Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de biodiversidad
versión On-line ISSN 2007-8706versión impresa ISSN 1870-3453
Rev. Mex. Biodiv. vol.81 no.3 México dic. 2010
Ecología
Distribution, genetic structure, and conservation status of the rare microendemic species, Guaiacum unijugum (Zygophyllaceae) in the Cape Region of Baja California, Mexico
Distribución, estructura genética y conservación de la especie microendémica Guaiacum unijugum (Zygophyllaceae) en la región de los Cabos de Baja California, México.
Ross A. McCauley*1, Aurea C. Cortés–Palomec, and Ken Oyama
Centro de Investigaciones en Ecosistemas, Universidad Nacional Autónoma de México. Antigua Carretera a Pátzcuaro No. 8701, Col. Ex–Hacienda de San José de la Huerta, 58190 Morelia, Michoacán, México. *Correspondent: rmccauley@oikos.unam.mx; mccauley_r@fortlewis.edu
1Current address: Department of Biology and Agriculture, Fort Lewis College, 1000 Rim Drive. Durango, Colorado, 81301 USA.
Recibido: 29 septiembre 2009
Aceptado: 06 febrero 2010
Abstract
Guaiacum unijugum is a rare shrub endemic to a 70 km stretch of coastline extending east from San José del Cabo in Baja California and is the least well–known of the 4 species of Guaiacum in Mexico. To increase our knowledge of this species and assess its conservation status we surveyed the extent of occurrence using both herbarium material and field work, assessed levels of genetic diversity, determined its phylogenetic relationships, and completed an evaluation of risk of extinction (MER). Herbarium material identified 5 known localities of occurrence with field work verifying the continued persistence of 4 of these with an additional site discovered. Genetic analysis across the small range using 17 microsatellite loci showed very low levels of genetic diversity with a mean expected heterozygosity (HE) of 0.162 over all polymorphic loci. Most loci were found to be monomorphic and genetic divergence was small, maintained by the presence of rare private alleles in widely–separated populations. Phylogenetic analysis indicated a sister group relationship to G. coulteri along the Pacific coast suggesting vicariance for the origin and occurrence of G. unijugum. The unique evolutionary history coupled with current small population sizes warrants increased conservation via listing as a critically endangered species.
Key words: Baja California Sur, conservation genetics, endemic species, Guaiacum unijugum, MER, Mexico, Zygophyllaceae.
Resumen
Guaiacum unijugum es un arbusto endémico en un área de aproximadamente 70 km en la región de Los Cabos, Baja California Sur, siendo la menos estudiada de las 4 especies de Guaiacum en México. Para incrementar nuestro conocimiento sobre esta especie y determinar su estatus de conservación se realizó un censo de sus poblaciones determinándose su estructura genética, su relación filogenética con otros miembros del género y se calculó su riesgo de extinción (MER). La revisión de material de herbario, confirmó la presencia de 4 poblaciones a las que se sumó el hallazgo de 1 más. Mediante el uso de 17 loci de microsatélites se encontraron bajos niveles de diversidad genética con un promedio de heterocigosidad (HE) de 0.162. La mayoría de los loci fueron monomórficos y la diferenciación genética entre poblaciones fue pequeña y atribuida a la presencia de alelos raros y únicos en algunas poblaciones. El análisis filogenético indicó que el de G. unijugum es un grupo hermano de G. coulteri de la costa del Pacífico en México, lo cual sugiere que la distribución de G. unijugum es el resultado de vicarianza. Su historia evolutiva y la presencia de poblaciones pequeñas indican la necesidad de considerarla como especie en peligro de extinción.
Palabras clave: Baja California Sur, genética de la conservación, especie endémica, Guaiacum unijugum, MER, México, Zygophyllaceae.
Introduction
The genus Guaiacum, often known commonly as "Lignum Vitae" or "Palo santo", is a group of 6 species native to the American dry tropics ranging from Sonora and the Florida Keys to northern Venezuela. The genus has a long history of human utilization. It was likely used as a medicinal plant for centuries by native peoples of the Caribbean and was adopted by Europeans by the late 15th century for the treatment of syphilis (Munger, 1949). It has been used in a variety of other medicinal treatments and the heartwood resin is currently used for the production of kits for detecting hidden gastrointestinal bleeding (Grow and Schwartzman, 2001; Oldfield, 2005). Its wood additionally has been of high value. It is one of the hardest and most dense woods in trade and has a self–lubricating quality making it useful for mechanical purposes such as wooden bearings and marine propeller–shaft bushings (Oldfield, 2005).
Overexploitation in conjunction with habitat loss and a slow rate of regeneration has led to the listing of most Guaiacum species on various state, national, and international lists of endangered species. In Mexico, Guaiacum sanctum and G. coulteri are classified as species under special protection (SEMARNAT, 2002) and both are classified in the IUCN Red List with G. sanctum listed as endangered (EN C2a) and G. coulteri listed as lower risk conservation dependent (LR/cd) (IUCN, 2007). Protection regulating international trade in all parts and derivatives is afforded by the 2003 listing of all species of Guaiacum in Appendix II of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) due to the inability to differentiate timber from among different species (CITES, 2003; UNEP–WCMC, 2007).
One of the rarer and least known species of the genus Guaiacum is G. unijugum Brandegee, a rare endemic of the Cape Region of Baja California. The species was first observed and collected by T. S. Brandegee in September of 1890 while surveying the flora of southern Baja California under the auspices of the California Academy of Sciences. He initially identified the shrub as Guaiacum sanctum L., a large tree native to tropical forests from southeastern Mexico to Costa Rica, although his notes at the time expressed doubts regarding this placement (Brandegee, 1891). The species, while showing similarities to G. sanctum, differed by its much smaller stature and in having only 1 or 2 pairs of oblique leaflets, indicating to Brandegee its uniqueness and thus suggesting designation as a discrete species (Brandegee, 1915).
Guaiacum unijugum is a divaricately branched shrub growing to about 2 meters in height on coastal dunes and sandy washes. It has small pinnately compound leaves with generally one or rarely 2 pairs of leaflets. It produces 5–merous flowers with light blue petals during mid–summer, maturing into a yellowish–brown capsule with generally one reddish–orange arillate seed by late summer to early fall (Porter, 1963) (Figure 1).
Due to the rarity of G. unijugum little is known regarding the basic biology of the species. Pollination is likely facilitated by bees which have been observed visiting flowers of G. coulteri (R. McCauley, personal observation) which are almost identical in both color and structure. Further information regarding the reproduction and establishment is however unknown. The relationship of G. unijugum to the remainder of the genus in Mexico is additionally uncertain. Porter (1963) hypothesized that G. unjugum was closely related to G. coulteri extending along the coast from Sonora to Oaxaca and that it could represent only a restricted subspecies or variety of this more widespread taxon. Its unique leaf structure and isolation by more than 200 km from the remainder of the genus have led to its continued recognition.
The isolation and rarity of G. unijugum has apparently limited its use in medicinal treatments and wood production. While it is likely that G. unijugum possesses many of the same chemical and wood characteristics as the other species of Guaiacum, it is not known to have ever been exploited commercially. Such lack of use has led to G. unijugum not being recognized on national or international lists of threatened or endangered species.
As the genus Guaiacum is a group of high conservation concern across its entire range, the assessment of conservation status for all of the species is equally important. Thus in this study we aim to provide a set of basic information regarding G. unijugum including a detailed survey of its precise distribution, an analysis of genetic diversity and differentiation, assessment of phylogenetic relationship, and a criteria–based analysis of extinction risk using the Method for Evaluation of Risk of Extinction for Mexican Wild Species (MER; SEMARNAT, 2002).
Materials and methods
Herbarium, field observations and tissue sampling. Herbarium material was sought from a large number of North American herbaria with the collections at DEK, HCIB, MEXU, SD and UC housing specimens of G. unijugum. These were consulted to compile the known distribution of the species (See Appendix for listing of all reviewed specimens). In August of 2007 a survey of the region was undertaken based on the information from previous collection localities as a guide to delimit the region of species occurrence and determine the persistence of the species in these locations. Areas comprising the potential vegetation type near previous collection localities were searched for individuals of G. unijugum. To determine abundance, qualitative observations of the number of adult individuals were made in each collection locality with sites demarcated as zones in which individuals of G. unijugum could be encountered within 0.5 km of each other. Leaf material was sampled from all observed adult plants in each population and placed in silica–gel desiccant for later genetic analysis.
DNA extraction and SSR amplification. Genomic DNA from each individual was isolated from 15 mg of silica–gel dried leaf tissue using the CTAB extraction procedure of Doyle and Doyle (1987) with modifications by Cullings (1992). SSR amplification was performed using the multiplexing procedure previously described in McCauley et al. (2008). A total of 17 loci developed in G. coulteri were used for genotyping, 14 of which were described previously (Gcoult_2 through Gcoult_15) (McCauley et al., 2008). Three additional loci (Gcoult_16, 17, and 18) were developed de novo in G. coulteri using the same procedure and found to amplify consistently with G. unijugum (Table 1). Multiplexed PCR products were diluted 1:1 in dH2O and run in an ABI–Prism 3100–Avant Genetic Analyzer with the GeneScan–500 LIZ™ size standard included (Applied Biosystems). Fragment analysis and final sizing was performed using Genemapper ver. 4 (Applied Biosystems).
Within population genetic diversity. Variation among the 17 microsatellite loci was initially tested for deviations from Hardy–Weinberg equilibrium (HWE) and for linkage disequilibrium using exact tests in Genetic Data Analysis (GDA) ver. 1.0 (Lewis and Zaykin, 2001). Individual allele frequencies in populations for the variable polymorphic loci were calculated in GenePop ver 3.4 (Raymond and Rousset, 1995) to illustrate the distribution of specific alleles and to identify the presence of private alleles. Descriptive statistics including the proportion of polymorphic loci (P), mean number of alleles per locus (A), average expected (HE) and observed heterozygosities (HO), and fixation index (FIS) were also determined using GDA. Effective number of alleles per locus (AE) was calculated empirically from HE following Weir (1990).
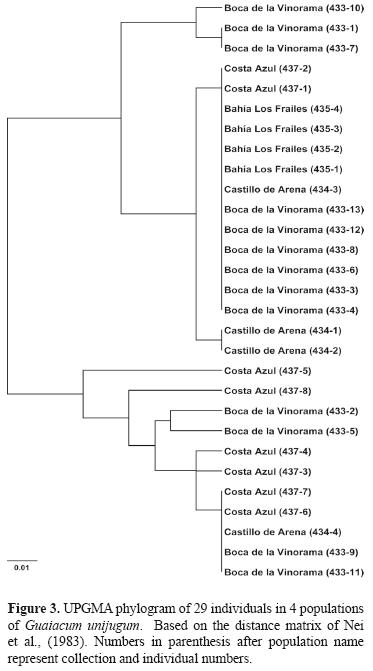
Genetic divergence among populations. Pairwise population differentiation was evaluated by using FST (Weir and Cockerham, 1984) and RST (Slatkin, 1995; Michalakis and Excoffier, 1996) to account for potential differences in microsatellite evolution based on both the infinite alleles model (IAM) (Kimura and Crow, 1964) and the stepwise mutation model (SMM) (Ohta and Kimura, 1973), which is generally more applicable to microsatellite data (Valdes et al., 1993), in Arlequin ver. 3.1 (Excoffier et al., 2005). Additional estimation of gene flow among populations was made by calculating the effective number of migrants (Nm) using the private allele method of Slatkin (1985) in Genepop reporting the corrected estimated value of Barton and Slatkin (1986). To illustrate the relationship among the individuals across populations, the genetic distance (DA) of Nei et al. (1983) was calculated among all individuals using the program Populations 1.2.28 (Langella, 2002). The program was also used to produce a UPGMA tree based on the genetic distance matrix. Branch support was assessed using 1000 bootstrap replicates.
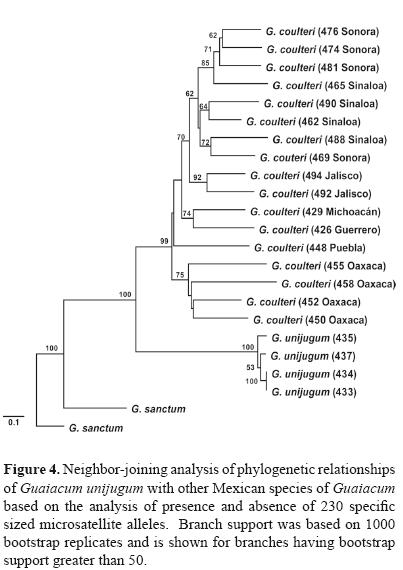
Reconstruction of evolutionary relationships. The relationship of G. unijugum to other species in the genus Guaicum in Mexico was assessed using a subset of populations used in a range–wide phylogeographic study of G. coulteri (McCauley et al., 2009) in conjunction with 2 natural populations of G. sanctum from Campeche. Due to polyploidy within G. coulteri, the microsatellite data matrix was reduced to a dataset of presence and absence of 230 specific sized alleles. Using the genetic distance estimator of Nei and Li (1979), a neighbor–joining tree was computed and branch support assessed with 1 000 bootstrap replicates in TreeCon ver. 1.3b (Van de Peer and De Wachter, 1994).
Assessment of extinction threat. In Mexico, the Method for Evaluation of Risk of Extinction for Mexican Wild Species (MER) has been required by law since 2002 for listing of species for protection (SEMARNAT, 2002), and consists of a combined assessment of geographical distribution, habitat conditions, intrinsic biological vulnerability, and human impact with an associated risk ranking (SEMARNAT, 2002; Olson et al., 2005). As geographic range is an important factor of the MER analysis and since determining the exact range of a rare and scattered species is problematic, we considered range as determined by the shape of a polygon corresponding to the limits of the known distribution determined through the analysis of herbarium collections. This was then compared to the total area of Mexico (1 964 375 km2, Presidencia de la República – México, 2008). The remaining criteria were each scored according to the scale presented by SEMARNAT (2002).
Results
Analysis of herbarium records indicated that G. unijugum inhabits a range of approximately 70 km along the coast from the region around San José del Cabo east to Cabo Pulmo within the sarcocaulescent shrublands east of the Sierra La Trinidad (Fig. 2). Field surveys within this region verified a total of 4 discrete populations, of which 3, Boca de la Vinorama, Bahía los Frailes, and Costa Azul corresponded to previous collection localities. The population at Castillo de Arena was a newly discovered location during this survey. The population size varied greatly among the populations with Boca de la Vinorama being the largest with 13 adult individuals. This site also exhibited the highest density of individuals, particularly in a small region on the north side of the arroyo. The population at Costa Azul was the next smallest in size with 8 individuals, however these were very widely scattered along a dry arroyo over a distance of approximately 3 km. The populations Castillo de Arena and Bahía los Frailes were both very small, containing only 4 individuals, with the individuals being widely separated.
Fruit and seed production was observed in all populations with almost all adult individuals bearing numerous fruits (Fig. 1). Successful reproduction, as evidenced by young seedlings, was observed only in the population at Boca de la Vinorama. Seedlings were observed in the small "thicket" of G. unijugum and Leguminosae along the north edge of the arroyo occurring beneath adult plants. The seedlings had the appearance of being present for some time and consistently differed from the adult plants by producing leaves with 3 pairs of leaflets in contrast to the normal 1 pair of leaflets of the adults.
The 17 microsatellite loci assayed in this study generated a total of 21 unique alleles across the 29 individuals with the average number of alleles per locus, A = 1.162 (range 1.05–1.24). Exact tests for HWE and linkage disequilibrium identified only one significant departure from HWE (locus Gcoult_9 in Boca de la Vinorama) due to an excess of homozygotes, and no instances of linkage disequilibrium. Most loci were monomorphic and fixed across the 4 populations. One locus, Gcoult_12 was heterozygous with the same 2 alleles expressed in all sampled individuals. Four loci, Gcoult_9, Gcoult_10, Gcoult_13, and Gcoult_16 were variable and polymorphic ((Table 2). The most northern sampled population Bahía los Frailes, was shown to be fixed for the most common of the alleles. The equal–sized population Castillo de Arena showed a small level of variation at the locus Gcoult_13. Greater variation in allele frequencies was seen in the 2 larger populations and private alleles were found in 2 separate loci in both populations. Genetic diversity was consistently low for all indices across the 4 populations ((Table 3). Mean values across the 4 populations calculated using only the polymorphic loci showed that values for both expected and observed heterozygosity (HE = 0.162, HO = 0.061) were much lower than the averages reported for endemic species (HE = 0.42, HO = 0.32) by Nybom (2004) using microsatellite data. The 2 largest populations did show almost equal and higher levels of variation but they were still lower than average. The fixation index (FIS) indicated divergence from Hardy–Weinberg equilibrium proportions; however, these were not consistent, with the smallest populations showing an excess of heterozygotes and the larger populations showing a deficit of heterozygotes.
Assessment of population differentiation via the pairwise comparison of FST and RST indicated significant divergence between the 2 largest populations at Boca de la Vinorama and Costa Azul which are separated by approximately 35 km ((Table 4). The negative values for all comparisons based on RST made interpretation of these values inconclusive. The pairwise comparisons of FST additionally showed divergence of low significance between the populations at Bahía los Frailes and Costa Azul separated by a distance of 54 km. While a low level of genetic differentiation was seen among some populations, the relationship among the populations as shown through a UPGMA analysis (Fig. 3) indicated that no population was segregated as distinct and no individuals or populations formed groups having bootstrap support greater than 50 indicating that gene flow among the populations was likely. The estimation of effective number of migrants (Nm) among populations using the private allele method showed a mean frequency of private alleles of p = 0.180 with a resulting Nm = 0.528, a value estimating 1 interpopulation mating per generation equal to that suggested by Ellstrand and Elam (1993), to be sufficient for preventing strong differentiation.
Phylogenetic analysis indicated that G. unijugum is clearly distinct from both G. sanctum and the widespread G. coulteri. The sampled populations of G. unijugum formed a highly supported clade with G. coulteri although it does show a high level of genetic divergence from this sister group (Fig. 4).
The MER assessment for G. unijugum considered the area of potential distribution along the SE tip of the peninsula to encompass approximately 350 km2 representing 0.018% of the total area of Mexico. This would thus classify G. unijugum as a microendemic being very restricted and occurring in less than 5% of Mexican territory and thus was rated at the highest risk level of 4. The habitat conditions and requirements of G. unijugum do not appear to be highly limiting, encompassing coastal dune and arroyo environments. Due to the abundance of this habitat type within the range, we assigned the lowest risk factor value of 1. The intrinsic biological vulnerability, as evidenced by the low population numbers, very slow growth rate, low recruitment, and low levels of genetic variation, was deemed to be high and was assessed a value of 3. The effects of human activity on G. unijugum are likely unequal across populations. Some populations, particularly that of Costa Azul in which the arroyo serves as a roadway and which is subject to the removal of gravel from the arroyo banks, human activity appears to be a very important threat. Other populations, including the largest and best preserved in Boca de la Vinorama and the population in Bahía los Frailes, do not currently show significant signs of human impact even though they are close to a road. The potential for future development and expansion of the adjacent road crossing these areas could, if completed, cause a severe disturbance to the already small populations. Due to the already high level of human impact seen in the southern–most population and the potential for future disturbance in other populations, we assign an intermediate value of 3. Addition of the individual risk criteria values gives a composite value of 11, indicating a conservation status of threatened. If increased human activity were to impinge on more populations, thus increasing this value from intermediate to high, this would raise the composite value to 12, a value in which a species is considered in danger of extinction.
Discussion
Population survey and site evaluation. Our population survey verified that most of the locations from which G. unijugum had been previously known are still extant. From the available information we cannot ascertain if there has or has not been a decrease in population numbers or size in the near past. Anecdotal evidence indicates that the abundance of G. unijugum has likely always been low. Brandegee (1891) remarked that the species was rare at the time he first collected it, a time much prior to the recent development and growth of the region surrounding San José del Cabo, and Porter (1963) also made the same observation.
The discovery of a new, unknown population of G. unijugum (Castillo de Arena) indicates that there is a possibility that additional isolated trees or small populations may occur within the range. The area between San José del Cabo and Cabo Pulmo, sometimes referred to as the "East Cape", is one of the least botanically known regions of the peninsula, and the adjacent Sierra de la Trinidad has historically not been well–collected (Lenz, 1992). While there is a distinct possibility of additional individuals within the range, further identification of these individuals would not significantly expand the naturally small range of the species. Additionally we feel confident that additional large populations are not present within the region. Even though the area is not as well collected as others on the peninsula, the paucity of herbarium specimens of a large, easy to identify and collect evergreen tree point strongly to its rarity across its entire range.
The 4 populations of G. unijugum observed all occurred in arroyos supporting xeric floodplain forests. The one at Costa Azul was in a degraded state due to human activity. In this site vehicular traffic was common, with the arroyo serving as a roadway to access a small tourist development and homes. Additional degradation in this site is due to the current extraction of gravel from the edges of the arroyo which could pose a direct threat to some of the individuals.
Phylogenetic history. Previous hypotheses regarding the origin of G. unijugum had proposed a close relationship with G. coulteri (Dertien and Duvall, 2007), with Porter (1963) suggesting that G. unijugum could represent a subspecies or variety of G. coulteri. Our analysis confirms the close relationship of between G. unijugum and G. coulteri but further suggests that it is likely not a direct descendant or segregate form of the western Mexico species. Rather it represents an evolutionary line sister to that of G. coulteri, both of which long–diverged from a G. sanctum–like ancestor. The timing of the divergence may be in parallel with the geologic origin of the peninsula of Baja California which began separating from the west coast of Mexico approximately 5.5 mya (Riddle et al., 2000). Therefore it is likely that G. unijugum represents a unique lineage within the genus which became isolated via vicariance in the Cape Region of the peninsula.
The occurrence of endemic plant species within the peninsula of Baja California is well known and has a direct link to this geologic history. Of the estimated 3 700 species of vascular plants known from the peninsula, approximately 20% are endemic with endemism occurring in 84 of the 155 native plant families (Reimann and Ezcurra, 2007).
The southern tip of Baja California, usually referred to as the Cape Region or San Lucas region occurring south of the La Paz Fault, roughly equaling a line between the city of La Paz and Todos Santos, is one of the areas exhibiting a particularly high level of species endemism (Lenz, 1992) (Fig. 2). The area was first recognized as a biogeographical unit by Bryant (1891) and the flora of this region has been long–recognized as unique and much differentiated from the drier desert regions to the north due to a large number of species with tropical affinities (Brandegee, 1891, 1892; Shreve, 1937; Shreve and Wiggins, 1964; Wiggins, 1980). Much of the uniqueness is due to the geological structure and history of the region which for a time period of approximately 2 million years during the Pliocene was isolated as an island, allowing its flora to evolve separate from that of the remainder of the peninsula (Wiggins, 1960; Riddle et al., 2000; Hellenes and Téllez–Duarte, 2002). The insular character of the flora was early recognized by Brandegee who noted the large proportion of genera to species in the region and estimated that 10% of the flora was endemic (Brandegee, 1892). Today 1,053 taxa of vascular plants distributed in 131 families are recognized from the Cape Region, with 132 local endemic vascular plant taxa (Lenz, 1992; Peinado et al., 1994).
Genetic diversity, differentiation, and evolutionary potential. A predominant finding of our study was the very low levels of variation and differentiation found across the range of G. unijugum. It is likely that a number of factors could be influencing this pattern of low genetic diversity and could include historical population size, breeding system, and phylogenetic history (Hamrick and Godt, 1996).
Given the historical biogeography of the Cape Region, and the unique morphology and phylogenetic history of G. unijugum, it is best viewed as a vicariant species long–isolated in an island–like environment. Such insular endemics are often at greater risk of extinction due to genetic effects such as drift and inbreeding depression that increase their susceptibility to environmental stochasticity, especially if populations are small (Ellstrand and Elam, 1993; Frankham, 1997). In small populations drift generally causes i) loss of heterozygosity and fixation of alleles and ii) greater differentiation (Ellstrand and Elam, 1993).
Differentiation was very low as indicated by the pairwise FST and RST comparisons although there were 2 instances of statistically significant differentiation between the Costa Azul population and the northern most 2 populations of Boca de la Vinorama and Bahía Los Frailes ((Table 4). The RST comparisons, generally more appropriate for the interpretation of microsatellite data, were particularly difficult to interpret due to their negative values. Negative RST values generally occur when differentiation is very low, thus leading to a condition in which the within–population variance can be larger, by chance alone, than the among–population variance (Meloni et al., 2007). Thus the values of RST indicate a condition of complete panmixia among the populations. The UPGMA analysis additionally supported this finding with multiple individuals from multiple populations expressing the same genotype. While this pattern could be a result of the low number of polymorphic loci thus resulting in repeated genotypes we believe that it is actually representing the relationships among individuals in a system of essentially non–differentiated populations, a condition differing from that in which drift over a long period of time is the primary force determining genetic structure.
The differentiation identified among the populations was completely due to the presence of private alleles and not to changes in allele frequencies ((Table 2). While differentiation is more often exhibited due to the changes in allele frequency, the occurrence of rare alleles can provide important evidence for the genetic segregation of groups of populations or taxa (Navarro–Quezada et al., 2003). These private alleles have likely arisen via the slow accumulation of mutations via genetic drift. We propose that it is likely that G. unijugum has always shown low levels of genetic diversity due to its isolation in a relatively small area of suitable habitat and that the genetic diversity values are not the result of a recent decline in population numbers.
It is likely that both the evolutionary history, centered on long–term isolation, and some aspect of the breeding system are maintaining these low levels of genetic diversity. Inbreeding via selfing or seed production via agamospermy could be processes serving to keep levels of genetic variation low. The deviations from Hardy–Weinberg proportions shown in the negative values for FIS calculated among all loci are consistent with the pattern seen in inbreed species ((Table 3)..While an excess of heterozygotes was shown in the 2 smallest populations in Castillo de Arena and Bahía Los Frailes, this is most likely due to random stochastic events due to their small size and not indicative of the patterns of reproduction in these populations.
While the pattern exhibited by the negative FIS values along with the high incidence of monomorphic loci indicate potential inbreeding, the behavior of one locus, Gcoult_12 suggests that a non–sexual process may be involved in the production of offspring. Locus Gcoult_12 was heterozygous with the same configuration of alleles across all individuals. If recombination was occurring via a form of sexual reproduction (including selfing) the consistent expression of this heterozygote condition would be unlikely. While this is speculative and based on circumstantial evidence, we suggest that asexual seed production via agamospermy may be a likely cause of the genetic pattern. While only limited reproduction was observed, it was in the form of small seedlings, indicating that reproduction is occurring via seed and that vegetative propagation is not likely. Such uniparental modes of reproduction have been shown to be more common in plants of both oceanic and offshore island systems (Barrett, 1996; Inoue and Amano, 1986) and species at the periphery of their range (Busch, 2005) due primarily to the limitations of pollinator service. Self–compatibility has been demonstrated in G. coulteri (Bullock, 1985), although it appears that outcrossing may also occur and the high levels of genetic variation in sampled populations of G. coulteri give no indication of widespread inbreeding in this species (McCauley et al., 2008). Further studies comparing the genetic structure of seedlings and parental plants and/or analysis of flower and fruit development could serve to evaluate this hypothesis.
Conservation implications. Given the micro–endemic nature, the small number of individuals and populations, the slow rate of growth and regeneration, and the low levels of genetic diversity, the threats to the persistence of G. unijugum are significant. The results of the MER analysis clearly indicate the potential vulnerability of this species. The MER analysis also clearly indicates that persistence is most closely tied to the extent of human activity. Currently the area where most G. unijugum occurs is a relatively undeveloped region which has primarily only seen small–scale development of isolated custom homes and small housing developments. The potential for future development of this area however is very great and with an improved infrastructure (i.e. paving the entire road San José del Cabo – La Ribera and extension of the electric grid) the level of development will undoubtedly increase. Currently the paved road extends from La Ribera south to the northern edge of the Cabo Pulmo Marine Reserve and plans are in place for its eventual extension.
Conservation and recognition of the unique evolutionary position of G. unijugum would be helped by its listing as an at–risk species. Recognition and protection in international trade is currently afforded due to the listing of all Guaiacum species under Appendix II of CITES, however, this designation does not give recognition to the rarity or afford protection outside of that relating to trade. Currently at the national level, G. unijugum is not listed on Mexico's list of threatened species. We would strongly advocate for the inclusion of G. unijugum on the national list of threatened species to bring the species condition of urgent conservation concern to the forefront. Beyond the national level, G. unijugum additionally meets the criteria drawn up by IUCN for inclusion on the Red List of Endangered Species under the category of Critically Endangered (CR) with an estimated population size numbering less than 250 individuals with an inferred decline (due to development threat) of mature individuals with the population structure such that no population contains more than 50 individuals (C2a(i)) (IUCN, 2001).
Conservation of G. unijugum can not be realized in isolation of the natural community of the southeast coast of the peninsula. In the entire peninsula of Baja California and surrounding islands, approximately 46% of the terrestrial vegetation is protected within some form of a reserve (Reimann and Ezcurra, 2005). This allows for the protection of about 75% of the endemic taxa. None of the low coastal cape sarcocaulescent scrublands of the southeastern tip of the peninsula occur inside any protected area, with the only area of protection along the southeast coast being the marine reserve at Cabo Pulmo. While the reserve further inland within the Sierra de la Laguna covers a large land area of the Cape Region, a total of 55 micro and meso–endemic vascular plants are still not afforded any protected habitat (Reimann and Ezcurra, 2005). Coastal corridors such as that represented in this region are often one of the areas with the highest species richness and/or occurrence of endemics and are particularly vulnerable to land–use change and development (Riemann and Ezcurra, 2007). This region of sarcocaulescent scrublands of the Cape Region shows a unique assemblage of more dry tropical elements and a higher level of species richness than the scrublands further to the north (León de la Luz et al., 2000). This fact, coupled with the current relatively pristine condition along the "East Cape" and the potential for imminent development, stresses the importance that conservation should be in this region. To properly conserve G. unijugum and other associated species, priority should be given to the establishment of a coastal terrestrial reserve or area with special environmental regulations extending from the marine reserve at Cabo Pulmo south to east of the outlet of the Rio San José which could serve to protect this ecosystem and the unique G. unijugum.
Acknowledgments
We would like to thank José Luis León–de la Luz (Centro de Investigaciones Biológicas del Noroeste, CIBNOR), Jon P. Rebman (San Diego Natural History Museum), Andrew S. Doran (University of California, Berkeley), Joseph R. Dertien (Northern Illinois University), and James C. Solomon (Missouri Botanical Garden) for assistance in locating herbarium material of G. unijugum. We would also like to thank Leonel López–Toledo for supplying samples of G. sanctum for the phylogenetic analysis and Ricardo Clark Tapia and 3 additional reviewers for their constructive comments on this and an earlier version of the manuscript. Financial support for this work was granted by the Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO), project number BS004.
Literature cited
Barrett, S. C. H. 1996. The reproductive biology and genetics of island plants. Philosophical Transactions of the Royal Society of London. Series B, Biological sciences 351:725–733. [ Links ]
Barton, N. H. and M. Slatkin. 1986. A quasi–equilibrium theory of the distribution of rare alleles in a subdivided population. Heredity 56:409–415. [ Links ]
Brandegee, T. S. 1891. Flora of the Cape Region of Baja California. Proceedings of the California Academy of Sciences, Ser. 2, Vol. III:108–182. [ Links ]
Brandegee, T. S. 1892. The distribution of the flora of the Cape Region of Baja California. Zoe 3:223–231. [ Links ]
Brandegee, T. S. 1915. Plantae Mexicanae Purpusianae VII. University of California Publications in Botany 6:177–197. [ Links ]
Bryant, W. E. 1891. The Cape Region of Baja California. Zoe 2:185–201. [ Links ]
Bullock, S. H. 1985. Breeding systems in the flora of a tropical deciduous forest in Mexico. Biotropica 17:287–301. [ Links ]
Busch, J. W. 2005. The evolution of self–compatibility in geographically peripheral populations of Leavenworthia alabamica (Brassicaceae). American Journal of Botany 92:1503–1512. [ Links ]
CITES (Convention on International Trade in Endangered Species of Wild Fauna and Flora). 2003. Follow–up of CoP12 Decisions, Guaiacum spp. [Decision 11.114 (Rev. COP12)], Report from the Thirteenth meeting of the Plants Committee, Geneva 12–15 August 2003, CITES PC13 Doc. 9.2. [ Links ]
Cullings, K. W. 1992. Design and testing of a plant–specific PCR primer for ecological and evolutionary studies. Molecular Ecology 1:233–240. [ Links ]
Dertien, J. R. and M. R. Duvall. 2007. Systematics and molecular conservation genetics of endangered trees of the dry neotropics (Guaiacum; Zygophyllaceae). Paper presented at Botany and Plant Biology 2007, Chicago, Illinois, 7–11 July. [ Links ]
Doyle J. J. and J. L. Doyle. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemistry Bulletin 19:11–15. [ Links ]
Ellstrand, N. C. and D. R. Elam. 1993. Population genetic consequences of small population size:implications for plant conservation. Annual Review of Ecology and Systematics 24:217–242. [ Links ]
Excoffier, L., G. Laval and S. Schneider. 2005. Arlequin ver. 3.0:An integrated software package for population genetics data analysis. Evolutionary Bioinformatics Online 1:47–50. [ Links ]
Frankham, R. 1997. Do island populations have less genetic variation than mainland populations? Heredity 78:311–327. [ Links ]
Grow, S. and E. Schwartzman. 2001. The status of Guaiacum species in trade. Medicinal Plant Conservation 7:19–21. [ Links ]
Hamrick J. L. and M. J. W. Godt. 1996. Conservation genetics of endemic plant species. In J .C. Avise and J. L. Hamrick (Eds.). Conservation genetics:case studies from nature. pp. 281–301. Chapman & Hall, New York, New York. [ Links ]
Hellenes, J. and M. A. Téllez–Duarte. 2002. Paleontological evidence of the Campanian to Early Paleocene paleogeography of Baja California. Palaeogeography, Palaeoclimatology, Palaeoecology 186:61–80. [ Links ]
Inoue, K. and M. Amano. 1986. Evolution of Campanula punctata Lam. in the Izu Islands:changes in pollinators and evolution of breeding systems. Plant Species Biology 1:89–97. [ Links ]
IUCN (International Union for Conservation of Nature). 2001. Categories and Criteria (version 3.1). IUCN, Gland, Switzerland. http://www.iucnredlist.org/info/categories_criteria2001; last access:6.XI.2007. [ Links ]
IUCN. 2007. IUCN Red List of Threatened Species. IUCN, Gland, Switzerland. http://www.iucnredlist.org; last access:6.XI.2007. [ Links ]
Kimura, M. and J. F. Crow. 1964. The number of alleles that can be maintained in a finite population. Genetics 49:725–738. [ Links ]
Langella, O. 2002. POPULATIONS 1.2.28:Population genetic software:individuals or populations distances based on allelic frequencies, phylogenetic trees, file conversions. Available at: http://bioinformatics.org/project/?group_id = 84. [ Links ]
Lenz, L. W. 1992. An Annotated Catalogue of the Plants of the Cape Region, Baja California Sur, Mexico. The Cape Press, Claremont, California. [ Links ]
León de la Luz, J. L., J. J. Pérez Navarro and A. Breceda. 2000. A transitional xerophytic tropical plant community of the Cape Region, Baja California. Journal of Vegetation Science 11:555–564. [ Links ]
Lewis, P. O. and D. Zaykin. 2001. Genetic Data Analysis: Computer program for the analysis of allelic data. Version 1.0 (d16c). Free program distributed by the authors over the internet from http://lewis.eeb.uconn.edu/lewishome/software.html. [ Links ]
McCauley R. A., A. C. Cortés–Palomec and K. Oyama. 2008. Isolation, characterization, and cross–amplification of polymorphic microsatellite loci in Guaiacum coulteri (Zygophyllaceae). Molecular Ecology Resources 8:671–674. [ Links ]
McCauley, R. A., A. C. Cortés–Palomec and K. Oyama. 2009. A range–wide population genetic study of Guaiacum coulteri (Zygophyllaceae), a threatened tree endemic to the Pacific coast of Mexico. Paper presented at Botany and Mycology 2009, Snowbird, Utah, 25–29 July. [ Links ]
Michalakis, Y. and L. Excoffier. 1996. A generic estimation of population subdivision using distances between alleles with special reference to microsatellite loci. Genetics 142:1061–1064. [ Links ]
Meloni, M., D. Perini and G. Binelli. 2007. The distribution of genetic variation in Norway spruce (Picea abies Karst.) populations in the western Alps. Journal of Biogeography 34:929–938. [ Links ]
Munger, R. S. 1949. Guaiacum, the Holy Wood from the New World. Journal of the History of Medicine and Allied Sciences 4:196–229. [ Links ]
Navarro–Quezada, A., R. González–Chauvet, F. Molina–Freaner and L. E. Eguiarte. 2003. Genetic differentiation in the Agave deserti (Agavaceae) complex of the Sonoran Desert. Heredity 90:220–227. [ Links ]
Nei, M. and W.–H. Li. 1979. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proceedings of the National Academy of Sciences of the United States 76:5269–5273. [ Links ]
Nei, M, F. Tajima and Y. Tateno. 1983. Accuracy of estimated phylogenetic trees from molecular data. II. Gene frequency data. Journal of Molecular Evolution 19:153–170. [ Links ]
Nybom, H. 2004. Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Molecular Ecology 13:1143–1155. [ Links ]
Oldfield, S. 2005. Analysis of trade in parts and derivatives of Guaiacum species from Mexico. Report Prepared for IUCN MPSG, February 2005. CITES PC15 Inf. 4. [ Links ]
Olson, M. E., J. A. Lomelí S. and N. Ivalú Cacho. 2005. Extinction threat in the Pedilanthus clade (Euphorbia, Euphorbiaceae), with special reference to the recently rediscovered E. conzattii (P. pulchellus). American Journal of Botany 92:634–641. [ Links ]
Ohta, T. and M. Kimura. 1973. A model of mutation appropriate to estimate the number of electrophoretically detectable alleles in a genetic population. Genetical Research 22:201–204. [ Links ]
Peinado, M., F. Alcaraz, J. Delgadillo and I. Aguado. 1994. Fitogeografía de la península de Baja California, México. Anales del Jardín Botánico de Madrid 51:255–277. [ Links ]
Porter, D. M. 1963. The taxonomy and distribution of the Zygophyllaceae of Baja California, Mexico. Contributions from the Gray Herbarium of Harvard University 192:99–135. [ Links ]
Presidencia de la República – México. 2008. México. Sistema Internet de la Presidencia; http://www.presidencia.gob.mx/mexico/. Acessed 1 December 2009. [ Links ]
Raymond, M. and F. Rousset. 1995. GENEPOP (version 1.2):population genetics software for exact tests and ecumenicism. Journal of Heredity 86:248–249. [ Links ]
Riemann, H. and E. Ezcurra 2005. Plant endemism and natural protected areas in the peninsula of Baja California, Mexico. Biological Conservation 122:141–150. [ Links ]
Riemann, H. and E. Ezcurra. 2007. Endemic regions of the vascular flora of the peninsula of Baja California, Mexico. Journal of Vegetation Science. 18:327–336. [ Links ]
Riddle, B. R., D. J. Hafner, L. F. Alexander and J. R. Jaeger. 2000. Cryptic vicariance in the historical assembly of a Baja California peninsular desert biota. Proceedings of the National Academy of Sciences of the United States 97:14438–14443. [ Links ]
SEMARNAT (Secretaría del Medio Ambiente y Recursos Naturales). 2002. Norma Oficial Mexicana, NOM–059–ECOL–2001, Protección ambiental:Especies nativas de México de flora y fauna silvestres. Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio. Lista de especias en riesgo. Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT). Diario Oficial de la Federacion, Mexico, D.F. [ Links ]
Shreve, F. 1937. The vegetation of the Cape Region of Baja California. Madroño 4:105–113. [ Links ]
Shreve, F. and I. L. Wiggins. 1964. Vegetation and flora of the Sonoran Desert, Vol 1. Stanford University Press, Stanford, California. [ Links ]
Slatkin, M. 1985. Rare alleles as indicators of gene flow. Evolution 39:53–65. [ Links ]
Slatkin, M. 1995. A measure of population subdivision based on microsatellite allele frequencies. Genetics 139:457–462. [ Links ]
UNEP–WCMC. 2007. UNEP–WCMC Species Database:CITES–Listed Species. http://www.cites.org; last access:13.VI.2007. [ Links ]
Valdes, A. M., M. Slatkin and N. B. Freimer. 1993. Allele frequencies at microsatellite loci:the stepwise mutation model revisited. Genetics 133:737–749. [ Links ]
Van de Peer, Y. and R. De Wachter. 1994. TREECON for Windows:a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Computer Applications in the Biosciences 10:569–570. [ Links ]
Weir, B. S. 1990. Genetic data analysis. Sinauer Associates, Inc, Sunderland, Massachusetts. [ Links ]
Weir, B. and C. Cockerham. 1984. Estimating F–statistics for the analysis of population structure. Evolution 38:1358–1370. [ Links ]
Wiggins, I. L. 1960. The origin and relationships of the land flora. Systematic Zoology 9:148–165. [ Links ]
Wiggins, I. L. 1980. Flora of Baja California. Stanford University Press, Stanford, California. [ Links ]














