Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de biodiversidad
versão On-line ISSN 2007-8706versão impressa ISSN 1870-3453
Rev. Mex. Biodiv. vol.80 no.3 México Dez. 2009
Taxonomía y sistemática
Ciliates (Protozoa) from dried sediments of a temporary pond from Argentina
Ciliados (Protozoa) de sedimentos secos de una charca temporaria de la Argentina
Gabriela Cristina Küppers1,*, María Cristina Claps1 and Estela Celia Lopretto2
1 Instituto de Limnología Dr. R. A. Ringuelet (CONICET–UNLP), Av. Calchaquí Km 23,5, (1888) Florencio Varela, provincia de Buenos Aires, Argentina.
2 Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, Paseo del Bosque s/n, (1900) La Plata, provincia de Buenos Aires, Argentina.
* Correspondent:
gkuppers@fcnym.unlp.edu.ar; gabrielakuppers@yahoo.com.ar
Recibido: 01 agosto 2008
Aceptado: 20 febrero 2009
Abstract
Temporary ponds represent special environments that are inhabited by organisms adapted to changing environmental conditions. Ciliates are able to survive complete loss of water in these transient habitats through cyst formation. However, ciliates from the Neotropical region in general have been poorly studied with modern techniques. The main goal of this study is to describe the ciliates in dried sediments of a temporary pond from Buenos Aires Province, Argentina, through sampling efforts that were performed 2003–2005. Soil samples were obtained during drought phases and re wetted in laboratory to establish raw and enriched cultures. Ciliates were then studied both in vivo and after impregnation with protargol. In this study, we present 4 new records for Argentina (Gonostomum affine (Stein, 1859) Sterki, 1878, Stylonychia bifaria (Stokes, 1887) Berger, 1999, Pleurotricha lanceolata (Ehrenberg, 1835) Stein, 1859, Meseres corlissi Petz and Foissner, 1992), 1 for South America (Blepharisma americanum (Suzuki, 1954) Hirshfield, Isquith and Bhandary, 1965), and 2 for the Neotropical Realm (Gonostomum strenuum (Engelmann, 1862) Sterki, 1878, Stylonychia lemnae Ammermann and Schlegel, 1983).
Key words: ephemeral freshwater environment, soil samples, Ciliophora, Buenos Aires Province.
Resumen
Los cuerpos de agua temporarios son ambientes particulares que se encuentran habitados por organismos adaptados a condiciones fluctuantes. Los ciliados son capaces de sobrevivir a la pérdida completa de agua del ambiente gracias a la formación de estructuras de resistencia. Por otra parte, los ciliados de la región Neotropical han sido poco investigados con técnicas modernas. El objetivo de este estudio es referir los ciliados que se desarrollaron a partir de los sedimentos secos de una charca temporaria de la provincia de Buenos Aires, Argentina, en la que se realizaron muestreos durante el período 2003–2005. Las muestras de suelo fueron obtenidas durante las fases de sequía y luego resuspendidas en el laboratorio para realizar cultivos naturales y enriquecidos. Los ciliados fueron estudiados in vivo y luego de la impregnación argéntica con protargol. En este trabajo se presentan 4 nuevos registros de especies para la fauna de ciliados de la Argentina (Gonostomum affine (Stein, 1859) Sterki, 1878, Stylonychia bifaria (Stokes, 1887) Berger, 1999, Pleurotricha lanceolata (Ehrenberg, 1835) Stein, 1859, Meseres corlissi Petz and Foissner, 1992), 1 para América del Sur (Blepharisma americanum (Suzuki, 1954) Hirshfield, Isquith and Bhandary, 1965) y 2 para la región Neotropical (Gonostomum strenuum (Engelmann, 1862) Sterki, 1878, Stylonychia lemnae Ammermann and Schlegel, 1983).
Palabras clave: limnótopo efímero, muestras de suelo, Ciliophora, provincia de Buenos Aires.
Introduction
Ciliate assemblages from temporary ponds have been scarcely studied (Andrushchyshyn et al., 2003), especially those populations from ponds whose water supply comes mainly from rainfall. Freshwater ciliates in general have been poorly investigated in Argentina and in South America in general with most studies being based solely on live observations (Cela, 1972; Dioni, 1972; Claps and Modenutti, 1984, 1988; Modenutti and Claps, 1986; Pettigrosso and Cazzaniga, 1987; Foggetta and Boltovskoy, 1995; Zaleski and Claps, 1999, 2001; Modenutti and Pérez, 2001; Guillén et al., 2003; among others). Only recently have some researchers employed modern techniques to study the microorganisms from such aquatic environments in Brazil (Paiva and da Silva–Neto, 2004a; 2004b; 2004c; 2005; 2006; 2007) and in Argentina (Küppers et al., 2006a; 2006b; 2007a; 2007b). In temporary ponds, the bed becomes part of the terrestrial habitat during drought periods and the species adapted to survive such stressful conditions are forced either to migrate to another body of water or to produce quiescent structures (Williams, 1987). Many ciliates are able to form resting cysts (Foissner, 1987) that enable them to persist in the sediments of transient habitats and thereafter colonize the water body during the filling phase through excystment. Bamforth (1980) has stated that the ciliate assemblages that develop from rewetted sediments are similar to those found during the initial stages of colonization of transient habitats.
This study has the aim of describing the morphology of Argentinean populations of certain ciliates collected from the dried sediments of a temporary pond using observations made both in vivo and after protargol staining, and comparing the characteristics of these ciliates to populations from different geographical locations.
Materials and methods
Samplings were made from a freshwater temporary pond in Buenos Aires Province, Argentina (35° 05' S, 57° 48' W) during 2003–2005. For a detailed description of the study site, see Küppers et al. (2006a). During droughts, which occur mainly in summer, dried sediments of the pond bed were collected along with leaf litter and the decomposing macrophytes from the pond. Some samples were re–suspended soon after sampling, while the rest of the samples were stored for subsequent re–suspension during the years 2006, 2007, and 2008. Some species were also recorded during hydric phases, when conductivity, temperature, and pH were measured with a multiparameter sensor and dissolved oxygen estimated by the Winkler method (Clesceri et al., 1998). In the laboratory, soil samples were air–dried for almost a month and then rewetted with distilled water in Petri dishes for qualitative examination, following the so–called nonflooded Petri dish method (Foissner, 1992). Crushed wheat kernels were added to the cultures, kept at room temperature (ca. 15 °C), to promote bacterial growth and thus facilitate ciliate development. Ciliates were taken from the cultures with micropipettes under the stereomicroscope in order to make live observations with a bright–field microscope at magnifications of 100X, 400X, and 1 000X. The organisms were also fixed in Bouin's solution and treated by the protargol technique according to the protocol of Wilbert (1975). Photographs were then taken under bright–field microscopy. Drawings of impregnated cells were made with the aid of a tracing device, while drawings of the live specimens were sketched freehand. Measurements were obtained with a calibrated ocular micrometer in the bright–field microscope. The abbreviations in the biometric tables are as follows: Ant., anterior; AZM, adoral zone of membranelles; M, median; N, number of observations; post., posterior; SD, standard deviation;  , arithmetic mean; Xm, minimum observation; XM, maximum observation. Voucher slides have been deposited in the Colección de Invertebrados from the Museo de La Plata, Argentina, and have the catalogue numbers: Blepharisma americanum MLP32; Gonostomum affine MLP39; G. strenuum MLP38; Stylonychia bifaria MLP35; S. lemnae MLP36; Pleurotricha lanceolata MLP40; Meseres corlissi MLP44.
, arithmetic mean; Xm, minimum observation; XM, maximum observation. Voucher slides have been deposited in the Colección de Invertebrados from the Museo de La Plata, Argentina, and have the catalogue numbers: Blepharisma americanum MLP32; Gonostomum affine MLP39; G. strenuum MLP38; Stylonychia bifaria MLP35; S. lemnae MLP36; Pleurotricha lanceolata MLP40; Meseres corlissi MLP44.
Results
Seven ciliate species from the sediments of the dried pond bed were recorded for the first time in Argentina, and in some instances these ciliates were also new for the Neotropical region as well. Their morphology is briefly described in the following paragraphs.
Blepharisma americanum (Suzuki, 1954) Hirshfield, Isquith and Bhandary, 1965 (Table 1; Figs. 1, 6a)


The body in vivo measured 182–280 µm in length and 42–84 µm in width and the cytoplasm was pale to dark pink–colored. The contractile vacuole was normal for the species. The nuclear apparatus had 5–8 interconnected macronuclear nodules and 6–15 micronuclei. The oral ciliature was composed of 53–63 membranelles and a paroral membrane typical of this genus. There were 20–29 somatic kineties, of which 12–17 abutted on the adoral zone membranelles, plus 3 short ventral postoral kineties.
Data on the frequency of occurrence and the physicochemical variables describing the conditions in which the species was found are detailed in Table 8.
Gonostomum affine (Stein, 1859) Sterki, 1878 (Table 2; Figs. 2a, b)

After protargol impregnation the body measured 56–80 µm in length and 26.6 µm in width. Unfortunately, the cell could not be measured in its living state. The contractile vacuole and nuclear apparatus were typical of the species. The oral ciliature was composed of 24–27 oral membranelles and paroral and endoral membranes typical of the genus. Somatic ciliature was normal for the species and consisted of 3 frontal cirri, 1 buccal cirrus, 5 anterior ventral cirri, 2 fronto–terminal cirri, 2 pretransverse cirri, 2 transverse cirri, 2 rows of marginal cirri, 3 rows of dikinetids, and 3 caudal cirri.
Data on the frequency occurrence of the species are detailed in Table 8.
Gonostomum strenuum (Engelmann, 1862) Sterki, 1878 (Table 3; Figs. 2c–e)
The body in vivo measured 98–119 µm in length and 21 –42 µm in width and had refractive cortical granules. The contractile vacuole and nuclear apparatus were typical of the species. The adoral zone of membranelles and paroral and endoral membranes were in a pattern characteristic of the genus. There were 25–30 oral polykineties. Somatic ciliature was normal for the species; being composed of 3 frontal cirri, 1 buccal cirrus, 10–11 anterior ventral cirri, 4–5 fronto–terminal cirri, 2–3 pretransverse cirri, 2 transverse cirri, and 2 rows marginal cirri. The dorsal side presented 3 rows of dikinetids and 3 caudal cirri.
Data on the occurrence frequency of the species are detailed in Table 8.
Stylonychia bifaria (Stokes, 1887) Berger, 1999 (Table 4; Figs. 3a–c, 6b)

The body in vivo measured 98–119 µm in length and 35–42 µm in width. The contractile vacuole and nuclear apparatus were typical of the species. The micronuclei were either faintly impregnated or not at all. One specimen (N = 32) presented 3 spherical macronuclear nodules (Fig. 6b). The oral ciliature consisted of 25–32 membranelles and paroral and endoral membranes in a pattern typical of the genus. The ventral and dorsal ciliature were characteristic of the species.
Data on the occurrence frequency of the species are detailed in Table 8.
Stylonychia lemnae Ammermann and Schlegel, 1983 (Table 5; Figs. 3d–f, 6c–e)
The body invivo measured 133–168 µm in length and 42–63 µm in width. The dorsal side presented a postperistomial bulge. The contractile vacuole and nuclear apparatus were characteristic of the species. The oral ciliature consisted of 38–58 polykineties and paroral and endoral membranes in a pattern typical of Stylonychia. The ventral and dorsal somatic ciliature were typical of the species, but the dorsal kinety 3 is composed of 21–27 (N = 6) dikinetids and the dorsal kinety 4 of 18–22 (N = 4) dikinetids.
Data on the occurrence frequency and the physicochemical variables pertaining to the conditions under which the species was found are detailed in Table 8.
Pleurotricha lanceolata (Ehrenberg, 1835) Stein, 1859 (Table 6; Figs. 4a–c, 7a)

The body in vivo measured 196 µm in length and 70 µm in width. Although these ciliates usually have 2 macronuclear nodules and 2 micronuclei, 3 specimens here possessed 3 macronuclear nodules and 1 micronucleus while another specimen 3 micronuclei. The adoral zone of membranelles consisted in 45–68 polykineties. The paroral and endoral membranes were arranged in a pattern typical of the genus. The ventral and dorsal somatic ciliature was normal for the species, but there were either 2 or 3 rows of marginal cirri on the right side of the body (27 specimens, though, with 2 and 11 with 3), the third of which rows (the innermost one), when present, possessed fewer and wider–spaced cirri.
Pleurotricha lanceolata was also collected during the filling phase of the pond and its food vacuoles contained small ciliates, such as Tetrahymena sp. and Cyclidium glaucoma, along with pennate diatoms.
Data on the occurrence frequency and the physicochemical variables pertaining to the conditions under which the species was found are detailed in Table 8.
Meseres corlissi Petz and Foissner, 1992 (Table 7; Figs. 5, 7b–c)
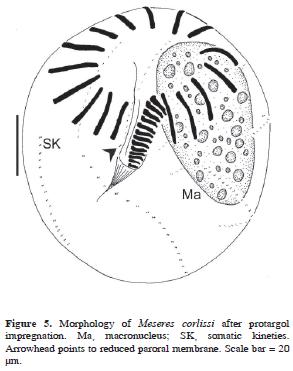
The body after protargol impregnation measured 84–126 µm in length and 66.5–112 µm in width. Unfortunately, the cell could not be measured in its living state. The contractile vacuole and the nuclear apparatus were typical of the species. The oral apparatus consisted of 16 anterior polykineties, 13–18 ventral polykineties, and the endoral membrane. A pair of cilia–bearing kinetosomes was observed to the right of the proximal end of the endoral membrane (reduced paroral membrane). The somatic ciliature was normal for the species, being composed of 7–8 kineties with 16–21 pairs of kinetosomes (N = 17) within each kinety.
The food vacuoles of M. corlissi contained pennate diatoms.
Data on the occurrence frequency of the species are detailed in Table 8.
Discussion
Temporary ponds constitute special kinds of habitats where organisms from various freshwater and soil communities can develop depending upon the conditions of the pond. During droughts, resistant structures from certain freshwater species remain in the sediments and are later able to colonize the pond during the filling phase. By contrast, a genuine soil community can possibly develop during prolonged drought periods. Soil ciliates from the Neotropical region have been scarcely investigated, with South America being almost completely unexplored. Only a few studies on soil ciliates can be cited, including one from Peru (Hemberger, 1985) and another from Venezuela, Peru, Brazil, and Costa Rica (Foissner, 1997a). Although most ciliates have a cosmopolitan distribution, species with restricted distributions also exist (Foissner et al., 2005a). In the end, the further survey of unexplored regions and particular habitats will serve to increase our knowledge about the true extent of the diversity of free–living ciliates.
Blepharisma americanum was described based on specimens cultured from North American freshwater samples (Suzuki, 1954), and Foissner and O'Donoghue (1990) presented a detailed description of the morphology and infraciliature of specimens from an Australian freshwater population. Aladro–Lubel et al. (2007) also recorded this species in freshwaters from Mexico. In terms of soil biotopes, although Aescht and Foissner (1998) found B. americanum in soil samples from Costa Rica in Central America, our finding here represents a new recording for South America. Its morphometric characters generally coincide with those observed bythe above cited authors and its infraciliature with the data of Foissner and O'Donoghue (1990). The number of somatic kineties observed by Wilfert (1972) in a German population, however, is lower than in the specimens studied in the present investigation (14 ± 2 vs. 23 ± 3).
Gonostomum affine is a cosmopolitan and very abundant species in terrestrial habitats or in limnetic environments with terrestrial influences (Berger, 1999). Moreover, several morphotypes exist (Foissner et al., 2001). Within South America, the species was previously found by Foissner (1997a) and Foissner et al. (2001) in soil samples from Brazil, Venezuela, and Peru. In Argentina, however, G. affine represents a new finding. Although few specimens were measured in vivo or were even well impregnated, the species could nevertheless be identified. Most of the morphological variability in this ciliate is in body size and in the number and arrangement of the fronto–ventral–transverse cirri. The morphometric characteristics of our Argentinean isolates coincide with those observed by other authors in other geographical regions, and particularly with the morphotypes recorded by Foissner et al. (2001) in Venezuela and Brazil (South America) and in Namibia (Africa).
Gonostomum strenuum was recorded by other authors in Mexico (Morelos State), Eurasia, Australia, and Africa (Madrazo–Garibay and López–Ochoterena, 1973; Berger, 1999; Foissner et al., 2001, 2005a). This species is, however, a new record for the Neotropical region. Generally, G. strenuum prefers limnetic environments, but it has also been found in edaphic biotopes (Berger, 1999; Foissner et al., 2001, 2005a). Its morphometric characteristics coincide with those observed by Foissner et al. (2001) in Australian populations.
Stylonychia bifaria is possibly cosmopolitan and was previously recorded by other authors for South America in Colombia, Venezuela, and Brazil, although these findings were not substantiated by morphometric data (Berger, 1999). The species is recorded here for the first time in Argentina. Stylonychia bifaria was generally found in stagnant freshwater environments (Berger, 1999), although records of this species in edaphic biotopes from Brazil do also exist (Foissner, 1997a). Its morphometric characteristics generally coincide with the observations of other authors (Berger, 1999).
Stylonychia lemnae has been cited by other authors in Germany, China, North America, and Japan (Berger, 1999); but the documentation of its presence here represents a new finding for the Neotropical region. This species is common in freshwater environments and has also previously been found in soil samples from Japan (Berger, 1999). With respect to its morphology, the most significant difference from that of S. mytilus is the position of the posteriormost frontoventral cirrus (Berger, 1999). Gupta et al. (2001) established a new Indian species, S. ammermanni, which belongs to the Stylonychia mytilus–lemnae complex, but differs from S. lemnae by lacking a postperistomial bulge and because there is a gap between the last frontoventral cirri and the anterior ones. Most morphometric characteristics of the specimens studied by us coincide with those observed by other authors, although the number of dikinetids in the third and fourth dorsal kineties was lower than those mentioned in Berger (1999; i. e., 21–27 and 18–22 vs. 33–40 and 30–39, respectively).
Pleurotricha lanceolata is probably cosmopolitan and was recorded by other authors in freshwater environments from Germany, Africa, Spain, China, India, Mexico, and the USA (Rico–Ferrat et al., 1987; Berger, 1999). Cunha (1913), moreover, recorded this species in Brazil, but without describing or illustrating its morphology. The present report constitutes the first finding of P. lanceolata in Argentina. The morphometric data of the specimens studied coincide with those observed by Jeffries and Mellot (1968), but with an important difference being in the number of right marginal rows of cirri. The ventral infraciliature is also variable, according to the cited authors, and coincides with specimens a–c from Fig. 189, p. 704 in Berger (1999).
Meseres corlissi is considered a rare species, although it does have a global distribution. It was previously found by other authors in Austria, Namibia, Australia, China, Venezuela, Brazil, and the Dominican Republic, in a variety of habitats such as in the sediments of a temporary pond, in a salt pan with regular floodings, in a river floodplain, in flooded soils, and within a bromeliad (Weisse, 2004; Müller et al., 2006). As in the present study, the species was sporadically found in unstable environments (Weisse, 2004). This present finding, however, is the first recording of M. corlissi in Argentina. Although its morphometric characteristics coincide with those observed by Petz and Foissner (1992), the Argentinean specimens presented a greater size (65.6 µm vs. 99.3 µm in their average length), and the numbers of their somatic kineties were more variable (8 constantly, N = 30 vs. 7–8, respectively). The morphology of the resting cysts and the processes of cyst formation and excystment have been well documented for this species (Foissner, 2005; Foissner et al., 2005b, 2006), while their resistance within completely dried edaphic material has been estimated to be several months and even 2 years in soil samples from the type locality (Müller et al., 2006). In the present study, the species was found only once in soil samples that were rewetted soon after its collection, but not in later resuspensions of the same soil material (1 year later). Although M. corlissi exhibits a global distribution, this species is not adapted to wide ranges of environmental conditions. Weisse (2004) stated that M. corlissi prefers unstable habitats with warm temperatures, where it survives through its characteristic resting cysts. As was shown by the cited author, the process of excystment of M. corlissi from a tree bromeliad was influenced by conditions of warm temperature. Other authors, however, proposed that the excystment of M. corlissi depended on a "soil factor", probably a soluble soil component and/or certain bacterial metabolites present (Müller et al., 2006).
Most species in the present report are cosmopolitan; nevertheless new isolates were also found among those published previously (Küppers et al., 2006b, 2007a, b). Although the majority of free–living ciliates are globally distributed, there are also unexplored geographical regions and specific environments that could be inhabited by organisms with restricted distributions. Within the soil biotope, ciliates remain as resting cysts most of the time, but the techniques used for the reactivation of these structures usually lead to an undersampling, thus resulting in an underestimation of the soil ciliate diversity (Foissner, 1997b). In spite of this limitation, almost 50% of the species discovered by Foissner (1997b) in African soils were new ones. This fact points to a great underestimation of soil ciliate biodiversity, an assessment that will increase as new regions are explored. Nevertheless, there are few ciliate taxonomists presently working in the Neotropical region, and soil ciliates in particular have been poorly investigated.
Acknowledgements
The authors wish to thank Santiago Nenda for his help with the photographs; the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) for its financial support; and Dr. Donald F. Haggerty, for review of our English.
Literature cited
Aescht, E. and W. Foissner. 1998. Divisional morphogenesis in Blepharisma americanum, B. undulans, and B. hyalinum (Ciliophora: Heterotrichida). Acta Protozoologica 37: 71–92. [ Links ]
Aladro–Lubel, M. A., M. Reyes–Santos, F. Olvera–Bautista and M. N. Robles–Briones. 2007. Ciliados y otros protozoos. In Guía ilustrada de la Cantera Oriente: caracterización ambiental e inventario biológico, Lot, A. (coordinador). Coordinación de la Investigación Científica. Secretaría Ejecutiva de la Reserva Ecológica del Pedregal de San Ángel de Ciudad Universitaria, Méjico. p. 97–122. [ Links ]
Andrushchyshyn, O., A. K. Magnusson and D. D. Williams. 2003. Ciliate populations in temporary freshwater ponds: seasonal dynamics and influential factors. Freshwater Biology 48: 548–564. [ Links ]
Bamforth, S. S. 1980. Terrestrial Protozoa. Journal of Protozoology 27: 33–36. [ Links ]
Berger, H. 1999. Monograph of the Oxytrichidae (Ciliophora, Hypotrichia). Monographiae Biologicae 78: 1–1080. [ Links ]
Cela, A. M. 1972. Algunos ciliados vinculados a la vegetación flotante. Physis 31: 559–577. [ Links ]
Claps, M. C. and B. E. Modenutti. 1984. Contribución al conocimiento de los ciliados (Ciliophora Peritricha) dulceacuícolas de Argentina, n. Limnobios 2: 581–585. [ Links ]
Claps, M. C. and B. E. Modenutti. 1988. Ciliados dulceacuícolas de Argentina. IV. Suctorios del río Luján. Iheringia, Série Zoologia 67: 127–136. [ Links ]
Clesceri, L. S., A. E. Greenberg and A. D. Eaton (eds.). 1998. Standard methods for the examination of water and wastewater. 20th edition. American Public Health Association, Washington, D.C. 1325 p. [ Links ]
Cunha, A. M. da. 1913. Contribuição para o conhecimento da fauna de protozoários do Brasil. Memórias do Instituto Oswaldo Cruz 5: 101–122. [ Links ]
Dioni, W. 1972. Un nuevo género de Folliculinidae de agua dulce: Botticula ringueleti nov. gen. nov. sp. del río Paraná medio. Acta Zoológica Lilloana 29: 304–313. [ Links ]
Foggetta, M. and A. Boltovskoy. 1995. Ciliated Protozoa from oxygen depleted waters from Cassaffousth reservoir (Córdoba, Argentina). Revista de la Asociación de Ciencias Naturales del Litoral 26: 25–31. [ Links ]
Foissner, W. 1987. Soil Protozoa: fundamental problems, ecological significance, adaptations in ciliates and testaceans, bioindicators, and guide to the literature. Progress in Protistology 2: 69–212. [ Links ]
Foissner, W. 1992. Estimating the species richness of soil protozoa using the "non–flooded petri dish method". In Protocols in Protozoology, Lee, J. J. and A. T. Soldo (eds.). Society of Protozoology, Allen Press, USA. p. B–10.1. [ Links ]
Foissner, W. 1997a. Soil ciliates (Protozoa: Ciliophora) from evergreen rain forests of Australia, South America and Costa Rica: diversity and description of new species. Biology and Fertility of Soils 25: 317–339. [ Links ]
Foissner, W. 1997b. Global soil ciliate (Protozoa, Ciliophora) diversity: a probability–based approach using large sample collections from Africa, Australia and Antarctica. Biodiversity and Conservation 6: 1627–1638. [ Links ]
Foissner, W. 2005. The unusual, lepidosome–coated resting cysts of Meseres corlissi (Ciliophora: Oligotrichea): transmission electron microscopy and phylogeny. Acta Protozoologica 44: 217–230. [ Links ]
Foissner W. and P. J. O'Donoghue. 1990. Morphology and infraciliature of some freshwater ciliates (Protozoa: Ciliophora) from western and south Australia. Invertebrate Taxonomy 3: 661–696. [ Links ]
Foissner, W., T. Stoeck, H. Schmidt and H. Berger. 2001. Biogeographical differences in a common soil ciliate, Gonostomum affine (Stein), as revealed by morphological and RAPD–fingerprint analysis. Acta Protozoologica 40: 83–97. [ Links ]
Foissner, W., S. Agatha and H. Berger. 2005a. Soil ciliates (Protozoa, Ciliophora) from Namibia (Southwest Africa), with emphasis on two contrasting environments, the Etosha Region and the Namib Desert. Denisia 5: 1–1063. [ Links ]
Foissner, W., H. Müller and T. Weisse. 2005b. The unusual, lepidosome–coated resting cysts of Meseres corlissi (Ciliophora: Oligotrichea): light and scanning electron microscopy, cytochemistry. Acta Protozoologica 44: 201–215. [ Links ]
Foissner, W., M. Pichler, K. Al–Rasheid and T. Weisse. 2006. The unusual, lepidosome–coated resting cyst of Meseres corlissi (Ciliophora: Oligotrichea): encystment and genesis and release of the lepidosomes. Acta Protozoologica 45: 323–338. [ Links ]
Guillén G., E. Morales and R. Severino. 2003. Adiciones a la fauna de protozooarios de los pantanos de Villa, Lima, Perú. Revista Peruana de Biología 10: 175–182. [ Links ]
Gupta, R., K. Kamra, S. Arora and G. R. Sapra. 2001. Stylonychia ammermanni sp. n., a new oxytrichid (Ciliophora: Hypotrichida) ciliate from the river Yamuna, Delhi, India. Acta Protozoologica 40: 75–82. [ Links ]
Hemberger, H. 1985. Neue Gattungen und Arten hypotricher Ciliaten. Archiv für Protistenkunde 130: 397–417. [ Links ]
Hirshfield, H. I., I. R. Isquith and A. V. Bhandary. 1965. A proposed organization of the genus Blepharisma Perty and description of four new species. Journal of Protozoology 12: 136–144. [ Links ]
Jeffries W. B. and J. L. Mellot. 1968. New observations on the anatomy of Pleurotricha lanceolata. Journal of Protozoology 15: 741–747. [ Links ]
Küppers, G. C., E. C. Lopretto and M. C. Claps. 2006a. Morphological aspects and seasonal changes of some planktonic ciliates (Protozoa) from a temporary pond in Buenos Aires Province, Argentina. Pan–American Journal of Aquatic Sciences 1: 74–90. [ Links ]
Küppers, G. C., E. C. Lopretto and M. C. Claps. 2006b. Pelagostrobilidium wilberti n. sp. (Oligotrichea, Choreotrichida): morphology and morphogenesis. Journal of Eukaryotic Microbiology 53: 477–484. [ Links ]
Küppers, G. C., M. C. Claps and E. C. Lopretto. 2007a. Description of Notohymena pampasica n. sp. (Ciliophora, Stichotrichia). Acta Protozoologica 46: 221–227. [ Links ]
Küppers, G. C., E. C. Lopretto and M. C. Claps. 2007b. Description of Deviata rositae n. sp., a new ciliate specie (Ciliophora, Stichotrichia) from Argentina. Journal of Eukaryotic Microbiology 54: 443–447. [ Links ]
Madrazo–Garibay, M. and E. López–Ochoterena. 1973. Protozoarios ciliados de México. XIX. Estudio biológico de algunas especies recolectadas en Salto San Antón, Estado de Morelos. Revista de la Sociedad Mexicana de Historia Natural 34: 63–69. [ Links ]
Modenutti, B. E. and M. C. Claps. 1986. Ciliados dulceacuícolas de la Argentina, III: Ciliophora–Peritricha. Revista de la Asociación de Ciencias Naturales del Litoral 17: 71–78. [ Links ]
Modenutti, B. E. and G. L. Pérez. 2001. Planktonic ciliates from an oligotrophic south Andean lake, Morenito lake (Patagonia, Argentina). Brazilian Journal of Biology 61: 389–395. [ Links ]
Müller, H., W. Foissner and T. Weisse. 2006. Role of soil in the life cycle of Me seres corlissi (Ciliophora: Oligotrichea): experiments with two clonal strains from the type locality, an astatic meadow pond. Aquatic Microbial Ecology 42: 109–208. [ Links ]
Paiva, T. da S. and I. D. da Silva–Neto. 2004a. Comparative morphometric study of three species of Apoamphisiella Foissner, 1997 (Ciliophora: Hypotrichea) from Brazilian locations, including a description of Apoamphisiella foissneri sp. n. Zootaxa 505: 1–26. [ Links ]
Paiva, T. da S. and I. D. da Silva–Neto. 2004b. Description of Parentocirrus brasiliensis sp. n. (Ciliophora: Spirotrichea), a new ciliate protist present in activated sludge. Zootaxa 504: 1–10. [ Links ]
Paiva, T. da S. and I. D. da Silva–Neto. 2004c. Ciliate protists from Cabiúnas Lagoon (Restinga de Jurubatiba, Macaé – Rio de Janeiro) with emphasis on water quality indicator species and description of Oxytricha marcili sp. n. Brazilian Journal of Biology 64: 465–478. [ Links ]
Paiva, T. da S. and I. D. da Silva–Neto. 2005. Deviata estevesi sp. n. (Ciliophora: Spirotrichea), a new ciliate protist from a restinga lagoon in Rio de Janeiro, Brazil. Acta Protozoologica 44: 351–362. [ Links ]
Paiva, T. da S. and I. D. da Silva–Neto. 2006. Pseudourostyla pelotensis sp. nov. (Ciliophora, Stichotrichia, Urostylida): a new psammophilic ciliate from the southern Brazil. Zootaxa 1247: 43–58. [ Links ]
Paiva, T. da S. and I. D. da Silva–Neto. 2007. Morphology and morphogenesis of Strongylidium pseudocrassum Wang and Nie, 1935, with redefinition of Strongylidium Sterki, 1878 (Protista: Ciliophora: Stichotrichia). Zootaxa 1559: 31–57. [ Links ]
Pettigrosso, R. E. and N. J. Cazzaniga. 1987. Registro de tres especies de Aspidisca (Ciliophora: Hypotrichida) en la Argentina. Anales del Museo de Historia Natural de Valparaíso 18: 5–12. [ Links ]
Petz, W. and W. Foissner. 1992. Morphology and morphogenensis of Strobilidium caudatum (Fromentel), Meseres corlissi n. sp., Halteria grandinella (Müller), and Strombidium rehwaldi n. sp., and a proposed phylogenetic system for oligotrich ciliates (Protozoa, Ciliophora). Journal of Protozoology 39: 159–176. [ Links ]
Rico–Ferrat, G., G. Vilchis–Canales and E. López–Ochoterena. 1987. Los protozoarios del suelo en la Reserva de la Biosfera de Mapimí, Durango. Revista de la Sociedad Mexicana de Historia Natural 39: 21–26. [ Links ]
Suzuki, S. 1954. Taxonomic studies on Blepharisma undulans japonicum Suzuki, with special reference to the macronuclear variation. Journal of Science of the Hiroshima University, Serie B–Division. I, Zoology 15: 205–220. [ Links ]
Weisse, T. 2004. Meseres corlissi: a rare oligotrich ciliate adapted to warm water and temporary habitats. Aquatic Microbial Ecology 37: 75–83. [ Links ]
Wilbert, N. 1975. Eine verbesserte Technik der Protargolimprägnation für Ciliaten. Mikrokosmos 64: 171–179. [ Links ]
Wilfert, M. 1972. Zytologische Untersuchungen an dem Ciliaten Blepharisma americanum Suzuki, 1954, Stamm Berlin (Heterotrichida, Spirostomatidae) sowie Bemerkungen zur Taxonomie und Systematik der Gattung Blepharisma Perti 1849. Archiv für Protistenkunde 114: 152–230. [ Links ]
Williams, D. D. 1987. The ecology of temporary waters. Croom Helm, London. 205 p. [ Links ]
Zaleski, M. and M. C. Claps. 1999. First records of epiphytic limnetic ciliates from Argentina. Natura Neotropicalis 30: 77–84. [ Links ]
Zaleski, M. and M. C. Claps. 2001. First record of some peritrichs ciliates for San Miguel del Monte pond (Buenos Aires, Argentina). Gayana 65: 39–49. [ Links ]














