Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de biodiversidad
versión On-line ISSN 2007-8706versión impresa ISSN 1870-3453
Rev. Mex. Biodiv. vol.79 no.2 México dic. 2008
Ecología
Genetic homogeneity between two populations of the parthenogenetic lizard Aspidoscelis cozumela
Homogeneidad genética entre dos poblaciones de la lagartija partenogenética Aspidoscelis cozumela
Norma L. Manríquez–Morán1* and Fausto R. Méndez–de la Cruz2
1 Laboratorio de Sistemática Molecular, Centro de Investigaciones Biológicas. Universidad Autónoma del Estado de Hidalgo. Km. 4.5 Carr. Pachuca–Tulancingo s/n. Col. Carboneras, 42182, Mineral de la Reforma, Hidalgo, México.
2 Laboratorio de Herpetología, Instituto de Biología, Universidad Nacional Autónoma de México. Tercer Circuito Exterior s/n, Ciudad Universitaria, 04510, México, D. F., México.
*Correspondent:
mnorma@uaeh.edu.mx
Recibido: 19 febrero 2007
Aceptado: 23 enero 2008
Abstract
We used skin–grafting to assess the genetic variability between 2 populations of the parthenogenetic lizard Aspidoscelis cozumela. We transplanted 238 skin fragments among individuals of 2 coastal populations at Cozumel Island. Grafts belonging to individuals that survive more than 60 days suggested genetic homogeneity between both populations, consistent with the existence of only 1 clone within this unisexual species.
Key words: histocompatibility, parthenogenesis, skin–grafting, Cozumel.
Resumen
Se utilizó el método de transplantes de piel para evaluar la variabilidad genética entre 2 poblaciones de la lagartija partenogenética Aspidoscelis cozumela. Se transplantaron 238 fragmentos de piel entre individuos de 2 poblaciones costeras de Isla Cozumel. Los transplantes pertenecientes a individuos que vivieron más de 60 días, mostraron homogeneidad genética entre ambas poblaciones, lo cual sugiere la existencia de 1 sólo clon dentro de esta especie unisexual.
Palabras clave: histocompatibilidad, partenogénesis, transplantes de piel, Cozumel.
Introduction
Immunological studies have demonstrated that grafts transplanted between members of the same gonochoristic species are rejected in response to genetic differences among histocompatibility genes (Cuellar and Smart, 1977; Zapata and Cooper, 1990). The transplanted graft acts as a trigger that promotes an immune response that will eventually destroy the foreign tissue (Zapata and Cooper, 1990). In contrast, various studies involving unisexual vertebrates have demonstrated absence of immunological response and hence genetic homogeneity among individuals of the same clone (Hernández–Gallegos et al., 1998; Abuhteba et al., 2000; Hernández–Gallegos et al., 2003). This homogeneity has been attributed to clonal reproduction (Cuellar and Smart, 1977). In this case, the transplanted graft establishes cell connections with the host tissue and is permanently retained.
Skin–grafting is a technique sensitive enough to detect slight differences in the Major Histocompatibility Complex genes (Vrijenhoek et al., 1977), which constitutes one of the most variable family of genes in all jawed vertebrates (Miller et al., 2006). For this reason, the method has been extensively used to establish genetic structure in clones of parthenogenetic lizards and to make inferences on their origin and evolution (Maslin, 1967; Cuellar, 1976, 1977; Cuellar and Wright, 1992; Hernández–Gallegos et al., 1998; Abuhteba et al., 2000, 2001; Hernández–Gallegos et al., 2003).
Previous skin–grafting studies have demonstrated that unisexual lizards reject grafts from individuals originated from different hybridization events (different clones). In contrast, individuals of parthenogenetic species normally accept grafts from individuals belonging to the same clone. Postformational mutations could provoke small rates of rejection among individuals that constitute an ancient clone (Maslin, 1967; Cuellar, 1976, 1977, 1984; Cuellar and Wright, 1992; Hernández–Gallegos et al., 1998; Abuhteba et al., 2000, 2001, Hernández–Gallegos et al., 2003).
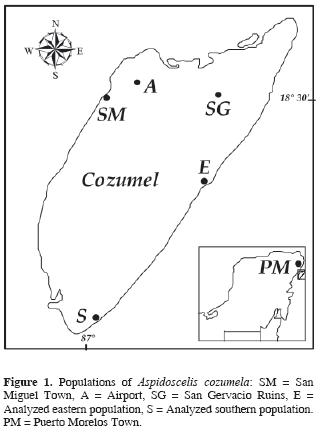
The Aspidoscelis cozumela complex is composed of 3 unisexual lizard species endemic to the Yucatán Peninsula. Earlier studies have shown that these species originated as a result of 2 independent hybridization events between females of A. angusticeps and males of A. deppii (Moritz et al., 1992; Hernández–Gallegos et al., 1998; Manríquez–Morán, 2002; Hernández–Gallegos et al., 2003): one of them originating A. rodecki and the other one A. maslini and A. cozumela, taxa that were recognized as distinct species based on morphological differences (Taylor and Cooley, 1995). Karyotypic and mtDNA sequencing data suggested that Aspidoscelis cozumela evolved from the unisexual A. maslini (Manríquez–Morán, 2002). The comparison of partial sequences of 2 mitochondrial genes showed that individuals belonging to A. cozumela exhibit the same haplotype as individuals of A. maslini from Puerto Morelos (Fig. 1), Quintana Roo (Manríquez–Morán, 2002), and possess a karyotype that evolved as a consequence of multiple centric fissions in the hybrid karyotype of A. maslini (Manríquez–Morán et al., 2000).
Although different studies have suggested the existence of only 1 major clone within Aspidoscelis cozumela (Hernández–Gallegos et al., 1998; Manríquez–Morán et al., 2000; Manríquez–Morán, 2002; Hernández–Gallegos et al., 2003), differences in size and color have been observed among individuals from different areas of Cozumel Island. We used skin–grafting to assess genetic variability between 2 populations of A. cozumela at the eastern side of Cozumel, as different color patterns may be the result of genetic change in clonal species (Taylor et al., 2003).
Material and methods
Individuals of Aspidoscelis cozumela were collected at Cozumel Island (20° 36' N and 86° 44' W; Fig. 1) during spring and summer of 1995 and 1996 from 2 populations, one of them located 20 km southeast of the town of San Miguel (N = 31; Fig. 1, E) and the other one at 34 km south of San Miguel (N = 14: Fig. 1, S). Although the 2 sites are located in the coastal zone of the island, they exhibit differences in vegetation and moisture. The southernmost area consists predominately of rocky soil covered with an association of halophytic vegetation and cacti. The eastern site exhibits halophytic vegetation characteristic of the coastal dunes, which is composed of erect and prostrate plants with shrubby and herbaceous life forms (Tellez et al., 1989).
Lizards were permanently marked by toe clipping in the laboratory. An average of 5 individuals were kept in each of a number of covered plastic terraria (78 x 48 x 21 cm) filled with 5 cm of sterile sandy substrate. Infrared and Vita–Lite lamps located at the top of terrariums were automatically controlled to supply 6–9 h. of heat and light per day. Lizards were fed with wax worms, crickets and tenebrionid larvae, and water was provided ad libitum.
Before being transplanted, animals were anesthetized by an injection of 10% ketamine. A biopsy punch was used to cut circular dorsal patches (2–2.5 cm diameter) of skin previously covered with surgical adhesive. Grafts were reciprocally transplanted within and between individuals of both populations of Aspidoscelis cozumela, and coated with adhesive to prevent premature detachment (Cuellar, 1976, 1977). Each donor graft was placed perpendicular to the recipient's dosolateral stripes for easier observation. Graft acceptation or rejection was evaluated according to the criteria of Cuellar (1976, 1977, 1984).
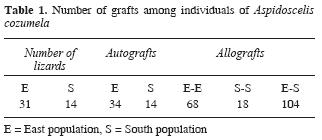
Nine experiments were conducted among 45 individuals (Fig. 2). A total of 238 grafts were transplanted, 48 were autografts, 86 intrapopulation allografts and 104 interpopulation allografts (Table 1).
Results
A previous skin–grafting analysis showed that individuals of Aspidoscelis cozumela exhibited an immunological response within the first 2 months, when they were transplanted with tissues from individuals of A. rodecki. The first evidence of rejection was present 15 days after transplantation when some of the grafts started to appear darker. After 2 months, the grafts sloughed off (Manríquez–Morán, 1998). Therefore, only those individuals that lived more that 60 days after transplantation were included in the analysis.
Nineteen of 45 individuals initially used were excluded from the analysis because of premature death (natural characteristic of A. cozumela; Hernández– Gallegos et al., 2003). The range of survivorship after transplantation of the 26 remaining individuals was from 60 to 251 days. The 124 grafts analyzed were characterized by the absence of any sign of rejection. The scales of the 24 autografts, 42 intrapopulation allografts and 58 interpopulation allografts maintained original characteristics of form, color, size and luster, indicating transplant acceptance (Table 2).
Discussion
Histocompatibility within (100%) and between (100%) populations of Aspidoscelis cozumela indicates that all individuals of this species share a same origin. A single origin may be assumed if individuals from different populations of a clonal species exhibit genetic homogeneity (Cuellar, 1976). Isogenicity has been demonstrated in several species of the genus Aspidoscelis throughout its geographical range: A. tesselata (Maslin, 1967), A. neomexicana (Cuellar, 1976, 1977), A. maslini (Hernández–Gallegos et al., 1998) and A. rodecki (Hernández–Gallegos et al., 2003).
The present evidence suggests that Aspidoscelis cozumela originated from a single female that derived from a continental population of A. maslini, as these species share histocompatibility genes (Hernández–Gallegos et al., 1998). Mitochondrial DNA sequencing revealed that the founder female possessed a haplotype characteristic of individuals from Puerto Morelos (Manríquez–Morán, 2002). Karyotypical and morphological differences between A. cozumela and A. maslini suggest that the parthenogenetic female that arrived to Cozumel suffered chromosomic and morphological transformations that were transmitted to individuals that colonized the island.
On the other hand, a morphological study carried out by Taylor and Cooley (1995), revealed that Aspidoscelis cozumela and A. maslini are the most variable species within the Aspidoscelis cozumela complex. Variability in A. maslini may be explained both by genetic differences produced via mutation (Manríquez–Morán, 2002) or environmental factors (Hernández–Gallegos et al., 2003). Neither option can be rejected, as populations genetically different from A. maslini are geographically isolated. In contrast, morphological variation in A. cozumela might be the result of small changes in environmental conditions within Cozumel Island. Several studies have shown that incubation temperatures (Flatt et al., 2001) and various other environmental factors (Sorci et al., 1996) can affect phenotypic and ecological characteristics of lizards. Moreover, field observations revealed that individuals inhabiting the beaches differ in color and size from lizards that occur in tropical forest (San Gervacio ruins; Fig. 1, SG). Individuals from tropical forests are longer and lighter in color than those from the beaches. Unfortunately, the tourist infrastructure of Cozumel Island has resulted in the disappearance of A. cozumela from the west side of the island (except around the airport; Figure 1, A) and therefore it was impossible to evaluate the complete genetic and morphological variability of this species.
Skin–grafting, like karyotypic (Manríquez–Morán et al., 2000) and mtDNA studies (Moritz et al., 1992; Manríquez–Morán, 2002), revealed that Aspidoscelis cozumela is an unisexual lizard originated from a single parthenogenetic female. However, unlike other uniparental species, A. cozumela is not a hybrid produced between 2 gonochoristic species, but a parthenogenetic species derived from a previously existing hybrid.
In the past few years many authors considered that it was not possible to demonstrate an independent origin between A. cozumela and A maslini, and proposed that these taxa were conspecific (Cole, 1985; Frost and Wright, 1988; Moritz et al., 1992; Hernández–Gallegos et al., 1998). However, existing evidence does not support that idea; the 2 parthenogenetic species are not product of the same hybridization event. Only the female that gave rise to A. maslini was produced by hybridization between A. angusticeps and A. deppii; A. cozumela was derived recently from a female from Puerto Morelos (Manríquez–Morán, 2002). Prior to 1995 Aspidoscelis maslini and A. cozumela were considered subspecies of A. cozumela (Fritts, 1969; Moritz et al., 1992). However, Taylor et al. (2005) have recently pointed out some reasons for rejecting this taxonomic arrangement: Karyotypic and mtDNA sequencing evidences suggest that A. cozumela is a mutational derivative of A. maslini (Manríquez–Morán, 2002), and this relationship might be ignored if A. maslini is viewed as a variant form of A. cozumela. Furthermore, these taxa show morphological differences comparable to those exhibited by A. maslini and A. rodecki, species that have originated through 2 independent hybridization events (Taylor and Cooley, 1995).
Genetic similarity has been considered as an indicator of coespecificity among parthenogenetic species; however, genetic change in the histocompatibility complex is not a requisite for speciation. Phenotypic variation and differential genetic expression produced by invasion to new environments can produce divergence and eventual speciation between 2 gonochoristic or parthenogenetic entities (West–Eberhard, 2005).
Acknowledgments
We thank DGAPA (project IN210594), DGEP–UNAM, CONACYT, PAEP–UNAM (projects 101315 and 201317) and Theodore Rooselvet Memorial Fund, for financial support and DGAPA–UNAM by the postdoctoral fellowship given to NLMM.
Literature Cited
Abuhteba, R. M., J. M. Walker and J. E. Cordes. 2000. Genetic homogeneity based on skin histocompatibility and the evolution and systematics of parthenogenetic Cnemidophorus laredoensis (Sauria: Teiidae). Canadian Journal of Zoology 78:895–904. [ Links ]
Abuhteba, R. M., J. M. Walker and J. E. Cordes. 2001. Histocompatibility between clonal complexes A and B of parthenogenetic Cnemidophorus laredoensis: evidence from separate hybrid origins. Copeia 2001:262–266. [ Links ]
Cole, C. J. 1985. Taxonomy of parthenogenetic species of hybrid origin. Systematic Zoology 34:359–363. [ Links ]
Cuellar, O. 1976. Intraclonal histocompatibility in a parthenogenetic lizard: evidence of genetic homogeneity. Science 193:150–153. [ Links ]
Cuellar, O. 1977. Genetic homogeneity and speciation in the parthenogenetic lizards Cnemidophorus velox and C. neomexicanus: evidence from intraspecific histocompatibility. Evolution 31:24–31. [ Links ]
Cuellar, O. and C. Smart. 1977. Analysis of histoincompatibility in a natural population of the bisexual whiptail lizard Cnemidophorus tigris. Transplantation 24:127–133. [ Links ]
Cuellar, O. 1984. Histocompatibility in Hawaiian and Polynesisan populations of the parthenogenetic gecko Lepidodactylus lugubris. Evolution 38:176–185. [ Links ]
Cuellar, O. and J. W. Wright. 1992. Isogenicity in the unisexual lizard Cnemidophorus velox. Compte–rendus des Séances de la Société de Biogéographie 68:157–160. [ Links ]
Flatt, T., R. Shine, P. A. Borges–Landaez and S. J. Downes. 2001. Phenotypic variation in an oviparous montane lizard (Bassiana duperreyi): the effects of thermal and hydric incubation environments. Biological Journal of the Linnean Society 74:339–350. [ Links ]
Fritts, T. H., 1969. The systematics of the parthenogenetic lizards of the Cnemidophorus cozumela complex. Copeia 1969:519–535. [ Links ]
Frost, D. R. and J. W. Wright. 1988. The taxonomy of uniparental species, with special reference to parthenogenetic Cnemidophorus (Squamata: Teiidae). Systematic Zoology 37:202–209. [ Links ]
Hernández–Gallegos, O., N. L. Manríquez–Morán, F. R. Méndez–de la Cruz, M. Villagrán–Santa Cruz and O. Cuellar. 1998. Histocompatibility in parthenogenetic lizards of the Cnemidophorus cozumela complex from the Yucatan Peninsula of México. Biogeographica 74:117–124. [ Links ]
Hernández–Gallegos, O., C. Ballesteros–Barrera, M. Villagrán–Santa Cruz, D. Alonzo–Parra and F. R. Méndez–de la Cruz. 2003. Actividad reproductora estacional de las hembras del género Aspidoscelis (Reptilia: Teiidae), en la Península de Yucatán, México. Biogeographica 79:1–17. [ Links ]
Hernández–Gallegos, O., F. R. Méndez–de la Cruz, M. Villagrán–Santa Cruz and O. Cuellar. 2003. Genetic homogeneity between populations of Aspidoscelis rodecki, a parthenogenetic lizard from Yucatán Peninsula. Journal of Herpetology 37:527–532. [ Links ]
McCoy, C. J. and T. P. Maslin. 1962. A review of the lizard Cnemidophorus cozumelus and the recognition of a new race, Cnemidophorus cozumelus rodecki. Copeia 1962:620–627. [ Links ]
Manríquez–Morán, N. L., 1998. Origen, histocompatibilidad y ciclo reproductor de la lagartija partenogenética Cnemidophorus cozumela (Reptilia: Teiidae). Tesis maestría, Facultad de Ciencias, Universidad Nacional Autónoma de México. México, D. F. 94 p. [ Links ]
Manríquez–Morán, N. L., M. Villagrán–Santa Cruz and F. R. Méndez–de la Cruz. 2000. Origin and evolution of the parthenogenetic lizards, Cnemidophorus maslini and C. cozumela. Journal of Herpetology 34:634–637. [ Links ]
Manríquez–Morán, N. L., 2002. Origen y diversidad clonal en las especies de lagartijas partenogenéticas del complejo Cnemidophorus cozumela (Reptilia: Teiidae). Tesis doctorado, Instituto de Biología, Universidad Nacional Autónoma de México. México, D.F. 108 p. [ Links ]
Maslin, T. P., 1967. Skin grafting in bisexual teiid lizard Cnemidophorus sexlineatus and the unisexual C. tesselatus. Journal of Experimental Zoology 166:137–149. [ Links ]
Miller, H. C., K. Belov and C. H. Daugherty. 2006. MHC class I genes in the tuatara (Sphenodon spp.): evolution of the MHC in an ancient reptilian order. Molecular Biology and Evolution 23:949–956. [ Links ]
Moritz, C., J. W. Wright, V. Singh and W. M. Brown. 1992. Mitochondrial DNA analyses and the origin and relative age of parthenogenetic Cnemidophorus. V. The cozumela species group. Herpetologica 48:417–424. [ Links ]
Sorci, G., J. Clobert and S. Belichon. 1996. Phenotypic plasticity of growth and survival in the common lizard Lacerta vivipara. Journal of Animal Ecology 65:781–790. [ Links ]
Taylor, H. L. and C. R. Cooley. 1995. A multivariate analysis of morphological variation among parthenogenetic teiid lizards of the Cnemidophorus cozumela complex. Herpetologica 51:67–76. [ Links ]
Taylor, H. L., C. J. Cole, H. C. Dessauer and E. D. Parker. 2003. Congruent patterns of genetic and morphological variation in the parthenogenetic lizard Aspidoscelis tesselata (Squamata: Teiidae) and the origins of color pattern classes and genotypic clones in eastern New Mexico. American Museum Novitates 3424:1–40 [ Links ]
Taylor, H. L., J. M. Walker, J. E. Cordes and G. J. Manning. 2005. Application of the evolutionary species concept to parthenogenetic entities: Comparison of postformational divergence in two clones of Aspidoscelis tesselata and between Aspidoscelis cozumela and Aspidoscelis maslini (Squamata: Teiidae). Journal of Herpetology 39:266–277. [ Links ]
Tellez–Valdéz, O., E. F. Cabrera–Cano, E. Linares–Mazari and R. Bye. 1989. The plants of Cozumel (touristic–botanic guide of the Cozumel Island, Quintana Roo). Instituto de Biología, Universidad, Nacional Autónoma de México. 75 p. [ Links ]
Vrijenhoek, R. C., R. A. Angus and R. J. Schultz. 1977. Variation and heterozygosity in sexual vs. clonally reproducing populations of Poeciliopsis. Evolution 31:767–781. [ Links ]
West–Eberhard, M. J. 2005. Developmental plasticity and the origin of species differences. Proceedings of the National Academy of Sciences 102:6543–6549. [ Links ]
Wright, W. J. and C. H. Lowe. 1967. Evolution of the alloploid parthenospecies Cnemidophorus tesselatus (Say). Mammalogy Chromosome Newsletter. 8:95–96. [ Links ]
Zapata, A. G. and E. L. Cooper. 1990. The immune system: comparative histophysiology. John Wiley and Sons. Chichester (England). 334p. [ Links ]














