interested in 1) how well the modeled distribution could predict the presence of related species and 2) how well that same distribution could predict the presence of P. macrocephala in unsampled areas. We chose GARP as the ENM for this study, as it is well suited to answer both questions. Peterson et al. (1999) showed that predicted distributions made by the GARP algorithm of 1 species could accurately predict the presence of its sister species. Also, in a comparison of GARP and Maxent, Peterson et al. (2007a) showed that GARP outperformed Maxent when predicting species' distributions into broad unsampled areas.
Materials and methods
The parasite. Paranoplocephala macrocephala is an anoplocephalid cestode. The cestode family Anoplocephalidae has a large number of described species, and includes forms that have no hooks on the scolex (holdfast), no apical organ or rostellum, a saccate or reticular uterus, and proglottids (segments) that are generally much wider than long. Species classified in this family occur in a wide variety of reptiles, birds, and mammals, with most species occurring in mammals. Stunkard (1937) was the first to work out the complete life cycle for a species in this family; it is now widely accepted that many members of the Anoplocephalinae use free–living grass mites (Oribatoidea) as intermediate hosts (Kates and Runkel 1948; Freeman, 1952). Paranoplocephala macrocephala has a Nearctic distribution, and is common in small intestines of voles (Rodentia: Arvicolidae) throughout their ranges (Spasskii, 1951; Haukisalmi and Henttonen, 2003).
The Ecological Niche Model. We used the Genetic Algorithm for Rule–set Production (GARP) model (Stockwell and Peters, 1999) to create the ENM for this analysis. Software is available free from the DesktopGARP website (http://www.lifemapper.org/desktopgarp). This method has seen extensive testing and validation (Stockwell and Peterson, 2002, 2003). Although GARP did not rank highly among methods for distribution modeling (Elith et al., 2006), these results have been seen to depend to an unknown degree on methodological artifact (Peterson et al., 2008). Regardless, the challenge of estimating ecological niches is distinct, and GARP has seen considerable success in niche modeling applications (Peterson, 2003).
To create a predictive model within GARP processing, available unique locality points are divided randomly and evenly into groups for model training and testing. The program uses a set of methods (logistic regression, bioclimatic rules, atomic rules, range rules) to make initial hypotheses of non–random associations between the training locality points, a population of randomly generated localities where the species has not been recorded, and the environmental layers provided. These rules are selected, evaluated, tested, and incorporated into or rejected from the final model through an iterative process that resembles chromosomal evolution (crossing over, additions, deletions, etc.): hence the term "genetic algorithm". The testing points are used to assess model accuracy via comparing the percentage of points correctly predicted present or absent by the current rule. The change in predictive accuracy determines whether a particular rule gets incorporated into the final rule set. The algorithm either completes 1000 iterations or runs until further iterations no longer increase predictive accuracy. The final rule set, defined in ecological dimensions, is projected back into geographic space to produce a map.
Because of the random–walk nature of the GARP algorithm, results of replicate runs based on the same input data vary, if subtly. As a result, we filtered the replicate solutions based on their error characteristics to create a best–subset model following Anderson et al. (2003). In particular, spatial predictive models can show error of 2 types: omission (prediction of absence at sites where the species is present) and commission (prediction of presence at sites where the species is believed absent). Because the 2 error components differ in important ways (i.e., commission error is much less serious than omission error), the best–subsets approach emphasizes minimization of omission error. Hence, from 400 random replicate models created by the program, we selected the 20 that showed no omission error as measured based on independent testing data; from this group, we selected the 10 models that deviated least from the median commission index (area predicted as suitable). We summed these 10 models pixel by pixel to produce a final prediction of the potential distribution of P. macrocephala. We present this prediction as a lowest presence threshold map (Pearson et al., 2007); a conservative estimate of the minimum predicted area for P. macrocephala with no omission error.
Environmental data sets used included 14 layers: topographic data (elevation, slope, aspect, topographic index, flow accumulation, and flow direction) from the United States Geological Survey's Hydro–1K data set (http://edcdaac.usgs.gov/gtopo30/hydro/) and climate data (annual mean of daily temperature range, frost days, wet days, vapor pressure, precipitation, and maximum, minimum, and mean temperatures for 1961–1990) from New et al. (1999). All environmental data sets were resampled to 0.1o spatial resolution for analysis to match the approximate spatial precision of the occurrence data available.
To obtain occurrence data for P. macrocephala, we collected the geographic information associated with published literature of this species over the past 100 years (Erickson, 1938; Rankin, 1945; Rausch and Tiner, 1949; Hansen, 1950; Kuns and Rausch, 1950; Hall and Sonnenberg, 1955; Leiby, 1961; Kinsella, 1967) and searched specimen databases associated with the U. S. National Parasite Collection (USNPC) in Beltsville, Maryland, and the Harold W. Manter Laboratory of Parasitology (HWML) at the University of Nebraska – Lincoln. Synonymous names were rectified based on Rausch (1976). Specimens or records without specific latitude/longitude coordinates were geo–referenced to the nearest 0.01o using the GeoLocate geo–referencing program (Tulane University, Belle Chasse, Louisiana). We found 33 references to this species, 23 of which were spatially unique.
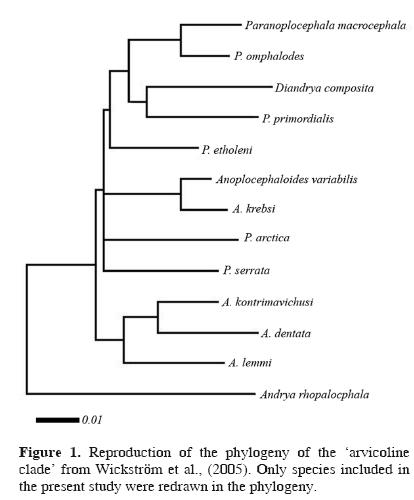
Predicting potential distribution of related species. Peterson et al. (1999) found that GARP was able predict distributions of sister species of their target species, and that this predictive ability was inversely proportional to time since divergence. We performed a similar test using museum records with locality data for closely related congeneric and confamilial cestodes from across North America. Wickström et al. (2005) found strong support for a close phylogenetic relationship among species of Anoplocephaloides, Paranoplocephala, and Diandrya. They called this assemblage the 'arvicoline clade' because of the common association with an arvicoline (vole) definitive host. Their phylogeny is simplified and redrawn as our Figure 1. As such, we gathered all occurrence data available for all species of Anoplocephaloides, Diandrya, and Paranoplocephala from the literature (see Rausch 1946, Hansen 1947, and Kamiya et al. 1979, in addition to the references listed above) and the USNPC and HWML databases. Entries with missing spatial data were geo–referenced as described above. In total, we found 83 spatially–unique localities of 24 parasite species occurring in 29 different definitive host species. Those parasite and host species are listed in Table 1.
To validate the ability of the model to predict the spatial arrangement of these new points, we used a one–tailed chi–square test to compare observed frequencies of successful prediction of test points across the range of thresholds of model agreement (0– 10) with expectations if points and predictions were to be assorted at random with respect to one another. Specifically, we calculated null expectations as the proportion of the area predicted present multiplied by the total number of testing points. Observed and expected values were compared using a chi–square statistic with 1 degree of freedom.
Results
Each of the 10 models that were summed to create the best–subset model predicted independent testing points more accurately than would be expected were testing points and predictions to be at random with respect to one another (all P < 0.001). The best–subset model presented (Fig. 2) shows the lowest presence threshold of models (6) needed to incorporate all locality points for P. macrocephala without omission error. As such, we are confident that this ENM has significant predictive power regarding the geographic distribution of P. macrocephala.
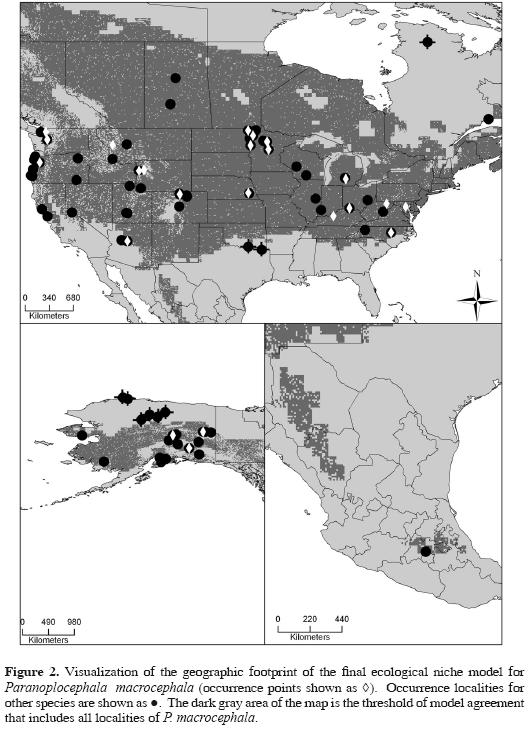
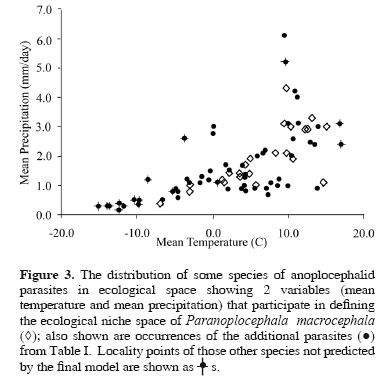
When the P. macrocephala ENM is compared with localities of the additional parasite species, the best–subset model was able to predict the spatial arrangement of those occurrence points more accurately than a null model assuming no association between occurrence and model prediction (P < 0.001). Of the 83 such points, 12 were not predicted successfully by the P. macrocephala ENM; otherwise, however, the best–subset model was able to predict the presence of 19 different parasites from 23 different hosts. Inspecting Figure 2 and Figure 3 in detail, omissions are found along the periphery of the geographic and ecological space outlined by the P. macrocephala ENM, occurring either in extremely cold and dry conditions at northern latitudes or in the warmer climates of the southern Nearctic region.
Discussion
Our results indicate that the ecological niche of P. macrocephala is similar to the ecological niches of 19 other parasite species included in the Anoplocephalidae. Put another way, the ecological conditions suitable for completion of the life cycle of P. macrocephala are variable, but overlap the conditions observed among the other species tested broadly (Fig. 3). This is not surprising, considering the proposed evolutionary relationships among microtine rodents (Conroy and Cook, 1999) and, similarly, among "arvicoline" cestodes (Wickström et al., 2005). The lack of resolution, i.e., polytomies, observed in the anoplocephalid phylogeny found by Wickström et al. (2005) (Fig. 1) appears to mirror the 'pulses of speciation' suggested for the Arvicolinae by Conroy and Cook (1999) and the 2 microtine invasions of North America from Asia around 1.3 million years ago (Conroy and Cook, 2000). The rapid diversification of Microtus during their Pleistocene invasion of the Nearctic region would be consistent with little time and opportunity for evolution of diverse ecological niches among their parasites, which likely accompanied the voles as they crossed Beringia.
Omission error among the other parasite species included in our test occurred mainly along the extremes of the climatic parameters in Figure 3. Ten of the points omitted correspond to cold and dry conditions above the Arctic Circle (~66o 30' N latitude), where the possibility of anoplocephalid life cycle completion on the surface might seem unlikely, given the extreme environment during winter months. At these sites, however, cestodes were found in lemmings (Dicrostonyx sp. and Lemmus sp.), arvicoline rodents known to maintain subnivial burrow systems in winter months (Wooding, 1982). The burrow systems allow these rodents to exist under the snow in a microclimate suitable for the intermediate host, offering opportunities for maintenance of the life cycle of the parasites. Since the maintenance of the life cycle in this area probably does not occur above ground during winter months, our predictive model based on broad scale climatic variables would be less likely to be able to predict their occurrence. The model also does not predict appropriate conditions to occur in the southeastern United States. However, it is known that confamilial species of Cittotaenia occur in cottontail rabbits (Sylvilagus spp.) in Florida, Georgia, and Alabama, and that this parasite also utilizes oribatid mites as intermediate hosts (Stunkard, 1941). As such, it would seem that the geographic extent of suitable habitat for this life cycle (as defined by P. macrocephala) may be limited by the definitive and not the intermediate host. This conclusion is supported by work on the historical ecology of other parasite taxa (Hoberg, 1996) and from the life cycle experiments that show that an anoplocephalid cestode not included in this study, Moniezia expansa, can utilize 73 species of oribatid mites as intermediate hosts and an oribatid mite, Scheloribates laevigatus, can serve as an intermediate host to 14 species of anoplocephalids (Denegri, 1993).
Our model predicted that the anoplocephalid life cycle would be present in 2 areas where, to our knowledge, there have been very few published reports of surveys for small–mammal parasites in decades. The first area predicted as suitable for the transmission of anoplocephalids is along the eastern edge of the Sierra Madre Mountains in northwest Mexico. To our knowledge there are no records of anoplocephalids along this mountain range. In the area predicted to have anoplocephalids present just south of Mexico City, Kamiya et al. (1979) reported on the presence of Anoplocephaloides romerolagi in the volcano rabbit, Romerolagus diazi. These results support the use of GARP when determining likely areas for targeted sampling of anoplocephalid cestodes during biodiversity surveys.
It has been suggested that P. macrocephala may actually be a complex of species, although this complex has not yet been separated and will likely require molecular data to understand the true species limits (Haukisalmi and Henttonen, 2003). If such is the case, the model may overestimate the distributional area of any one species. The likelihood of an overestimation of the niche does not seem high, since the present study shows conserved life cycle characteristics across the subfamily.
Parasites offer a unique problem in niche modeling as parasites from highly vagile hosts may be collected in areas far from the areas in which the parasite infection was obtained (see Hoberg, 1996). We feel that this element does not greatly affect the current study, as movement by hosts in this study is unlikely to exceed the spatial resolution of the data set (0.1o pixels), with any movement between adjacent areas having minimal impact on the coarse environmental variables used. Consideration of this point must be made when making generalizations as to the geographic extent of parasite transmission.
Of the 2 methods outlined by Peterson (2006) for analyzing disease transmission, the present study treats the complex species interactions leading to parasite transmission as a 'black box,' (inductive approach). The alternative (deductive approach)––modeling each host in the life cycle separately––is not feasible in this case since the geographic range of the intermediate host (or hosts) of P. macrocephala are not well characterized. The deductive approach would also not consider incidental transmission of the parasite among suitable, but rare, hosts. Both systems may have merit, depending on the parasite species of interest in the study. For example, the inductive approach may be enlightening when facets of a life cycle are unknown or the parasite is known to show plasticity in its life cycle, e.g., utilizing multiple definitive and intermediate hosts, truncating or bypassing life cycle stages. The deductive approach would be beneficial if that parasite is known to show high levels of host specificity (such as pocket gophers and their ectoparasitic lice, see Hafner et al., 2003).
The creation of such inductive maps in the future may lead to a better understanding of the biogeography of parasites and other host/symbiont associations. Combination of maps across multiple species may prove useful when analyzing geographic patterns of parasite community ecology. Since detailed investigations of ecological characteristics of parasite life cycles of non–human hosts are rare, the inductive approach may prove more fruitful. For many groups or species of parasites, comprehensive life cycle studies, taxonomic and systematic studies, and rigorous field sampling are incomplete or lacking. Although this information is required for creating reliable range maps of parasites, the expertise required to evaluate the quality of these studies critically is receding. However, biogeographic studies of free–living animals and plants are relying less on expertly derived maps and more on existing museum records, bioinformatics, and GIS technologies to provide information relative to conservation of biodiversity. Thus far, parasitic taxa have been largely excluded from these analyses, even though they constitute a dominating presence in the environment (Lafferty et al., 2006). We present this suite of analyses as an exploration of the potential of ecological niche modeling of parasites, to capture their distributions and perhaps contribute more prominently to evaluations of biodiversity.
Acknowledgments
Thanks to Dave Tinnin and Gabor Racz for assistance during this project. This work was partially supported by grants (DEB–0717214, BSR–9024816, DEB–0097019, and DBI–0646356) from the National Science Foundation to S.L. Gardner.
Literature cited
Anderson, R. P., D. Lew and A. T. Peterson. 2003. Evaluating predictive models of species' distributions: criteria for selecting optimal models. Ecological Modelling 162:211–232. [ Links ]
Conroy, C. J. and J. A. Cook. 1999. MtDNA evidence for repeated pulses of speciation within arvicoline and murid rodents. Journal of Mammalian Evolution 6:221–245. [ Links ]
Conroy, C. J., and J. A. Cook. 2000. Molecular systematics of a Holarctic rodent (Microtus: Muridae). Journal of Mammalogy 81:344–359. [ Links ]
Denegri, G. M. 1993. Review of oribatid mites as intermediate hosts of tapeworms of the Anoplocephalidae. Experimental & Applied Acarology. 17:567–580. [ Links ]
Elith, J., C. H. Graham, R. P. Anderson, M. Dudik, S. Ferrier, A. Guisan, R. J. Hijmans, F. Huettman, J. R. Leathwick, A. Lehmann, J. Li, L. G. Lohmann, B. A. Loiselle, G. Manion, C. Moritz, M. Nakamura, Y. Nakazawa, J. M. Overton, A. T. Peterson, S. J. Phillips, K. Richardson, R. Scachetti–Pereira, R. E. Schapire, J. Soberón, S. E. Williams, M. S. Wisz, N. E. Zimmermann. 2006. Novel methods improve prediction of species' distributions from occurrence data. Ecography. 29:129–151. [ Links ]
Erickson, A. B. 1938. Parasites of some Minnesota Cricetidae and Zapodidae, and a host catalogue of helminth parasites of native American mice. American Midland Naturalist 20:575–589. [ Links ]
Freeman, R. S. 1952. The biology and life history of Monoecocestus Beddard, 1914 (Cestoda: Anoplocephalidae) from the porcupine. Journal of Parasitology 38:111–129. [ Links ]
Hafner, M. S., J.W. Demastes, T. A. Spradling and D. L. Reed. 2003. Cophylogeny between pocket gophers and chewing lice. Pages 195–220 In Tangled trees: phylogeny, cospeciation and coevolution, R. D. M. Page (ed.). University of Chicago Press, Chicago, Illinois. 350 p. [ Links ]
Hall, J. E. and B. Sonnenberg. 1955. Some helminth parasites of rodents from localities in Maryland and Kentucky. Journal of Parasitology 41:640–641. [ Links ]
Hansen, M. F. 1947. Three anoplocephalid cestodes from the prairie meadow vole, with description of Andrya microti n. sp. Transactions of the American Microscopical Society. 66:279–282. [ Links ]
Hansen, M. F. 1950. A new dilepidid tapeworm and notes on other tapeworms of rodents. American Midland Naturalist 43:471–479. [ Links ]
Haukisalmi, V. and H. Henttonen. 2003. What is Paranoplocephala macrocephala (Douthitt, 1915) (Cestoda: Anoplocephalidae)? Systematic Parasitology 54:53–69. [ Links ]
Hoberg, E. P. 1996. Faunal diversity among avian parasite assemblages: the interaction of history, ecology, and biogeography in marine systems. Bulletin of the Scandanavian Society of Parasitology 6:65–89. [ Links ]
Kamiya, M., H. Suzuki and B. Villa–R. 1979. A new anoplocephaline cestode, Anoplocephaloides romerolagi sp.n. parasitic in the volcano rabbit, Romerolagus diazi. Japanese Journal of Veterinary Research. 27:67–71. [ Links ]
Kates, K. C. and C. E. Runkel. 1948. Observations on oribatid mite vectors of Moniezia expansa on pastures, with a report of several new vectors from the United States. Proceedings of the Helminthological Society of Washington 15:18–33. [ Links ]
Kimura, M., A. A. Dhondt and I. J. Lovette. 2006. Phylogenetic structuring of Plasmodium lineages across the North American range of the house finch (Carpodacus mexicanus). Journal of Parasitology 92:1043–1049. [ Links ]
Kinsella, J. M. 1967. Helminths of Microtinae in western Montana. Canadian Journal of Zoology 45:269–274. [ Links ]
Kuns, M. L. and R. L. Rausch. 1950. An ecological study of helminths of some Wyoming voles (Microtus spp.) with a description of a new species of Nematospiroides (Heligmosomodiae: Nematoda). Zoologica 35:181–188. [ Links ]
Lafferty, K. D., A. P. Dobson and A. M. Kuris. 2006. Parasites dominate food web links. Proceedings of the National Academy of Sciences of the United States of America. 103: 11211–11216. [ Links ]
Leiby, D. A. 1961. Intestinal helminths of some Colorado mammals. Journal of Parasitology 47:311. [ Links ]
New, M., M. Hulme and P. Jones. 1999. Representing twentieth–century space–time climate variability. Part I: development of a 1961–90 mean monthly terrestrial climatology. Journal of Climate 12:829–856. [ Links ]
Pearson, R. G., C. J. Raxworthy, M. Nakamura and A. T. Peterson. 2007. Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. Journal of Biogeography 34:102–117. [ Links ]
Peterson, A. T. 2003. Predicting the geography of species' invasions via ecological niche modeling. Quarterly Review of Biology 78:419–433. [ Links ]
Peterson, A. T. 2006. Ecological niche modeling and spatial patterns of disease transmission. Emerging Infectious Diseases 12:1822–1826. [ Links ]
Peterson, A. T. 2007. Ecological niche modelling and understanding the geography of disease transmission. Veterinaria Italiana. 43:393–400. [ Links ]
Peterson, A. T., J. Soberón and V. Sánchez–Cordero. 1999. Conservation of ecological niches in evolutionary time. Science 285:1265–1267. [ Links ]
Peterson, A. T., V. Sánchez–Cordero, B. Beard and J. M. Ramsey. 2002. Ecological niche modeling and potential reservoirs for Chagas disease, Mexico. Emerging Infectious Diseases 8:662–667. [ Links ]
Peterson, A. T., J. T. Bauer and J. N. Mills. 2004. Ecological and geographic distribution of filovirus disease. Emerging Infectious Diseases 10:40–47. [ Links ]
Peterson, A. T., M. Papes, D. S. Carroll, H. Leirs and K. M. Johnson. 2007. Mammal taxa constituting potential coevolved reservoirs of filoviruses. Journal of Mammalogy 88:1544–1554. [ Links ]
Peterson, A. T., M. Papes and M. Eaton. 2007a. Transferability and model evaluation in ecological niche modeling: a comparison of GARP and Maxent. Ecography 30:550–560. [ Links ]
Peterson, A. T., M. Papes and J. Soberón. 2008. Rethinking receiver operating characteristic analysis applications in ecological niche modelling. Ecological Modelling. 213:63–72. [ Links ]
Rankin, Jr., J. S. 1945. Ecology of the helminth parasites of small mammals collected from Northrup Canyon, upper Grand Coulee, Washington. Murrelet 26:11–14. [ Links ]
Rausch, R. L. 1946. Paranoplocephala troeschi, new species of cestode from the meadow vole, Microtus p.pennsylvanicus Ord. Transactions of the American Microscopical Society 65:354–356. [ Links ]
Rausch, R. L. 1976. The genera Paranophlocephala Luhe, 1910 and Anoplocephaloides Baer, 1923 (Cestoda: Anoplocephalidae) with particular reference to species in rodents. Annales de Parasitologie Humaine et Comparee 51:513–562. [ Links ]
Rausch, R. L. and J. D. Tiner. 1949. Studies on the parasitic helminths of the north central states. II. Helminths of voles (Microtus spp.). American Midland Naturalist 41:665–694. [ Links ]
Spasskii, A. A. 1951. Essentials of cestodology, Anoplocephalate tapeworms of domestic and wild animals. A. Birron and Z. S. Cole (translators). The Academy of Sciences of the USSR, Moscow. 783 p. [ Links ]
Stockwell, D. and D. Peters. 1999. The GARP modeling system: problems and solutions to automated spatial prediction. Journal of Geographic Information Systems 13:143–158. [ Links ]
Stockwell, D. and A. T. Peterson. 2002. Effects of sample size on accuracy of species distribution models. Ecological Modelling. 148:1–13. [ Links ]
Stockwell, D. and A. T. Peterson. 2003. Comparison of resolution of methods used in mapping biodiversity patterns from point–occurrence data. Ecological Indicators 3:213–221. [ Links ]
Stunkard, H. W. 1937. The life cycle of Moniezia expansa. Science 86:312. [ Links ]
Stunkard, H. W. 1941. Studies on the life history of the anoplocephaline cestodes of hare and rabbits. Journal of Parasitology 27:299–325. [ Links ]
Wickström, L. M., V. Haukisalmi, S. Varis, J. Hantula and H. Henttonen. 2005. Molecular phylogeny and systematics of anoplocephaline cestodes in rodents and lagomorphs. Systematic Parasitology. 62:83–99. [ Links ]
Wilson, D. E. and D. M. Reeder. 2005. Mammal species of the world. Third edition. Johns Hopkins University Press, Baltimore. 2142 p. [ Links ]
Wooding, F. 1982. Wild mammals of Canada. McGraw–Hill Ryerson Limited, Toronto, Ontario, Canada. 272 p. [ Links ]
Zelmer, D. A. and G. W. Esch. 1998. The odonate naiad as a paratenic host for Halipegus occidualis (Trematoda: Hemiuridae). Journal of Parasitology. 84:94–96. [ Links ]














