Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de biodiversidad
versión On-line ISSN 2007-8706versión impresa ISSN 1870-3453
Rev. Mex. Biodiv. vol.79 no.1 México jun. 2008
Ecología
Chemical composition of the inflorescence odor of Malaxis rzedowskiana (Orchidaceae)
Composición química del olor de la inflorescencia de Malaxis rzedowskiana (Orchidaceae)
Geoffrey C. Kite1 and Gerardo A. Salazar2*
1 Jodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3DS, United Kingdom
2 Departamento de Botánica, Instituto de Biología, Universidad Nacional Autónoma de México, Apartado postal 70–367, 04510 México, D.F., Mexico
* Correspondent:
g.salazar@ibiologia.unam.mx
Recibido: 24 enero 2007
Aceptado: 27 septiembre 2007
Abstract
Malaxis rzedowskiana R.González (Malaxideae, Orchidaceae) from Mexico produces a pleasant floral odor reminiscent of violets in contrast to the unpleasant odors noted for several other members of Malaxideae. Analysis of the floral odor of M. rzedowskiana by headspace trapping and thermal desorption–gas chromatography–mass spectrometry revealed the presence of kaurene (76%), (E)–β–ionone (18%) and (E)–α–ionone (4%) as the main components. This is the first report of a floral odor containing a high proportion of kaurene.
Key words: floral fragrance, gas chromatography–mass spectrometry, β–ionone, kaurene, Malaxis, Orchidaceae.
Resumen
Malaxis rzedowskiana R.González (Malaxideae, Orchidaceae) de México produce un agradable olor floral reminiscente del de violetas, en contraste con los olores desagradables que han sido detectados en varios otros miembros de Malaxideae. El análisis del olor floral deM. rzedowskiana a partir del aire que rodeaba la inflorescencia en un espacio cerrado ("headspace trapping") y cromatografía de gases–espectrometía de masas por deabsorción térmica reveló la presencia de kaureno (76%), (E)–β–ionona (18%) y (E)–a–ionona (4%) como sus principales constituyentes. Éste es el primer registro de un olor floral conteniendo una alta proporción de kaureno.
Palabras clave: fragrancia floral, cromatografía de gases–espectrometría de masas, β–ionona, kaureno, Malaxis, Orchidaceae.
Introduction
The genus Malaxis Sw. (Malaxideae, Orchidaceae) encompasses about 300 species found throughout the tropics and subtropics of the Old and New World, with a few representatives in temperate regions of Asia, Europe and North America (Cribb, 2005). It is characterized by a predominantly terrestrial habit, pseudobulbous or cormous stems, soft bifacial leaves, minute greenish flowers and short gynostemium with erect or suberect anther (e.g., Williams, 1951; McVaugh, 1985; Dressler, 2003). Mexico is an important center of diversity of Malaxis and about 60 species have been recorded so far in the country (Soto et al., 2007).
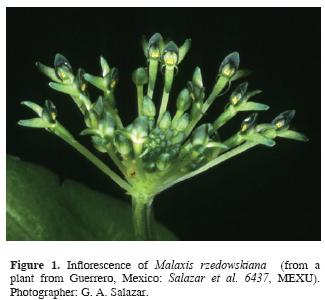
Flowers of Mexican Malaxis are colored in various tones of green, and commonly range from ca. 3 to 10 mm in span, although exceptionally they can be as large as 20 mm or more (in M. lepanthiflora and its allies; e.g., Salazar and Soto, 1990). The labellum is usually darker than the rest of the perianth and distinctly concave near the base but more or less flat above. At least in some species, droplets of fluid (presumably nectar) can be present either on the cavity or on the flat portion (pers. obs. of G. A. Salazar). Malaxis rzedowskiana R.González has an umbellate inflorescence in which the pale green flowers with dark green labellum open in succession and there can be up to about a dozen flowers open at the same time (Fig. 1). This species is restricted to the Sierra Madre del Sur (Guerrero) and the Eje Neovolcánico (Estado de México) in south–central Mexico, where it grows in ravines and shady mountain slopes with pine–oak forest at 2 200–2 400 m elevation.
The chemical composition of floral odors in Malaxis has not been studied previously, and among other members of the tribe Malaxideae only the odor of Liparis viridiflora (Bl.) Lindl. has been analyzed (Kaiser, 1993). However, anecdotal reports suggest that strange or unpleasant odors are frequent in the tribe (Fay, 1986; Kaiser, 1993). Thus we were surprised to notice that M. rzedowskiana produced an agreeable scent from its inflorescence, somewhat reminiscent of violets. This prompted us to analyze its chemical composition with the aim of establishing a starting point for more objective comparisons of floral odors in Malaxis, which may improve our understanding of the reproductive biology of this diverse but poorly studied orchid group.
Materials and methods
Study species. The analysis was performed on a live plant of M. rzedowskiana that flowered in cultivation. The plant sprouted from a corm removed from a recently pressed herbarium specimen (voucher: Mexico, state of Guerrero, Diego et Gispert 8479, MEXU). The corm was planted in a plastic pot with a mixture of leaf mould and pine bark in March 1999; a few months later it had developed two leaves and an inflorescence and the first flowers started to open by mid–June. The inflorescence produced the scent during the light hours of the day. A wild–growing plant of Malaxis rzedowskiana was found in flower in Guerrero state, Mexico, by one of us (GS) in the summer of 2004 and is shown in the accompanying photograph (Fig. 1). This plant produced the same agreeable floral scent but was not analyzed chemically.
Chemical analysis. The inflorescence still attached to the plant was covered in a glass Quickfit adapter into which was inserted a 100 mm × 3 mm diameter trap (SGE Ltd.) packed with 100 mg Tenax TA (35/60 mesh). Air was sucked through the trap at a rate of 50 ml/min by means of a portable pump. The odor was sampled for 2.5 h from 12:00 to 14:30. The volatiles collected on the trap were analyzed by thermal–desorption gas chromatography–mass spectrometry using a Perkin–Elmer Model 8 500 gas chromatograph fitted with thermal desorption injector (SGE Ltd) and a 25 m × 0.2 mm i.d. x 0.25 μm BPX5 capillary column (SGE Ltd.); the mass spectrometer was a Finnigan–MAT 800 series ion trap detector. The trap was desorbed directly onto the capillary column by inserting the trap into the thermal desorption injector set at 250°C. Desorption was allowed to proceed for 3 min during which time the front end of the column was immersed in liquid nitrogen to focus the desorbed volatiles. After 3 min the trap was replaced with a blank and the column was removed from the liquid nitrogen, and chromatography proceeded using an oven temperature programme of 40–260°C at 4°C/min with helium at 20 psi as the carrier gas. Mass spectra were recorded in the range m/z 38–400 at 1 scan/s. Percentage compositions were estimated from peak areas in the total ion chromatogram and compound assignments were achieved by comparing mass spectra and retention indices (RIs) to those published in Adams (1995). RIs were calculated from a series of n–alkanes (Supelco) analyzed in the same manner.
Results
The major compound trapped from the inflorescence was the diterpenoid kaurene (= 16–kaurene), which occurred with lesser amounts of the irregular terpenoids a– and β–ionone and several other minor constituents (Table 1; Fig. 2). Identification of a– and β–ionone was unambiguous from their characteristic mass spectra but there were several diterpenoids having similar mass spectra to kaurene. The published spectra of kaurene and its stereoisomer phyllocladene had the closest match to the sample spectrum. The sample compound was assigned to kaurene on the basis of its relative elution to a minor constituent, epi–13–manoyl oxide. Phyllocladene co–elutes with epi–13–manoyl oxide whereas kaurene elutes just after it (Adams, 1995). Epi–13–manoyl oxide could be assigned unambiguously from its mass spectrum and its elution after a C20 n–alkane used as a retention time standard (manoyl oxide, which has the same mass spectrum, elutes just before the C20 alkane).
Discussion
(–)–Kaurene is a precursor of gibberellins (Bohlmann, 1998), nevertheless kaurene it is not commonly detected as a headspace volatile from plants and was not listed as a constituent of flower scents by Knudsen et al. (1993; 2006). Transgenic Arabidopsis thaliana (L.) Heynh. overexpressing kaurene synthase produced (–)–kaurene as a headspace volatile (Otsuka et al., 2004) and it is possible that high kaurene synthase activity in the flowers of M. rzedowskiana results in its presence in the flower headspace volatiles. β–Ionone is a common fragrance ingredient with a woody, violet (Viola spp.) odor and it is likely to be responsible for the perceived violet–like note in the floral odor. Being a breakdown product of carotenoids (Winterhalter and Rouseff, 2002), β–ionone is a constituent of the floral headspace volatiles from many species (Knudsen et al., 2006) giving rise to scents with an 'ionone–floral image' (Kaiser, 1993). Among orchids, β–ionone is a prominent component of the floral odor of Houlletia odoratissima Linden ex Lindl. et Paxton, Maxillaria nigrescens Lindl. and Oncidium tigrinum La Llave et Lex. and present in the odor of numerous other species (Kaiser, 1993).
In containing kaurene and ionones as the major components, the odor of Malaxis rzedowskiana differs markedly from the odor of Liparis viridiflora (Kaiser, 1993), the only other member of Malaxideae for which there is chemical data. Analysis of the strange odor of L. viridiflora identified 25 compounds with the most abundant being (E,E)–a–farnescene (65%), which is unlikely to be responsible for the dominant spermatic note in the odor. Kaiser (1993) noted that other species of Liparis also produced strange odors "frequently reminiscent of algae, yeast, crustacean and similar odors". The agreeable scent of Malaxis rzedowskiana also differs from other Mexican Malaxis. During the course of this study, one of us (GS) noted that M. brachyrrhynchos (Rchb.f.) Ames, M. brachystachys (Rchb.f.) Kuntze, and M. histionantha (Link, Klotzsch et Otto) Kuntze, among others, produce rather unpleasant odors reminiscent of stale raw eggs. Elsewhere, Fay (1986) reported that the flowers of M. maclaudii (Finet) Summerh., from the Central African Republic, produce a distinct odor of fresh fi sh. Reeves and Reeves (1984), however, noted that Malaxis (Hammarbya) paludosa (L.) Sw. in Minnesotta, USA, produce a sweet cucumber scent.
Lack of detailed information on natural pollination in species of Malaxis makes it difficult to comment on the biological significance of such differences in floral odor. The only reports of pollinators are for M. paludosa for which the fly Phronia digitata (Mycetophilidae) was observed as a pollinator (Reeves and Reeves, 1984). Kaiser (1993) postulated that the strange odors of Liparis are likely to be attractive to fly and midge species. Wallace (1974) found that an unidentified fly of family Sarcophagidae pollinates the dull, greenish–yellow flowers of Liparis reflexa (R. Br.) Lindl. while browsing for fluids on the labellum, being attracted to the flowers by a smell resembling that of stale egg yolk. A flowering plant of L. viridiflora in cultivation outside of its natural range was visited by mosquitoes (Culex sp., Culicideae), one of which was seen to carry a pollinium on its back (H. Petersen, quoted by Christensen, 1994).
It would be of interest to determine the pollinator of M. rzedowskiana, not least because of the high proportion of kaurene in the floral odor. This intermediate in gibberellin synthesis seems not to have been exploited in the floral odors of other plants. Of course, the predominant constituent in the odor is not necessarily the cue used by the pollinator. Among other consituents identified in the odor of M. rzedowskiana, β–ionone has been shown to be attractive to male euglossine bees (Nemésio, 2003).
We hope that this report stimulates further research focused on both the chemical characterization of floral odors and plant–pollinator interactions in Malaxideae. Such studies might suggest the processes that have favoured the noticeable diversification undergone by these orchids in the tropics.
Literature cited
Adams, R. P. 1995. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. Allured, Illinois. [ Links ]
Bolhmann, J., Meyer–Gauen, G., and R. Croteau. 1998. Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proceedings of the National Academy of Sciences of the United States of America 95:4126–4133. [ Links ]
Christensen, D. E. 1994. Fly pollination in the Orchidaceae. In Orchid biology: reviews and perspectives. VI, J. Arditti (ed.). John Wiley, New York. p. 415–454. [ Links ]
Cribb, P. J. 2005. Malaxis. In Genera Orchidacearum, vol. 4: Epidendroideae, part 1, A. M. Pridgeon, P. J. Cribb, M. W. Chase, and F. N. Rasmussen (eds.). Oxford University Press, New York. p. 471–475. [ Links ]
Dressler, R. L. 2003. Orchidaceae. In Manual de plantas de Costa Rica, vol. III, B. E. Hammel, M. H. Grayum, C. Herrera, and N. Zamora (eds.). Missouri Botanical Garden, St. Louis. p. 1–595. [ Links ]
Fay, J. M. 1986. Malaxis maclaudii: a fishy orchid. The Orchid Review 94:278. [ Links ]
Kaiser, R. 1993. The scent of orchids: olfactory and chemical investigations. Roche, Basel. [ Links ]
Kudsen, J. T., L. Tollsten, and L. G. Bergstrom. 1993. Floral scents – a checklist of volatile compounds isolated by head–space techniques. Phytochemistry 33:253–280. [ Links ]
Knudsen, J. T., R. Eriksson, J. Gershenzon, and B. Ståhl. 2006. Diversity and distribution of floral scent. The Botanical Review 72:1–120. [ Links ]
McVaugh, R. 1985. Orchidaceae. In Flora Novo–Galiciana: a descriptive account of the vascular plants of western Mexico, vol. 16, W. R. Anderson (ed.). The University of Michigan Press, Ann Arbor. p. 1–363. [ Links ]
Nemésio, A. 2003. Preliminary sampling of Euglossina (Hymenoptera: Apidae: Apini) of Reserva Particular do Patrimônio Natural "Feliciano Miguel Abdala", Caratinga, Minas Gerais, southeastern Brazil. Lundiana 4:121–124. [ Links ]
Otsuka, M., H. Kenmoku, M. Ogawa, K. Okada, W. Mitsuhashi, T. Sassa, Y. Kamiya, T. Toyomasu, and S. Yamaguchi. 2004. Emission of ent–kaurene, a diterpenoid hydrocarbon precursor for gibberellins, into the headspace from plants. Plant Cell Physiology 45:1129–1138. [ Links ]
Reeves, L. M. and T. Reeves. 1984. Life history and reproduction of Malaxis paludosa in Minnesota. American Orchid Society Bulletin 53:1280–1281. [ Links ]
Salazar, G. A. and M. A. Soto. 1990. Una nueva especie de Malaxis (Orchidaceae) de flores grandes del norte de Chiapas. Acta Botanica Mexicana 10:45–49. [ Links ]
Soto, M. A., E. Hágsater, R. Jiménez, G. A. Salazar, R. Solano, R. Flores, and I. Ruiz. 2007. Las orquídeas de México: catálogo digital (DVD). Instituto Chinoin, Mexico, D. F. [ Links ]
Wallace, B. J. 1974. The pollination of Liparis reflexa (R.Br.) Lindl. The Orchadian 4:106–108. [ Links ]
Williams, L. O.1951. The Orchidaceae of Mexico. Ceiba 2:1–321. [ Links ]
Winterhalter, P. and R. Rouseff. 2002. Carotenoid–derived aroma compounds: an introduction. In Carotenoid–derived aroma compounds, P. Winterhalter and R. Rouseff (eds.). ACS Symposium Series 802P. American Chemical Society, Washington, D. C. p. 1–17. [ Links ]














