Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de biodiversidad
versión On-line ISSN 2007-8706versión impresa ISSN 1870-3453
Rev. Mex. Biodiv. vol.81 no.1 México abr. 2010
Ecología
Appendicularian distribution and diversity in the southern Gulf of Mexico
Distribución y diversidad de apendicularias en el sur del golfo de México
César Flores–Coto1 *, Laura Sanvicente–Añorve1 and Marina Sánchez–Ramírez2
1 Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, Circuito Exterior, Cd. Universitaria, 04510, México, D.F., México.
2 Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional. Prolongación de Carpio S/N, 11340 México, D.F., México.
*Correspondent author:
coto@cmarl.unam.mx
Recibido: 07 mayo 2008
Aceptado: 04 noviembre 2009
Abstract
The diversity and distribution of appendicularians on the continental shelf and upper part of the oceanic sea in the southern Gulf of Mexico is analyzed here for the first time. Samples were collected in September 2003 using a fine mesh net. Twenty species were identified, of which Fritillaria venusta and Pelagopleura oppressa are first records for the Gulf of Mexico. Oikopleura species occur throughout the area, with greatest abundances in the upwelling waters of the inner shelf off Yucatán and Campeche. The greatest abundances of Fritillaria species and of the other genera were recorded in the mid, outer shelf, and oceanic areas of Campeche and Tabasco that are influenced by continental water discharges and an oceanic gyre. Diversity and abundance varied in differing directions, with high abundance and low diversity characterizing the costal areas and low abundance and high diversity in the mid, outer shelf, and oceanic areas. The distribution of appendicularian species appears to be influenced by upwelling currents, gyres, water column depth, continental water discharges, salinity, and temperature. However, it could be assumed that the reasons behind these physical environmental factors include the food supply, a short life cycle, and high reproductive efficiency, factors that are commonly associated with distribution.
Key words: pelagic tunicates, upwelling, continental shelf, ecology.
Resumen
Se analiza por primera vez la diversidad y distribución de las apendicularias en la capa superficial de la columna de agua de la plataforma continental del sur del Golfo de México. Las muestra se recolectaron en septiembre de 2003 usando una malla fina– Se identificaron 22 especies de las cuales Fritillaria venusta y Pelagopleura opresa tienen aquí su primer registro para el Golfo de México. Las especies de Oikopleura ocurrieron en toda el área con sus mayores abundancias en aguas de surgencia que corren sobre la plataforma de Yucatán y Campeche. La mayor abundancia de las especies de Fritillaria y de los otros géneros se registro en la plataforma media, externa y área oceánica de Campeche y Tabasco que están influenciadas por las descargas de aguas continentales y un giro oceánico. La diversidad y abundancia varían en sentido contrario una de otra; alta abundancia y baja diversidad caracterizan a las áreas costeras, en tanto baja abundancia y alta diversidad ocurren en la plataforma media, externa y área oceánica. La distribución de las especies parece estar influenciada por las corrientes de la surgencia, giros, profundidad del área, descargas de aguas continentales, salinidad y temperatura. Sin embargo, puede asumirse que detrás de estos factores físicos están la disponibilidad de alimento, el corto ciclo de vida y la alta eficiencia reproductiva, como los factores mas comúnmente asociados a su distribución.
Palabras clave: tunicados pelágicos, surgencia, plataforma continental, ecología.
Introduction
Appendicularians constitute one of the small zooplankton groups that have received little attention, in spite of their relative abundance and ecological importance. Factors that make this group important include the part that it plays in the rapid transfer of energy toward higher trophic levels through filtering of nanoplankton and picoplankton particles and of dissolved organic matter (Flood et al., 1992; Deibel, 1998; Gorsky and Fenaux, 1998), and the high reproductive rates of the species (Hopcroft and Roof, 1995; Hopcroft et al., 1998).
Most of the studies that have been carried out during the past 15 years on this group have focused on population and physiological aspects (Flood et al., 1992; Hopcroft and Roff, 1995, 1998; Nakamura et al., 1997; Cima et al., 2002; Brena et al., 2003), and few have dealt with its distribution.
Basic studies on the composition, distribution, and seasonality of appendicularians in the Gulf of Mexico are scarce. In fact, only 5 papers are related to these topics, and include those of Brooks and Kelner (1908) for the Tortugas, Florida, a study which also includes the description of a new species; Tokioka and Suarez–Caabro (1956) for Cuba, which reported 13 species; Flores–Coto (1965, 1974) for the reef system off Veracruz, which reported 20 species; and Lohmann (1916; fide Tokioka and Suarez–Caabro, 1956) who mentioned 8 species.
Thus, many aspects of the diversity, distribution, seasonal abundance and environmental factors that influence these matters are yet to be investigated.
This study analyzes the diversity and species distribution of appendicularians in the southern Gulf of Mexico, mainly on the continental shelf, in relation to the environmental parameters.
Materials and methods
The study area is located at 18–22°N and 88–95°W, and includes the continental shelf off the coasts of Tabasco, Campeche and Yucatán (Fig. 1).
The shelf north of the Yucatán Peninsula is very wide (about 150 km). The hydrodynamics on the shelf are regulated by a branch of the Yucatán Current with upwelling off Cabo Catoche (Besonov et al., 1971; Furnas and Smayda, 1987; Merino, 1997; Zavala–Hidalgo et al., 2003). The Tabasco shelf is only 50 km wide and is influenced by a variety of continental water outflows (Shirasago–German, 1991, Rivera–Arriaga and Borges–Souza, 2006). Water circulation in Campeche Bay is predominantly cyclonic (Monreal–Gómez and Salas de Leon, 1990).
Samples were collected at 107 stations during the SGM8 cruise from September 3rd to October 8th. A Neil Brown Mark III CTD was used to record conductivity, temperature and pressure throughout the water column. Biological samples were obtained by vertical sampling along the first 50 m of the water column, using a 30 cm diameter and 200 µm mesh size conical net with a flow meter to calculate the volume of filtered water. The samples were fixed with 4% formalin and were processed in the laboratory to separate the appendicularians.
Correlations between species abundance and the environmental parameters were determined by means of a multiple regression and a canonical correspondence analysis, in order to evaluate the influence of environmental factors on the distribution of the species. The Shannon–Wiener diversity index was also obtained.
Results
The highest temperatures were recorded on the inner and mid shelf of Campeche, and the lowest in 2 areas, one off the eastern Yucatán shelf that is influenced by upwelling and the other in the coastal area of Tabasco that receives freshwater discharges (Fig. 2a).
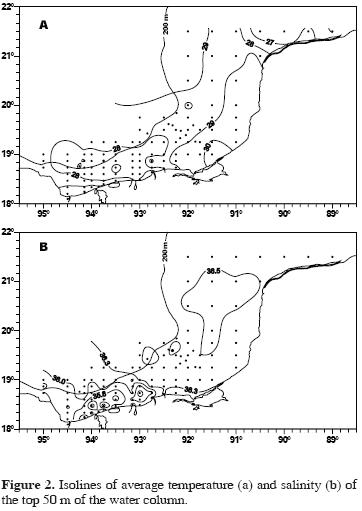
The highest salinities were recorded on the wide shelf off Yucatán and the lowest on the Tabasco shelf off the main freshwater discharge areas (Fig. 2b).
Twenty species were collected, of which Oikopleura longicauda (70.3%), O. fusiformis (13.6%), Fritillaria haplostoma (10.1%), F. borealis (2.3%), and O. rufescens (1.0%) represented >97% of the total abundance. The other 15 species were very scarce, each representing <1%. Eight of these were present in 5 or less sampling stations (Table 1).
The 2 most abundant species, Oikopleura longicauda (Fig. 3A) and O. fusiformis (Fig. 3B), were widely distributed throughout the study area and occurred in 95.3% and 71.9% of the stations respectively.
Other species including O. rufescens (Fig. 3C), O. dioica (Fig. 3D), O. cophocerca (Fig. 4A) and O. parva (Fig. 4B) were much less abundant but had relatively wide distributions. Oikopleura gracilis (Fig. 4C) was collected only on the Tabasco–Campeche shelf, in contrast with O. intermedia (Fig. 4D) that was restricted to the Yucatán shelf.
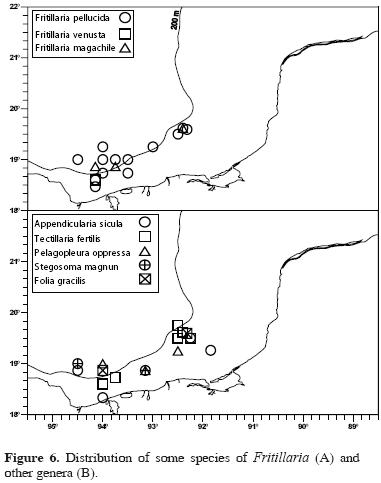
The remaining species were less widely distributed. Fritillaria venusta was collected at only 1 station but was more abundant than Tectillaria fertilis, F. megachile, Pelagopleura oppressa, Stegosoma magnum, and Folia gracilis (Table 1).
Fritillaria species were practically absent on the Yucatán shelf, and their greatest densities were recorded on the Tabasco shelf. The species F. haplostoma (Fig. 5A), F. borealis (Fig. 5B), and F. formica (Fig. 5C) were collected at 1 or 2 stations on the Yucatán shelf with very low abundances. Fritillaria haplostoma, the most abundant species of the genus, was relitvely abundant on the Campeche shelf, while F. fraudax (Fig. 5D) and the other species of this genus (Fig. 6) were present in low numbers in this area, and were found mainly on the narrow Tabasco shelf. Fritillaria venusta was found at only 1 station on the Tabasco shelf (Figs. 6). The species of the other genera had low abundances and frequencies of occurrence, and were collected at the oceanic and outer shelf stations off Tabasco (Fig. 6).
The diversity index (Shannon–Wiener) fluctuated from zero at some stations to 3.4 bits/individual at those where 10 species were recorded. The lowest values were at the coastal stations at depths of 30 m, mainly in the Yucatán and Campeche areas, with other notably low values occurring at depths between 30 and 75 m (Fig. 7). One to 3 species were recorded at these stations. One species only, O. longicauda, was found in 9 stations. The low diversity is a reflection of the high density and wide distribution of the Oikopleura genus.
The greatest diversity values (>2.75 bits/individual) were recorded for the stations located off Tabasco, particularly the outer shelf and oceanic stations where very low densities were recorded, as well as for a few stations in the adjacent areas of the Campeche shelf. These values are related to the presence of species of genera different from Oikopleura and Fritillaria.
The results of the multiple regression analysis between species abundance and the parameters indicate a significant negative correlation (p<0.05) between depth and O. longicauda, O. parva and F. haplostoma, between salinity and F. borealis, F. formica and O. dioica, and between temperature and F. fraudax. The very uncommon species were not included in the analysis.
Similarly, the canonical correspondence analysis indicated that F. formica, F. borealis, A. sicula and O. dioica occurred in areas of lower salinity, whereas F. megachile was present in areas of higher salinity. Oikopleura longicauda tended to be found in shallow low temperature areas, while S. magnum, F. pellucida, O. gracilis and Folia gracilis occurred in the outer shelf and oceanic stations at higher temperatures (Fig. 8).
Discussion
Of the 20 identified species, Fritillaria venusta and Pelagopleura oppressa are first records for the Gulf of Mexico. Apart from these 20 species, Flores–Coto (1971) had previously recorded the species O. albicans, Althofia tumida, F. aequatorialis, K. oceánica and T. veracruzana for the Veracruz reefs, which provide a total of 25 species recorded for the Gulf of Mexico.
The recording of O. longicauda as a dominant species is added to the list of studies that have found it to be the dominant species in tropical and subtropical waters around the world (Tokioka and Suarez–Cabro, 1956; Flores–Coto, 1974; Esnal, 1979, 1999; Hopcroft and Roof, 1998; Aravena and Palma, 2002).
The oceanic versus neritic nature for most species has not been definitively established, but P. oppressa, S. magnum, T. fertilis, A. sicula and F. gracilis have most frequently been found in oceanic as opposed to neritic waters, whereas O. dioca has been found more frequently in coastal areas (Tokioka and Suarez–Cabro, 1956; Fenaux, 1963; Zoppi de Roa, 1971; Esnal, 1972; Castellanos et al., 1994; Esnal and Castro, 1977; Capitanio and Esnal, 1997), in agreement with ourr findings.
It should be noted that, with respect to the distribution of this group, diversity and abundance presented opposite trends and divided the study area into 2 regions. Whereas the greatest diversity and lowest abundance occurred in the outer shelf and oceanic stations off Tabasco, the lowest diversity and greatest abundance was observed at the shallow stations off Campeche and Yucatán. The latter region, where Oikopleura species were abundant and Fritillaria species and other genera were practically absent, lies under the influence of a branch of the Yucatán current that is formed by clear upwelling waters (Besonov et al., 1971; Merino, 1997) that reach the shallow areas of Campeche and occasionally those of Tabasco (Inoue and Welsh, 1997; Zavala Hidalgo et al., 2003). In contrast, the mid and outer shelf of Tabasco, where most of the Fritillaria species and other genera occurred, is subject to continental water discharges, as well as a cyclonic gyre (Besonov et al., 1971; Salas de León et al., 1998).
The results indicate a significant negative relationship between O. longicauda, O. parva and F. haplostoma and depth, between F. fraudax and temperature, and between O. dioca, F. borealis and F. formica and salinity. Nevertheless, these relationships did not explain the dissimilar genera and species distribution between the deep and shallow areas, as mentioned above.
All the observed species have been characterised as tropical and subtropical inhabitants of neritic and oceanic waters, throughout wide ranges of salinity and temperature.
Different populations of the same species may be adapted to different ranges depending on the locality (Fenaux et al., 1998; López–Urrutia et al., 2005). Tomita et al. (2003) suggested that the spatial patterns of appendicularians in Toyama Bay were more likely the result of biological factors than of physical factors.
It is probable that the salinity and temperature in the study area could have a more evident effect on distribution as a consequence of large seasonal variations in continental water discharges, mixing processes, currents, gyres and other climatologically related activities, as previous studies have suggested for meso–scale distribution patterns of ichthyoplankton (Salas de León et al., 1998; Sanvicente et al., 2000).
Although currents, oceanic gyres, continental water discharges, depth, salinity and temperature appear to be the main factors that determined the distributions and abundances recorded in this study, similarly uneven distributions have been observed elsewhere (Bojorbern and Forneris 1956a, 1956b; Fenaux, 1963; Nakamura et al., 1977; Aldridge, 1982; Capitanio and Esnal, 1998; Brena et al., 2003). These being independent of the local Gulf of Mexico physical factors, other factors must be considered. We assume that the food supply, a short life cycle, and high reproductive efficiency (massive production) are factors commonly associated with distribution (Hopcroft and Roof, 1995, 1998; Capitanio and Esnal, 1998; Cima et al., 2002). Food supply could probably be the main factor, as appendicularians do not store energy in lipid deposits (Gorky and Fenaux, 1998). Consequently, the presence and abundance of this group of animals should indicate environments rich in detritus of ingestible size and quality.
Acknowledgements
The authors thank Faustino Zavala for the technical support provided. The samples for this study were collected during the "Monitoreo Ambiental del Sur del Golfo de México", PEMEX Program SGM8.
Literature cited
Alldredge, A. L. 1982. Aggregation of spawning appendicularians in surface windrows. Bulletin of Marine Science 32:250–254. [ Links ]
Aravena, G., S. Palma. 2002. Taxonomic identification of appendicularians collected in epipelagic waters off northern Chile (Tunicata, Appendicularia). Revista Chilena de Historia Natural 75:307–325. [ Links ]
Besonov, N., O. González and A. Elizarov. 1971. Resultados de las investigaciones Cubano–soviéticas en el Banco de Campeche. Coloquio sobre Investigaciones y Recursos del Mar Caribe y Regiones Adyacentes, Curasao, Antillas Holandesas, 1968. UNESCO, Paris. 318–323 p. [ Links ]
Björnberg, T. K. and L. Forneris. 1956a. On the uneven distribution of the copelata of the Fernando Noronha area. Boletin Instituto Oceanografico São Paulo 7:105–111. [ Links ]
Björnberg, T. K. and L. Forneris. 1956b. On the uneven distribution of the copelata of the Alcatrazes area. Boletin Instituto Oceanografico São Paulo 7:113–115. [ Links ]
Brena, C., Cima, F. and P. Burighel. 2003. The highly specialized gut of Fritillaridae (Appendicularia: Tunicata). Marine Biology 143:57–71. [ Links ]
Brooks, W. K. and C. Kellner. 1908. Tunicata of the Gulf Stream. Part IV. Oikopleura tortugensis, a new appendicularian from the Tortugas, Florida, with notes on its embryology. Papers of Tortugas Laboratory. Carnegie Institute of Washington 1:89–94. [ Links ]
Capitanio, F. L. and G. B. Esnal. 1997. Appendicularian distribution in the Rio de la Plata estuary and adjacent areas. Neritica Curitiva 11:37–48. [ Links ]
Castellanos, I., R. Gasca and G. Esnal. 1994. Apendicularias de dos sitemas costeros del mar Caribe. Sian Kaán Serie Documentos 2:18–22. [ Links ]
Cima, F., C. Brena and P. Burighel. 2002. Multifarius activities of gut epithelium in an appendicularian (Oikopleura dioca: Tunicata). Marine Biology 1421:479–490. [ Links ]
Deibel, D. 1998. Feeding and metabolism of appendicularia. In The Biology of Pelagic Tunicates, Q. Bone (ed.). Oxford University Press, Oxford. p. 139–149. [ Links ]
Esnal, G. 1972. Apendicularias de la desembocadura del Río de la Plata. Physis 31:259–272. [ Links ]
Esnal, G. 1979. Características generales de la distribución de los tunicados pelágicos del Atlántico sudoccidental, con algunas observaciones morfológicas. Physis 38:91–102. [ Links ]
Esnal, G. 1999. Appendicularia. In South Atlantic Zooplankton, D. Boltovskoy (ed.). Backhuys Publishers Netherlands. p. 1375–1399. [ Links ]
Esnal, G. and R. J. Castro. 1977. Distributional and biometrical study of appendicularians from the west South Atlantic Ocean. Hydrobiology 56:241–246. [ Links ]
Fenaux, R. 1963. Ecologie et Biologie des Appendiculaires Mediterraneens (Villefranche–sur–mer). Laboratorie Arago. Vie et Milieu 16:1–142. [ Links ]
Fenaux, R., Q. Bone and D. Deibel. (1998). Appendicularian distribution and zoogeograpgy. In The biology of pelagic tunicates, Q. Bone (ed.). Oxford University Press, Great Britain. p. 259–264. [ Links ]
Flood, P. R., D. Deibel and C. C. Morris. 1992. Filtration of colloidal melanin from the sea water by planktonic tunicates. Nature 355:630–632. [ Links ]
Flores–Coto, C. 1965. Notas preliminares sobre la identificación de las apendicularias de las aguas veracruzanas. Anales del Instituto de Biología, Universidad Nacional Autónoma de México 36:293–296. [ Links ]
Flores–Coto, C. 1974. Contribución al conocimiento de las apendicularias del arrecife "La Blanquilla" Veracruz, México, con descripción de una nueva especie. Anales del Centro de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México 1:41–60. [ Links ]
Furnas, M. J. and T. J. Smayda. 1987. Inputs of subthermocline waters and nitrate onto the Campeche Bank. Continental Shelf Research 7:161–175. [ Links ]
Gorsky, G. and R. Fenaux. 1998. The role of appendicularia in marine food webs. In The Biology of Pelagic Tunicates, Q. Bone (ed.). Oxford University Press, Oxford. p. 161–169. [ Links ]
Hopcroft, R. R. and J. C. Roff. 1995. Zooplankton growth rates: extraordinary production by the larvacean Oikopleura dioca in tropical waters. Journal of Plankton Research 17:205–220. [ Links ]
Hopcroft, R. R. and J. C. Roff. 1998. Production of tropical larvaceans in Kingston Harbour, Jamaica: Are we ignoring an important secondary producer. Journal of Plankton Research 20:557–569. [ Links ]
Hopcroft, R. R., J. C. Roff and H. A. Bouman. 1998. Zooplankton growth rates: The larvaceans Appendicularia, Fritillaria and Oikopleura in tropical waters. Journal of Plankton Research 20:539–555. [ Links ]
Inoue, M. and S. E. Welsh. 1997. Numerical simulation of Gulf of Mexico circulation under present and glacial climatic conditions. U.S. Deptartament of the Interior. Minerals Management Service, Gulf of Mexico OCS Region. p. 27–30. [ Links ]
López–Urrutia, A., R. P. Harris, J. L. Acuña, U. Bamstedt, P. R. Food, H. J. Fyhn, B. Gasser, G. Gorsky, X. Irigoien and M. B. Martinussen. (2005). A comparison of appendicularian seasonal cycles in 4 distinct European coastal environments. In Response of marine ecosystems to Global change, G. Gorsky, M. J. Youngblut and D. Dibel (eds.). Contemporary Publishing International, Paris. p. 255–276. [ Links ]
Merino, M. 1997. Upwelling on the Yucatan shelf: hydrographic evidence. Journal of Marine Systems 13:101–121. [ Links ]
Monreal–Gómez, M. A. and D. A. Salas de León. 1990. Simulación de la circulación en la Bahía de Campeche. Geofisica Internacional 29:101–111. [ Links ]
Nakamura, Y., K. Suzuki, S. Suzuki and J. Hiromi. 1997. Production of Oikopleura dioica (Appendicularia) following a picoplankton "bloom" in a eutrophic coastal area. Journal of Plankton Research 19:113–124. [ Links ]
Rivera–Arriaga, E. and G. Borges–Souza. 2008. El gran ecosistema marino del Golfo de México. Perspectivas para su manejo. Jaina Boletín informativo 16:30–48. [ Links ]
Salas de León, D., Monreal–Gómez, M. A., L. Sanvicente–Añorve, and C. Flores–Coto. 1998. Influence de la circulation à long terme sur la répartition des organismes zooplanctoniques dans la Baie de Campeche, Mexique. Oceanologica Acta 21:87–93. [ Links ]
Sanvicente–Añorve, L., C. Flores–Coto and X. Chiappa–Carrara. 2000. Temporal and spatial scales of ichthyoplankton distribution in the southern Gulf of Mexico. Estuarine, Coastal and Shelf Science 51:463–475. [ Links ]
Shirazago–German, B. 1991. Hidrografía y análisis frontognenico en el sur de la bahía de Campeche. Tesis maestría, Colegio de Ciencias y Humanidades, Universidad Nacional Autónoma de México. 160 p. [ Links ]
Tokioka, T. 1960. studies on the distribution of appendicularians and some thaliaceans of the nprth Pacific, with some morphological notes. Publications of Seto Marine Biological Laboratory VII:351–443. [ Links ]
Tokioka, T. y J. Suárez–Caabro. 1956. Apendicularias de los mares cubanos. Memorias de la Sociedad Cubana de Historia Natural 23:37–95. [ Links ]
Tomita, M., N. Shiga and T. Ikeda. 2003. Seasonal occurrence and vertical distribution of appendicularians in Toyama Bay, southern Japan Sea. Journal of Plankton Research 25:579–589. [ Links ]
Zavala–Hidalgo, J., S. L. Morey and J. O'Brien. 2003. Seasonal circulation on the western shelf of the Gulf of Mexico using a high–resolution numerical model. Journal of Geophysical Research 108: NO. C12, 3389, doi: 10.1029/2003JC001879. [ Links ]
Zoppi de Roa, E., 1971. Apendicularias de la región oriental de Venezuela. Studies on the fauna of Curaçao and other Caribbean islands 38:77–109. [ Links ]














