Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de biodiversidad
versión On-line ISSN 2007-8706versión impresa ISSN 1870-3453
Rev. Mex. Biodiv. vol.80 no.2 México ago. 2009
Taxonomía y sistemática
The parasitic wasp genus Hecabolus (Hymenoptera: Braconidae: Doryctinae), with the description of a new species from Mexico
El género de avispas parasíticas Hecabolus (Hymenoptera: Braconidae: Doryctinae), con la descripción de una especie nueva de México
Alejandro Zaldívar–Riverón1,* and Sergey A. Belokobylskij2,3
1 Museo Nacional de Ciencias Naturales (CSIC), c/ José Gutiérrez Abascal 2, 28006, Madrid, Spain. Domicilio actual: Departamento de Zoología, Instituto de Biología, Universidad Nacional Autónoma de México, Apartado postal 70153, 04510 México D. F., México.
2 Zoological Institute, Russian Academy of Sciences, Universitetskaya nab. 1, St. Petersburg 199034.
3 Museum and Institute of Zoology, Polish Academy of Sciences, Wilcza 64, Warsaw 00–679, Poland.
*Correspondent:
azaldivar@ibiologia.unam.mx
Recibido: 26 agosto 2008
Aceptado: 13 octubre 2008
Abstract
The doryctine wasp genus Hecabolus Curtis and its type species, H. sulcatus Curtis, are redescribed. A new species from Mexico, H. mexicanus sp. nov., is described and illustrated. The new species is distinguished from H. sulcatus and the other known species of the genus, H. costaricensis Marsh, in having a narrower pterostigma, longer discoidal (discal) cell, basal (1M) and recurrent (m–cu) veins diverging posteriorly, first abscissa of mediocubital (M+CU) vein of hind wing as long as the second abscissa (1M), and first metasomal tergite with a very small dorsope. A key for identification of the 3 known species of Hecabolus is provided.
Key words: Hymenoptera, Braconidae, Doryctinae, Hecabolus, Hecabolini, new species.
Resumen
Se redescriben el género de avispas doryctinas Hecabolus Curtis y su especie tipo, H. sulcatus Curtis. Se describe e ilustra una especie nueva procedente de México, H. mexicanus sp. nov. La nueva especie se distingue de H. sulcatus y de la otra especie conocida del género, H. costaricensis Marsh, por presentar un pterostigma más angosto, una celda discoidal más larga, las venas basal y recurrente divergiendo posteriormente, la primer abscisa de la vena mediocubital del ala posterior tan larga como la segunda abscisa, y el primer tergo metasomal con un dorsopo muy pequeño. Se presenta una clave para identificar las 3 especies conocidas de Hecabolus.
Palabras clave: Hymenoptera, Braconidae, Doryctinae, Hecabolus, Hecabolini, especie nueva.
Introduction
The subfamily Doryctinae is a large, cosmopolitan group of braconid wasps, mainly composed of idiobiont ectoparasitoids of xylophagous or bark–boring coleopteran larvae, though some species also attack larvae of other insect orders and in a few cases are known to be gall formers themselves (Belokobylskij, 1992; Wharton and Hanson, 2005). This subfamily contains to date about 180 recognized genera, some of which are highly speciose and widely distributed (e.g. Heterospilus Haliday, Notiospathius Marsh, Ecphylus Foerster). Several other doryctine genera, however, are only known by a small number of species, as in the case of Hecabolus Curtis, which is currently represented by 2 described species, H. sulcatus Curtis (type species) and H. costaricensis Marsh.
Hecabolus sulcatus is widely distributed along the western part of the Palaearctic region, but it has also been reported in the southern USA, including localities in Florida, Colorado, Arizona and California (Shenefelt and Marsh, 1976). The latter records, however, were not mentioned in the World Ichneumonoidea Catalogue (Yu et al., 2005). Hecabolus costaricensis appears to have a much more restricted distribution. It is only known from Costa Rica and can be distinguished from H. sulcatus by having the third tergite basally acinose and the ovipositor distinctly short (Marsh, 2002). Some other species had also been originally described within Hecabolus, though most of them were subsequently transferred to other braconid genera or even subfamilies. For instance, H. cinctus Walker, described from Japan, actually represents a species of the braconine genus Bracon (Tobias and Belokobylskij, 2000). Hecabolus hungaricus Szepligeti, on the other hand, belongs to the doryctine genus Leluthia Cameron (Doryctosoma Picard) (Belokobylskij and Tobias, 1986), and therefore is not a synonym of Polystenus rugosus Foerster, as suggested by Papp (1984) and Yu et al. (2005). Moreover, an examination of the type specimen of H. quadricolor Cameron revealed that this species belongs to the genus Syngaster Brulle (van Achterberg, 1980; Belokobylskij et al., 2004). Finally,H. tetrastigmus Kieffer and Jorgensen was described from Argentina (Kieffer and Jorgensen, 1910), though its type specimens are probably lost and its generic assignation is questionable.
A recent examination of the braconid material deposited at the Entomological Collection of the Museo Nacional de Ciencias Naturales (CSIC) in Madrid, Spain, revealed the existence of a clearly distinctive, undescribed species of Hecabolus. Unfortunately, only 1 specimen of this species was deposited in the above collection, which was collected at the beginning of the twentieth century and has ambiguous locality data (labeled only as "Mexico, Méndico"). Nevertheless, the considerable morphological distinctiveness of this species in comparison to the 2 currently described species of the genus has prompted us to describe it, hoping that future collections will allow us to document its morphological variation and precise geographic distribution. We also redescribe the genus Hecabolus and its type species, H. sulcatus, and give a key for determination of its 3 known species.
Materials and methods
Photographs were taken using a Philips® XL–20 scanning electron microscope. Measurements are expressed in millimeters. The wing venation nomenclature follows Belokobylskij and Tobias (1998) and Sharkey and Wharton (1997) (in parentheses). The following abbreviations are employed: POL – postocellar line; OOL – ocular–ocellar line; Od – maximum diameter of lateral ocellus.
Specimens are held in the following collections: Museo Nacional de Ciencias Naturales, CSIC, Madrid, Spain (MNCN); Institut Royal des Sciences Naturelles, Brussels, Belgium (IRSN); Hungarian Natural History Museum, Budapest, Hungary (HNMB); Muséum National d' Histoire Naturelle, Paris, France (MNHN); Naturhistorisches Museum, Vienna, Austria (NHMW); Zoologische Staatssumlung, Munich, Germany (ZSM); Museum der Naturkunde für Humboldt Universität zu Berlin, Germany (MNHB): Institute of Zoology NANU, Kiev, Ukraine (IZNANU); Insect Museum, Department of Renewable Resources, University of Wyoming, Laramie, WY, USA (ESUW); and Zoological Institute, Rusian Academy of Sciences, St. Petersburg, Russia (ZISP).
Descriptions
Hecabolus Curtis, 1834
Hecabolus Curtis, 1834: 507 (type species: H. sulcatus Curtis, 1834).
Anisopelma Wesmael, 1838: 134 (type species: A. belgicum Wesmael, 1838).
Diagnosis. The main diagnostic characters that have traditionally defined the genus Hecabolus include an opened brachial (first subdiscal) cell, absence of second radiomedial (r–m) vein, antefurcal position of recurrent (m–cu) vein, and a distinctly wide hind femur (Tobias, 1971, 1976; Marsh, 2002). A hind coxa distinctly protruding forward and without a ventro–anterior tooth, as well as a deep and wide mesosternal suture, have also been proposed as diagnostic features that help to distinguish Hecabolus from other doryctine genera (Belokobylskij and Tobias, 1986).
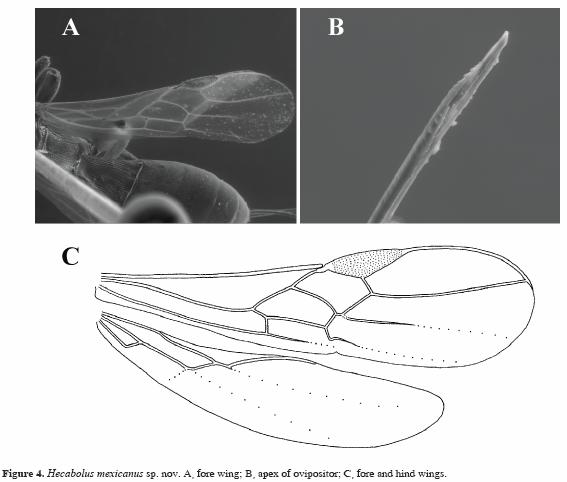
Head subcubical to weakly transverse (Figs. 1B, 2C). Temple long (Fig. 2C). Occipital carina wide (Fig. 1C), ventrally joining hypostomal carina. Postgenal bridge rather wide. Vertex often entirely transversely striate, but sometimes almost smooth. Maxillary and labial palpi 6 and 4–segmented, respectively. Antennae thick (Fig. 2E) and setiform. First flagellar segment never shorter than second segment. Mesoscutum high, almost round and elevated above pronotum. Notauli complete, shallow to very shallow posteriorly (Fig. 2F). Sternauli distinct (Figs. 1C, 3A). Mesosternal suture deep, wide and coarsely crenulate. Metanotal tooth absent. Propodeum without delineated areas. Fore wing (Figs. 1E, 4A, C): radial (marginal) cell long. Second radiomedial (r–m) vein absent. Recurrent (m–cu) vein usually antefurcal, but sometimes interstitial. Brachial (first subdiscal) cell open apico–posteriorly. Hind wing (Fig. 4C): first abscissa of mediocubital (M+CU) vein never shorter than second abscissa (1M). Submedial (subbasal) cell rather short, widened apically. Legs robust. Fore tibia on inner side with short and numerous spines arranged in a narrow stripe or a single line (Fig. 3F). Hind coxa without baso–ventral tubercle, strongly protruding forward in ventro–anterior corner (Figs. 1D, F, 3E). Hind femur distinctly broad. First tergite with distinct or faint (Fig. 3C) dorsope and with short or long (Fig. 3D) acrosternite. Second to fourth tergites with separated laterotergites. Second suture distinct and narrow (Figs. 1E, F, 3B). Second and third tergites densely striate or aciculate entirely and at least in basal half, respectively (Fig. 1E, F, 3B). Ovipositor often as long as body (Fig. 2A), with a double nodus on the upper valve (Fig. 4B).
Distribution. This genus has been recorded for the western Palaearctic, southern Nearctic and northern Neotropical regions.
Comments. A recent molecular phylogenetic analysis of a large number of doryctine genera recovered Hecabolus (represented by H. sulcatus) within a clade mainly composed of Central and South American taxa (Zaldívar–Riverón et al., 2008). This suggests that Hecabolus originated in the latter region and subsequently dispersed to the Palaearctic. Moreover, in the same study the remaining examined members of the tribe Hecabolini, to which Hecabolus belongs, were also nested within the 'South American' clade; however, they did not constitute a monophyletic group but instead appeared to be more closely related to members of other putative tribes. Further morphological and molecular studies will help clarify the relationships between the members of this group.

Hecabolus sulcatus Curtis, 1834
Hecabolus sulcatus Curtis, 1834: 507.
Anisopelma belgicum Wesmael, 1838: 134.
Female. Body length 3.0–5.3 mm; fore wing length 2.2–2.8mm (Fig. 1A).
Head (Fig. 1B). Head width 1.2–1.3 times median length (dorsal view), 1.0–1.1 times width of mesoscutum. Head behind eyes (dorsal view) weakly convex in anterior half, and weakly, roundly narrowed in posterior half. Transverse diameter of eye 0.8–0.85 times as long as temple. Ocelli rather small, arranged in almost equilateral triangle; POL 1.0–1.2 times Od, 0.25–0.3 times OOL. Eye with sparse and rather long setae, with very shallow or indistinct emargination opposite antennal sockets, 1.25–1.3 times higher than broad. Malar space height 0.5–0.6 times height of eye, 0.8–1.0 times basal width of mandible. Face convex, its width 1.3–1.4 times height of eye and 1.2–1.4 times height of face and clypeus combined. Malar suture absent. Clypeus high, with rather distinct lower flange. Hypoclypeal depression round, its width 0.55–0.7 times distance from edge of depression to eye, 0.35–0.4 times width of face. Occipital carina wide, dorsally complete, ventrally joined with hypostomal carina. Hypostomal flange wide. Head below eyes roundly narrowed. Antennae thickened, weakly setiform, 24–26–segmented, 0.7–0.8 times as long as body. Scapus long and narrow, 1.7–1.8 times longer than its maximum width. First flagellar segment practically straight, widened anteriorly, 2.0–2.2 times longer than its apical width, 1.05–1.1 times longer than second segment. Penultimate segment 1.7–2.1 times longer than wide, 0.5–0.6 times as long as first flagellar segment, 0.8–1.0 times as long as apical segment; the last pointed apically.
Mesosoma (Figs. 1A, C). Length 2.2–2.3 times its height. Pronotum short, dorsally weakly convex, with distinct submedian pronotal carina; side of pronotum with narrow, shallow, weakly curved submedian depression. Mesoscutum (lateral view) highly and roundly elevated above pronotum; its length (dorsal view) 0.9 times maximum width. Median lobe of mesoscutum shortly protruding forward, without anterolateral shoulders. Notauli rather wide, deep anteriorly and shallow posteriorly, densely crenulate. Prescutellar depression rather shallow, more or less short, with 3–5 carinae, finely or distinctly rugulose between carinae, 0.4–0.5 times as long as scutellum. Scutellum weakly convex and with fine lateral carinae. Sternaulus distinct, rather shallow, weakly S–shaped, distinctly rugose–crenulate, running along 0.7–0.8 of lower length of mesopleuron. Metanotal tooth almost indistinct. Metapleural lobe rather long and narrow, rounded apically. Propodeum with very small and wide lateral tubercles.
Wings (Fig. 1E). Fore wing 3.2–3.3 times longer than its maximum width. Pterostigma 2.9–3.1 times longer than wide. Radial vein (r) arising before middle of pterostigma. Radial (marginal) cell long, its length 3.3–3.6 times maximum width. Metacarpus (R1) 1.3–1.4 times longer than pterostigma. First radial abscissa (r) about 0.6 times as long as maximum width of pterostigma. Second radial abscissa (3RSa) slightly curved anteriorly and straight in posterior half, 8.5–10.0 times longer than first radial abscissa (r), 3.6–4.0 times longer than first radiomedial vein (2RS). First radiomedial vein (2RS) 2.4–2.6 times longer than first radial abscissa (r) and 2.0–2.1 times longer than recurrent vein (m–cu). Recurrent vein (m–cu) antefurcal or sometimes interstitial. First medial abscissa ((RS+M)a) weakly curved. Discoidal (discal) cell 1.9–2.1 times longer than wide. Basal (1M) and recurrent (m–cu) veins slightly convergent posteriorly. Posterior abscissa of basal vein (1M) 2.1–2.5 times longer than anterior abscissa (1RS), 2.4– 2.5 times longer than recurrent vein (m–cu). Nervulus (cu–a) curved, distance from nervulus (cu–a) to basal vein (1M) 0.6–0.7 times nervulus length. Mediocubital vein (M+CU) distinctly S–shaped. Brachial (2cu–a) vein entirely absent. Hind wing 4.0–4.5 times longer than wide. First abscissa of costal vein (C+Sc+R) almost as long as second abscissa (Sc+R). Medial (basal) cell rather narrow, weakly widened in apical half, its length 7.5–9.0 times maximum width, 0.3–0.33 times length of wing. First abscissa of mediocubital vein (M+CU) 1.3–1.4 times longer than second abscissa (1M). Recurrent vein (m–cu) unsclerotized, almost straight, weakly oblique toward base of wing.
Legs (Figs. 1D, F). Fore tibia with numerous slender spines arranged in narrow row. Hind coxa 1.6–1.7 times longer than maximum width. Hind femur wide, 2.7–2.9 times longer than wide. Hind tibia rather wide. Hind tarsus 0.9 times as long as hind tibia. Basitarsus about 0.5 times as long as second–fifth segments combined. Second segment f hind tarsus 0.5–0.6 times as long as basitarsus, 1.0–1.2 times as long as fifth segment (without pretarsus).
Metasoma (Figs. 1E, F). 1.2–1.5 times longer than head and mesosoma combined. First tergite with short acrosternite, which is 0.25 times as long as first tergite; with small but distinct dorsope, with small spiracular tubercles in basal 0.3; tergite distinctly and linearly widened from base to apex. Maximum width of first tergite 2.5 times its minimum width; length 1.3 times its apical width, 1.5 times length of propodeum. Second tergite with wide and shallow lateral subparallel depression delineated by distinct carinae on their inner sides. Median length of second tergite 0.9–1.0 times its basal width, 1.2–1.25 times length of third tergite. Combined length of second and third tergites 1.1–1.2 times their maximum width. Second suture shallow, slightly evenly curved. Ovipositor sheaths slender, 1.6–2.3 times longer than metasoma, 2.8–3.7 times longer than mesosoma, 0.8–1.2 times as long as body, 1.6–2.0 times longer than fore wing.
Sculpture and pubescence (Figs. 1C, D). Vertex entirely and rather finely striate, sometimes smooth medially; frons distinctly, entirely, densely and weakly–curvedly and transversely striate, with fine rugulose microsculpture. Face entirely, distinctly, densely, and weakly–curvedly and transversely striate, with very fine rugulose microsculpture; temple almost smooth in anterior half and distinctly densely and curvedly or finely and almost vertically striate in posterior 0.2–0.5. Sides of pronotum distinctly longitudinally striate in upper half and rugose–reticulate with striae in lower half. Mesoscutum densely and finely reticulate–coriaceous (in large specimens) or almost smooth (in small specimens), lateral lobes sometimes with fine transverse curved aciculation, rather coarsely and widely rugose with lateral striation in medioposterior 0.6. Scutellum almost entirely smooth. Mesopleuron almost entirely longitudinally curvedly striate, but in small specimens glabrous in lower 0.5 upper sternaulus. Metapleuron entirely and coarsely reticulate–areolate. Propodeum entirely densely and mostly longitudinally striate with dense rugulose microsculpture, without delineated areas. Hind coxae almost entirely or mostly, densely, semi–circularly or almost linearly striate. Hind femur almost entirely longitudinally striate. First and second tergites entirely, densely and longitudinally striate with dense rugulose microsculpture; third tergite in basal 0.4 very densely longitudinally striate and with extremely fine rugulose microsculpture. Remaining tergites smooth. Vertex with sparse short and semi–erect setae, glabrous medio–anteriorly. Mesoscutum almost entirely or widely with rather dense, short and semi–erect pale setae, with narrow median glabrous areas on lateral lobes. Mesopleuron medially widely glabrous. Hind tibia dorsally with very short, rather dense semi–erect setae and ventrally with very short and very dense (especially apically) setae.
Color. Body black or dark reddish brown, median or apical half of metasoma dark reddish brown. Antenna reddish brown, apically evenly infuscate. Palpi yellowish brown. Legs reddish brown or light reddish brown, hind coxa dark, mostly reddish brown to dark reddish brown or black, all tibiae subbasally paler. Fore wing faintly infuscate. Pterostigma dark brown, pale at very short part basally and apically.
Male. Body length 2.3–4.1 mm, fore wing length 1.5–2.4 mm. Temple in small specimens long, transverse diameter of eye 0.7 times length of temple. Antenna 22–segmented. First flagellar segment as long as the second one. Recurrent vein usually distinctly antefurcal. Hind wing with brown stigma–like enlargement, its length 1.5–1.8 times distance between enlargement and base of wing. First tergite slender, its maximum width 2.1–2.3 times minimum width; length 1.5–1.7 times its apical width. Median length of second tergite 1.1–1.2 times its basal width, 1.2–1.4 times length of third tergite. Otherwise similar to female.
Distribution. Hecabolus sulcatus is distributed along the western part of the Palaearctic region, with records including Austria (new record), Azerbaijan, Belgium, Belarus (new record), Bulgaria (new record), Croatia, Czech Republic, Finland, France, Georgia, Germany, Hungary, Italy, Israel, Latvia, Moldova, Poland, Serbia (new record), Slovakia, Sweden, Switzerland, UK, and Ukraine (Yu et al., 2005). For the European part of Russia, it has been recorded for the following provinces: Daghestan, Krasnodarskiy kray, Leningrad, Yaroslavl', Lipetzk, Perm', and Chelyabinsk. This species has also been recorded in the southern USA (Shenefelt and Marsh, 1976) and is here recorded for the African continent for the first time: 1 female, Morocco, "Tanger, M. Escalera" (MNCN).
Biology. This species has been reported to be an idiobiont ectoparasitoid of coleopteran larvae of the families Anobiidae, Buprestidae, Cerambycidae, Lyctidae, Curculionidae, Ptinidae, and possibly Chrysomelidae (Belokobylskij and Tobias, 1986; Yu et al., 2005). Material examined. MNCN.—Morocco: 1 female, "Tanger". IRSN.—Belgium: 1 female, "environs de Liege" (holotype of A. belgicum). HNMB.—Bulgaria: 1 female, 1 male, "Madara"; 3 females. 1 male, "Kuleftse"; Croatia: 1 male, "Vaganski Vrh"; Italy: 1 male, "Piemonte"; Serbia: 1 female, 3 males, "Maa Dragusa". MNHN.—France: 1 female, "Corwtan–tiaeph"(?); 1 female, "Prat., 15 mai, Saule."; 1 male, "Arest." (?); 1 female, "brauche, I. hitri" (?), "maium Eaff. cilm (?)"; 1 female, 1 male, "Basses–Pyrenees, env. d'Arcangues, Oyambouroubehere"; 1 female, "parasite de Ptilinus pectinicornis", "Chevannes, Yonne, R. Comon"; 1 male, "Fouesnant (Finistere)"; 8 females, 8 males, "Versailles"". U. K.: 1 female, "Angleterre", "Marshall"; Austria: 1 female, "Vienna". NHMW.—Austria: 1 female, "Austr. infer., Dürrnstein, Wachtl", "ex Hadera heleii (?)"; 9 females, 4 males, "Lambach, O Ö, Butz, e. Ptilinus pectinicornis in acere"; 4 females, 2 males, "Wien Img. A.i., C. Holzschuh", "Prater, ex Vi scum album L. and ex Gastrallus laevigatus"; 1 female, "Weisskirchen, Mahren"; France: 1 male, "Avignon (Vse), Sl. Lierre, "parasite de Anobium domesticum L.". ZSM.— Germany: 1 female, "Buche", "Marburg, Gründlev"; 1 male, "Weidaruck (?), ex Malus". MNHB.—Germany: 2 females, 2 males, "Coll. Reinhard", 2 female, 1 male, "Berlin. Klug"; 2 females, 2 males, "Rhein–provinz"; 2 females, 2 males, "Sendelbach b. Lohr, Romberg, Bischoff S.G."; 2 females, "München", "Aus Ptilinus pectinicornis"; 1 female, "Mähren, Staudinger V."; Latvia: 1 female, "Kurland, Werkukkul". IZNANU.—Georgia: 1 female, Vashlovanskiy Nature Reserve; Azerbaijan: 1 male, Lerikskiy Region, Gosmalyan; Ukraine: 1 female, Donetzk Province, "Khomutovskaya step'" Nature Reserve. ZISP.—Czech Republic: 1 male, Praha; Hungary: 2 females, Marmaros and Szovata; Moldova: 1 male, Kishinev; Ukraine: 3 females, Khar'kov Province, Kupyansk; Ukraine: 1 female, Krym; Belorussia: 4 females, 2 males, Khoiniki; Azerbaijan: 1 female, Kuba; Abkhazia:, 1 female, Pitzunda; Russia: 4 females, 1 male, Yaroslavl' Province, Berditzyno; 1 female, Lipetzk Province, "Salich'ya gora" Nature Reserve; 1 female, 1 male, Krasnodarskiy kray, Sochi; 3 females, Dagestan, Akhty.; 1 female, Leningrad Province, Luga; 2 females, Perm' Province, Kungur; 1 female, Chelyabinsk Province, Miass.
Hecabolus mexicanus sp. nov.
Female. Body length 5.5 mm; fore wing length 3.9 mm (Fig. 2A).
Head (Figs 2B–E). Head width 1.4 times median length (dorsal view), 0.9 times width of mesoscutum. Head behind eyes (dorsal view) weakly convex in anterior half, weakly roundly narrowed in posterior half. Transverse diameter of eye 0.7 times as long as temple. Ocelli rather small, arranged in triangle, with base 1.2 times its sides. POL 1.5 times Od, 0.35 times OOL. Eye with sparse and very short setae, with very shallow emargination opposite antennal sockets, 1.3 times higher than broad. Height of malar space 0.7 times height of eye, 1.1 times basal width of mandible. Face convex, its width 1.35 times height of eye and 1.35 times height of face and clypeus combined. Malar suture absent. Clypeus high, with rather distinct lower flange. Hypoclypeal depression round, its width 0.9 times distance from edge of depression to eye, 0.5 times width of face. Occipital carina wide, dorsally complete, ventrally joined with hypostomal carina. Hypostomal flange wide. Head below eyes roundly narrowed. Antennae more or less thickened, more than 16–segmented (apical segments missing). Scapus long and rather narrow, 2.1 times longer than its maximum width. First flagellar segment practically straight, slightly widened anteriorly, 2.2 times longer than apical width, 1.1 times longer than second segment.
Mesosoma (Figs 2F, 3A). Length 2.2 times its height. Pronotum short, dorsally weakly convex, with distinct submedial pronotal carina; side of pronotum with rather wide, deep, weakly S–curved submedian depression. Mesoscutum (lateral view) highly and roundly elevated above pronotum; its length (dorsal view) 0.85 times maximum width. Median lobe of mesoscutum shortly protruding forward, without distinct anterolateral shoulders. Notauli rather narrow, deep anteriorly and shallow posteriorly, densely crenulate. Prescutellar depression rather shallow, long, with median carina, rugulose–crenulate, about 0.5 times as long as scutellum. Scutellum weakly convex. Sternauli distinct, rather shallow, straight, weakly sculptured, running along whole length of lower part of mesopleuron. Metanotal tooth short, wide and obtuse. Metapleural lobe rather large and narrow, rounded apically. Propodeum with small lateral tubercles.
Wings (figs 4A, C). Fore wing 3.6 times longer than its maximum width. Pterostigma 3.3 times longer than wide. Radial vein (r) arising before middle of pterostigma. Radial (marginal) cell long, its length 3.6 times maximum width. Metacarpus (R1) 1.5 times longer than pterostigma. First radial abscissa (r) 0.75 times as long as maximum width of pterostigma. Second radial abscissa (3RSa) slightly curved anteriorly and straight in posterior half, 8.0 times longer than first radial abscissa (r), 4.0 times longer than first radiomedial vein (2RS). First radiomedial vein (2RS) twice longer than first radial abscissa (r) and twice longer than recurrent vein (m–cu). Recurrent vein (m–cu) antefurcal. First medial abscissa [(RS+M)a] distinctly curved. Discoidal (discal) cell 2.7 times longer than wide. Basal (1M) and recurrent (m–cu) veins slightly divergent posteriorly. Posterior abscissa of basal vein (1M) 1.6 times longer than anterior abscissa (1RS), 2.3 times longer than recurrent vein (m–cu). Nervulus (cu–a) curved, distance from nervulus (cu–a) to basal vein (1M) equal to nervulus length. Mediocubital vein (M+CU) distinctly S–shaped. Brachial (subdiscal) cell open distally, brachial (2cu–a) vein almost entirely absent. Hind wing 4.5 times longer than wide. First abscissa of costal vein (C+Sc+R) 1.1 times longer than second abscissa (Sc+R). Medial (basal) cell narrow, weakly widened in apical half, its length 8.0 times maximum width, 0.35 times length of wing. First abscissa of mediocubital vein (M+CU) as long as second abscissa (1M). Recurrent vein (m–cu) unsclerotized, almost straight, weakly oblique toward base of wing.
Legs (Figs 3E, F). Fore tibia with several slender spines arranged more or less in a line. Hind coxa without basoventral tubercle, strongly protruding forward in ventro–anterior corner, 1.7 times longer than maximum width. Hind femur considerably wide, 2.3 times longer than wide. Hind tibia rather wide. Hind tarsus 0.85 times as long as hind tibia. Basitarsus 0.55 times as long as second–fifth segments combined. Second tarsal segment 0.5 times as long as basitarsus, 0.8 times as long as fifth segment (without pretarsus).
Metasoma (Figs 3B, D). Metasoma longer than head and mesosoma combined. First tergite with long acrosternite, 0.35 times as long as first tergite; with very small dorsope, with small spiracular tubercles in basal 0.3; tergite distinctly and linearly widened from base to apex. Maximum width of first tergite 1.6 times its minimum width; length 1.7 times its apical width, 1.6 times length of propodeum. Second tergite without any furrows. Median length of second tergite 0.85 times basal width of second tergite, 1.2 times length of third tergite. Combined length of second and third tergites 1.2 times their maximum width. Second suture shallow, slightly evenly curved. Ovipositor sheaths slender, twice as long as metasoma, 2.8 times longer than mesosoma, about as long as body, 1.4 times as long as fore wing.
Sculpture and pubescence (Figs 2B–D, F, 3A–D). Vertex very finely striate to smooth medially, rather distinctly aciculate laterally; frons distinctly, entirely and densely transversely striate, with fine rugulose microsculpture. Face entirely, distinctly, densely, and weakly–curvedly and transversely striate, with fine rugulose microsculpture; temple smooth. Sides of pronotum almost entirely rugose–reticulate, with distinct numerous crenulae on oblique depression. Mesoscutum densely and finely reticulate–coriaceous, median lobe with finee transverse undulate striation, coarsely and rather widely rugose with lateral striation in medioposterior half. Scutellum entirely coriaceous. Mesopleuron smooth in lower 0.6, coarsely longitudinally striate with rugosity in upper 0.4, finely and densely striate in posterior lower corner. Metapleuron entirely and coarsely reticulate–areolate. Propodeum entirely densely and mostly longitudinally striate with dense rugulose microsculpture, without delineated areas. Hind coxae densely and semi–circularly striate in dorsal 0.3, very finely striate to almost smooth on rest part. Hind femur smooth in basal half and finely punctate–coriaceous in apical half. First tergite entirely, densely and longitudinally striate with dense rugulose microsculpture; second tergite entirely and third in basal 0.2–0.3 very densely longitudinally striate (acinose) and with extremely fine rugulose microsculpture. Remaining tergites smooth. Vertex with very sparse short and semi–erect setae, setae dense along occipital carina. Mesoscutum almost entirely with rather dense, short and semi–erect pale setae. Metapleuron medially widely glabrous. Hind femur sparsely setose in basal half and densely setose in apical half. Hind tibia dorsally with very short and sparse and ventrally with very short and very dense (especially apically) setae.
Color. Body black, apical part of first tergite, almost entire second tergite and basal part of third tergite reddish brown. Antenna brown to dark reddish brown, basally light reddish brown. Palpi yellow. Fore and middle coxae and trochanters brownish yellow, femora brown, tibiae yellow in basal 0.2–0.3 and brown on rest part, tarsi reddish brown. Hind coxa almost black, trochanter yellow, femur dark brown, tibia yellow in basal 0.4 and brown in apical 0.6, tarsus entirely brown. Fore wing very faintly infuscate. Pterostigma dark brown, pale at very short part basally and apically. Male. Unknown.
Distribution. The locality of the only known specimen is labeled as "Mexico, Méndico".
Biology. Unknown.
Comments. The new species differs from the other 2 known species of Hecabolus, H. sulcatus and H. costaricensis, in the very long temple, narrow pterostigma, long discoidal (discal) cell, the basal (1M) and recurrent (m–cu) veins being slightly divergent posteriorly, the first abscissa of mediocubital (M+CU) vein of hind wing being as long as second abscissa (1M), and the first tergite being long and with very small dorsope.
Taxonomic summary
Type material. Holotype female: "Mexico, Méndico, Mai 1911, Dr. M. Draudt" (MNCN).
Other examined material. Hecabolus costaricensis. ESUW.– Costa Rica: 1 female, San José Province, Ciudad Colón, 800 m.
Acknowledgments
We thank Scott R. Shaw for helping with the morphological examination of the holotype of H. costaricensis Marsh. AZR thanks the Ministry of Education and Science (Spain) for contract "Juan de la Cierva". SAB is very thankful to Isabel Izquierdo and Mercedes Paris for their kind hospitality during his work in the Entomological Collection of the MNCN. This study was supported by grants from the European Commission's Research Infrastructure Action via the SYNTHESYS project at the MNCN and the Russian Foundation for Basic Research (No. 07–04–00454) to SAB.
Literature cited
Achterberg, C. van. 1980. The Cameron types of Braconidae in the Netherlands (Hymenoptera, Ichneumonoidea). Bulletin Zoologisch Museum Universiteit van Amsterdam 7:209–214. [ Links ]
Belokobylskij, S. A. and V. I. Tobias. 1986. Subfam. Doryctinae. In Key to insects of the European part of the USSR. Hymenoptera, Vol. 4. G. S. Medvedev (ed.). Nauka, Leningrad. p. 21–72. (In Russian). [ Links ]
Belokobylskij, S. A. 1992. On the classification and phylogeny of the Braconid wasps subfamilies Doryctinae and Exothecinae (Hymenoptera, Braconidae). Part I. On the classification, 1. Entomologicheskoe Obozrenie 71: 900–928. (In Russian). English translation, Entomological Review 1993:72, 109–137. [ Links ]
Belokobylskij, S. A. and V. I. Tobias. 1998. Fam. Braconidae. Introduction. In Key to insects of the Russian Far East. Neuropteroidea, Mecoptera, Hymenoptera, Vol. 4. P. A. Lehr (ed.). Dal'nauka, Vladivostok. p. 8–26. (In Russian). [ Links ]
Belokobylskij, S. A., M. Iqbal and A. D. Austin. 2004. Systematics, distribution and diversity of the Australasian diryctine wasps (Hymenoptera, Braconidae, Doryctinae). Records of the South Australian Museum. Monograph Series 8. 150 p. [ Links ]
Kieffer, J. J. and P. Jörgensen. 1910. Gallen und Gallentier aus Argentinien. Zentrablatt für Bakteriologie, Parasitenkunde und Infektionskrankheiten 27:362–442. [ Links ]
Marsh, P. M. 2002. The Doryctinae of Costa Rica (excluding the genus Heterospilus). Memoirs of the American Entomological Institute 70:1–319. [ Links ]
Papp, J. 1984. Contribution to the braconid fauna of Hungary, V. Doryctinae (Hymenoptera, Braconidae). Folia Entomologica Hungarica 45:173–185. [ Links ]
Sharkey, M. J. and R. A. Wharton. 1997. Morphology and terminology. In Manual of the New World genera of the family Braconidae (Hymenoptera), R. A. Wharton, P. M. Marsh and M. J. Sharkey (eds.). International Society of Hymenopterists, Special Publication 1, Washington, D. C. p. 21–40. [ Links ]
Shenefelt, R. D. and P. M. Marsh 1976. Hymenopterorum Catalogus. Pars 13. Braconidae 9. Doryctinae. 's–Gravenhage: Dr W. Junk. p. 1263–1424. [ Links ]
Tobias, V. I. 1971. Review of the Braconidae (Hymenoptera) of the USSR. Proceeding of the All–Union Entomological Society 54:156–268. (In Russian). [ Links ]
Tobias, V. I. 1976. Braconidae of Caucasus (Hymenoptera). Leningrad, Nauka. 286 p. (In Russian). [ Links ]
Tobias, V. I. and S. A. Belokobylskij. 2000. Subfam. Braconinae. In Key to insects of the Russian Far East. Neuropteroidea, Mecoptera, Hymenoptera, Vol. 4. P. A. Lehr (ed.). Dal'nauka, Vladivostok. p. 109–192. (In Russian). [ Links ]
Wharton, R. A. and P. E. Hanson. 2005. Gall wasps in the family Braconidae (Hymenoptera). In Biology, Ecology, and Evolution of Gall–inducing Arthropods, A. Raman, W. C. Schaefer, and T. M. Withers (eds.). Science Publishers, Enfield, New Hampshire. p. 321–383. [ Links ]
Yu, D. S., C. van Achterberg and K. Horstman. 2005. World Ichneumonoidea 2004. Taxonomy, biology, morphology and distribution. CD/DVD. Taxapad, Vancouver, Canada. taxapad.com [ Links ]
Zaldívar–Riverón, A., S. A. Belokobylskij, V. León–Regagnon, R. Briceño–G. and D. L. J. Quicke. 2008. Molecular phylogeny and historical biogeography of the cosmopolitan parasitic wasp subfamily Doryctinae (Hymenoptera: Braconidae). Invertebrate Systematics 22:345–363. [ Links ]














