Serviços Personalizados
Journal
Artigo
Indicadores
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de biodiversidad
versão On-line ISSN 2007-8706versão impressa ISSN 1870-3453
Rev. Mex. Biodiv. vol.77 no.2 México Dez. 2006
Ecología
Phylogeny, ecological fitting and lung flukes: helping solve the problem of emerging infectious diseases
Filogenia, flexibilidad ecológica y digéneos de pulmones: ayudando a resolver la crisis de las enfermedaes infecciosas emergentes
Daniel R. Brooks1*, Deborah A. McLennan1, Virginia León–Règagnon2 and Eric Hoberg3
1 Department of Zoology, University of Toronto, 25 Harbord Street, Toronto, ON M5S 3G5, Canada.
2 Instituto de Biología, Universidad Nacional Autónoma de Mexico, Mexico D.F. Mexico 04510.
3 US National Parasite Collection and Animal Parasitic Disease Laboratory, USDA, Beltsville, Maryland 20705
*Correspondent:
dbrooks@zoo.utoronto.ca
Recibido: 06 septiembre 2005
Aceptado: 02 junio 2006
Abstract
Traditional wisdom, based on assumptions of species–specific coevolutionary interactions between hosts and parasites, suggests that pathogens with multi–host life cycles are unlikely to move with their definitive hosts because their transmission requirements are so specialized. Ecological fitting provides a theory of diffuse coevolution, which allows introduced pathogens with complex life cycles to become established and spread rapidly into native hosts if the resource required at each stage of the life cycle is both phylogenetically conservative (distributed among numerous species) and geographically widespread. The external appearance of life cycle complexity does not, therefore, on its own, predict the potential for an organism to become an emerging infectious disease. We apply this concept to explain a potential enigma, the presence of a lung fluke, Haematoloechus floedae, endemic to North American bullfrogs, in Costa Rican leopard frogs, even though there are no bullfrogs extant in the country today, and none ever occurred where the parasite has been discovered. We then discuss how the integration of ecological and life history information within a phylogenetic framework can help biologists move from attempts to manage emerging infectious disease outbreaks to the ability to predict and thus circumvent the outbreak in the first place.
Key words: ecological fitting, emerging infectious diseases, pathogen pollution, introduced species, leopard frogs, bullfrogs, Haematoloechus floedae.
Resumen
Con base en el supuesto de coevolución a nivel de especies de parásitos y hospederos, tradicionalmente se asume como poco probable que aquellos patógenos con ciclos de vida que involucran varios hospederos acompañen a su hospedero definitivo a un nuevo ambiente, por lo especializado de sus requerimientos de transmisión. El fenómeno de flexibilidad ecológica aporta una teoría de coevolución difusa, que permite a los patógenos con ciclos de vida complejos, que han sido introducidos, establecerse y dispersarse de una manera rápida en hospederos nativos, si el recurso requerido en cada etapa del ciclo de vida es filogenéticamente conservado (se distribuye en numerosas especies) y a la vez, tiene una distribución geográfica amplia. Por lo tanto, la complejidad de un ciclo de vida no predice, por sí misma, el potencial de un organismo para provocar una enfermedad infecciosa emergente. Aplicamos este concepto para explicar el caso particular de un digéneo del pulmón de anfibios, Haematoloechus floedae, endémico de ranas toro de Norteamérica, que fue recolectado en ranas leopardo de Costa Rica, aún cuando actualmente no existen ranas toro en ese país, y nunca existieron en la región en donde se encontró al parásito. Asimismo, se discute de qué manera la integración de la información ecológica y de ciclos de vida, en un marco filogenético, puede ayudar a los biólogos a pasar de los intentos para controlar brotes de enfermedades emergentes, hacia la predicción y el impedimento de dichos brotes en primera instancia.
Palabras clave: flexibilidad ecológica, enfermedades infecciosas emergentes, contaminación por patógenos, especies introducidas, ranas leopardo, ranas toro, Haematoloechus floedae.
Introduction
Many people consider the biodiversity crisis to be solely one of extinction. It is, however, also a crisis of emerging infectious diseases (EIDs) (Daszak et al., 2000; Harvell et al., 2002; Woolhouse 2002; Epstein et al., 2003). More than 50% of the known species on this planet are parasites or pathogens of some form, many of which are agents of diseases affecting humans, livestock, crops, and wildlife. From an anthropocentric perspective, parasites are evolutionary paradoxes. On the one hand, they may control host populations, playing a central role in the maintenance of genetic diversity and structuring of metazoan communities. On the other hand, they may represent threats to human health, agriculture, natural systems, conservation practices and the global economy via interactions such as faunal disruption and ecological release (Altizer et al., 2003; Horwitz and Wilcox, 2005). Knowledge of the natural history and distribution of known and potential pathogens is thus a critical prerequisite for optimizing their positive, while minimizing their negative, effects on conservation, restoration and sustained development programs (Brooks and Hoberg, 2000, 2001, 2006; Brooks, 2003). Unfortunately, our knowledge remains patchy, particularly concerning patterns of pathogen diversity, biogeography and host associations; so patchy that we continue to make substantial discoveries about the distribution of often hidden diversity and potential EIDs in even relatively well–studied regions (Hoberg et al., 2003; Kutz et al., 2004).
Anticipating a problem is always more time– and cost–effective than responding to a crisis, no matter how effective the response. Anticipation, in turn, requires that we be able to predict the effects of policy implementation. Phylogenetic classification systems, incorporating data from all aspects of an organism's biology from molecules to ecology and behavior, are the most predictive information systems about organisms and their places in the biosphere currently available. The predictable parts of biological systems are the stable elements, form and function, autecological and synecological, which have persisted through evolutionary time. For example, we can predict with almost 100% confidence that the females of any newly described species of eutherian mammal will be viviparous and will suckle their offspring on milk produced in specialized mammary glands. Shared history allows us to make such predictions, and this buys us time and saves money, two resources that are in short supply in battling the EID crisis (Brooks and McLennan, 2002; Brooks and Hoberg, 2006). In the following paper we present an example illustrating how combining phylogenetic and natural history/ecological data can help biologists predict which species are likely to pose a threat as an emerging infectious disease should they be introduced to a novel habitat.
We begin the story with a potential enigma: A parasite from North American bullfrogs has been recently discovered in two species of Costa Rican leopard frogs, even though there are no bullfrogs extant in the country today, and none ever occurred where the parasite has been discovered. We then show how the concept of ecological fitting, viewed within the framework of phylogeny, can help solve the riddle. We are asking three questions, First, How did this parasite get into the Costa Rican frogs? Second, using information about parasite–host interactions (systematics and ecology), could we have predicted the problem might occur, and thus prevented the spread of a potential amphibian EID? Third, Where do we go from here?
The Concept of Ecological Fitting. Many researchers have emphasized problems that result from the anthropogenic encroachment into new areas by hosts susceptible to endemic pathogens (Audy, 1958; Elton, 1958; Rausch, 1972). More recently, however, biologists have begun to examine the opposite interaction, the introduction of a novel pathogen to endemic hosts (Hoberg, 1997; Daszak et al., 2000; Prenter et al., 2004). The most well known example of this second dynamic is the chytrid fungus, Batrachochytrium dendrobatidis, which is currently threatening amphibian populations and species worldwide (Hanselmann et al., 2004). Accidental introduction of this pathogen, and its subsequent establishment, were facilitated by the chytrid's natural history – spores are long–lived, resistant to environmental extremes, and easily transported, while the fungus itself is able to persist without clinical symptoms in the keratinized mouthparts of amphibian larvae after adult die–offs (Daszak et al., 1999, 2003).
Pathogen pollution, the negative impact of anthropogenically–introduced diseases on endemic biodiversity (Daszak et al., 2000), has been documented for species with relatively simple life cycles like fungi and viruses (Daszak et al., 1999; Hanselmann et al., 2004). In theoretical discussions, pathogens with complex life cycles requiring two or more different host species (e.g., many digeneans) are considered less of a threat for introduction outside their native ranges because of the specialized nature of their transmission dynamics (see e.g., Torchin et al., 2003; Prenter et al., 2004). This consideration is based upon the assumption that parasite species with complex life cycles require particular species of intermediate hosts to complete their life cycle; that they are ecological specialists. This assumption is only valid if the evolution of host–parasite interactions has generally been driven by a tight coevolutionary interaction between the host and the parasite species; that is, parasites should, in general, be fairly host specific so specialists should greatly outnumber generalists. Recent studies have, however, demonstrated that the ability to colonize many different hosts is more widespread within many parasite taxa than previously thought (Brooks and McLennan, 1993; Brooks and Ferrao, 2005). These data mirror those being uncovered for a variety of herbivorous insect–plant associations (see e.g., Janz and Nylin, 1998; Janz et al., 2001; Nylin and Janz, 1999; Nylin et al., 2000).
The general explanation for such widespread host switching is that the associate (parasite or insect) is tracking a resource that is distributed across many host species. From the associates' perspective, it is the resource, not the way that resource is packaged (the particular host species), that represents the specialized requirement. In this kind of interaction, the associate will look like either a generalist, if many different host species carrying the required resource are available within its range, or a specialist, if only one suitable host occurs within its range (for an extensive review with references, see Brooks and McLennan, 2002). The first scenario may explain the large reservoir host–range of many zoonoses and the observation that most pathogens can infect multiple hosts (Woolhouse, 2002). The second scenario is potentially a more difficult one for conservation biologists because, despite appearances in the field, the specialist is not host specific (Brooks and McLennan [2002] called such associates "faux specialists"), and may thus be difficult to control if moved to an area containing new hosts with the appropriate resource (Louda et al., 2003; Louda and Stiling, 2004). Following introduction the specialist will colonize as many of these hosts as it can, spreading rapidly against all "predictions" based upon its apparently restricted one host association.
The ability of organisms to track the same resource distributed among many different host species is termed "ecological fitting" (Janzen, 1985). Although ecological fitting has been documented in insect–host plant interactions (Futuyma et al., 1995; Janz and Nylin, 1998; Janz et al., 2001), researchers working with pathogen–host associations have been slower to appreciate its significance. For example, if the specialized needs of pathogens with complex life–cycles are phylogenetically conservative enough to be geographically widespread, then such pathogens may be more successful at moving with their introduced hosts, and spreading in the novel ecosystem, than previously thought (Brooks and McLennan, 2002; Brooks and Ferrao, 2005). Indeed, in this context the conservative dynamics of parasite transmission (e.g., Hoberg et al., 2000) may define the potential for rapid dissemination when functional guild associations occur across disjunct geographic localities. Ecological fitting thus explains why the external appearance of life cycle complexity does not, on its own, predict the potential for an organism to become an EID.
Case Study
The parasite. Haematoloechus floedae is a digenean species native to the southeastern United States, where it lives in the lungs of the bullfrog, Rana catesbeiana. When bullfrogs were introduced to the southwestern United States and the Yucatán Peninsula, Mexico the parasite went with them, and is now found in bullfrogs in southwestern U. S., as well as other groups of frogs in Yucatán (Rana brownorum – leopard frog– and R. vaillanti –palmipes group). The lung fluke has recently been reported in two leopard frogs, R. taylori and R. cf. forreri, from the Area de Conservación Guanacaste, Costa Rica (voucher specimens 5065, 5066, deposited in Colección Nacional de Helmintos, IBUNAM, México City). The only consistent differences between the North American and Mesoamerican populations of H. floedae are 3 synonymous substitutions in COI sequences, indicating that the meso–American lung flukes are recent introductions rather than ancient relictual populations (for details see León–Règagnon et al., 2005).
Introduction of the parasite. Although we have not been able to find official records of R. catesbeiana being introduced to Costa Rica, biologists in the country recall attempts in the 1960s to farm bullfrogs in the San Jose region (R. Puschendorf, pers. comm., from conversations with Sr. Gerardo Chávez, Universidad de Costa Rica). This circumstantial evidence is supported by two specimens of R. catesbeiana, which were collected in Alajuela, a suburb of San Jose (accession numbers 1944, 1945, Universidad de Costa Rica). Since that time, there have been no reports of bullfrogs in Costa Rica, despite intensive amphibian monitoring projects across the country.
Establishment of the parasite. Haematoloechus transmission dynamics, although specialized, are conservative across the genus, involving a freshwater pulmonate snail, a dragonfly nymph and a relatively large aquatic frog (Dronen, 1975). Although most lung flukes are known from only a single snail species in natural infections, a number are capable of infecting a broader range of snails from the superfamily Lymnaeoidea in the laboratory. Haematoloechus medioplexus, H. parviplexus and H. longiplexus are limited to one subfamily, the Planorbinae; H. medioplexus infects the closely related Planorbula armigera and Promenetus exacuous (Morgan et al., 2002) and all three parasites can utilize Gyraulus parvus (Lozano, 1994; Snyder and Janovy, 1994, 1996). Haematoloechus complexus occurs naturally in Pseudosuccinea columella (Lymneidae) and Physella virgata (Physidae) (Krull, 1933), and has been experimentally reared in Physella virgata, P. gyrina, and P. heterostropha (Snyder and Janovy, 1994, 1996). The cercariae that emerge from the snail infect the second intermediate host, which, for all species studied to date (H. longiplexus, H. medioplexus, and H. parviplexus [reported as H. varioplexus]) is an anisopteran odonate (dragonflies in the genera Libellula and Erythemis); H. complexus adds an array of aquatic arthropods to that repertoire (Snyder and Janovy, 1994; Wetzel and Esch, 1996).
Haematoloechus species are thus tracking resources that are relatively widespread through North and Central America (http://www.ups.edu/biology/museum/CRodonates.html). This allows the parasites to expand into novel territory because all they require is any one of a number of lymnaeoid pond snails and anisopteran dragonfly species, rather than specific species Evidently this is what happened with H. floedae, which, despite having a supposedly complex, specialized life cycle, has become established in a number of localities where bullfrogs have been introduced.
Expanding definitive host ranges. Optimization of hosts onto the phylogeny for Haematoloechus indicates that leopard frogs (Rana pipiens clade) are the plesiomorphic hosts for this clade of lung flukes (Fig. 1; for details see León–Règagnon et al., 2005). Although the ancestor of H. floedae switched to bullfrogs, the presence of the fluke in R. taylori andR. cf. forreri in Costa Rica and R. brownorum in Yucatán indicates that the parasite has retained its plesiomorphic ability to infect leopard frogs. This is the first demonstration that parasites, like phytophagous insects (Janz and Nylin 1998; Nylin and Janz 1999; Nylin et al., 2000; Janz et al., 2001) may display ancestral host preferences, and thus develop "new" associations through retro–colonization (Hoberg, 2005), under certain circumstances.
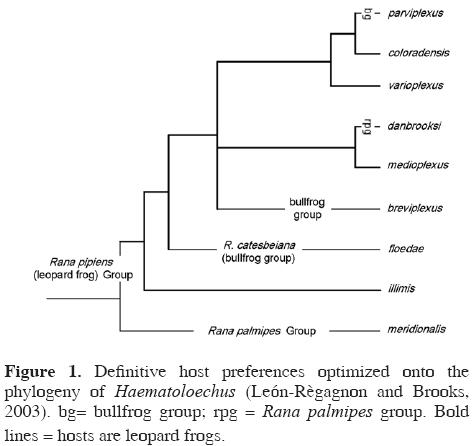
The observation that H. floedae is able to switch definitive hosts according to a pattern of ancestral associations represents the third manifestation of ecological fitting in its life cycle. Since the bullfrog definitive host no longer exists in Costa Rica, the contemporary occurrence of H. floedae in the country indicates that the parasite is capable of persisting in native hosts, in this case in leopard frogs, because those hosts represent the plesiomorphic resource for the parasite clade. The intersection between guild dynamics and evolutionary history (ecologically and phylogenetically conservative transmission patterns) defines the potential for host–switching among an array of available hosts. The diversification of parasites among definitive hosts may thus occur against a background of ecological continuity established by intermediate hosts.
Dispersal of the parasite. Haematoloechus floedae was discovered in both wet and dry forest habitats in the ACG in 1997. Not only are there no bullfrogs currently in the area (intensive monitoring and collection projects have been underway in the conservation area for a decade), there is no indication that bullfrogs were ever introduced there in the past. So, how did the parasite move from the environs of San Jose to the ACG, some 250 km distant, in no more than 40 years? Although bullfrogs are considered to be relatively rapid dispersers for amphibians, their ability to move 4 – 5km in, perhaps, 3 – 4 years (Hanselmann et al., 2004), cannot explain the presence of the parasite in the ACG. Even if the frogs had managed to move that far, dispersion in other localities is accompanied by large population sizes, so we would have to postulate rapid dispersal and population expansion followed by complete extinction to explain the Costa Rica pattern, which seems unnecessarily ad hoc.
There is, however, a component of the Haematoloechus life cycle that could be associated with rapid long–distance dispersal, the dragonfly intermediate host. For example, some Libellula species (a genus used by Haematoloechus) can migrate several hundred kilometers, with swarms of migrants often estimated to range from hundreds of thousands to billions of individuals (Artiss, 2004). Dispersal is hypothesized to occur as a response to strong, seasonal changes in weather systems such as the movement of cold fronts or monsoon rains (Russell et al., 1998). Dragonflies have indeed been observed moving en masse through valleys within the ACG, although which species were involved and where they were going are as yet unknown (W. Hallwachs pers. comm., based on videotapes of these migrations).
If dragonflies are indeed the medium of dispersal, is it possible that infected odonates are migrating southwards from North America? According to this scenario it would be the natural expansion of the dragonfly intermediate host, not the introduction of the bullfrog, that was responsible for the introduction of the parasite to Costa Rica. As mentioned previously, Haematoloechus floedae is found in the Yucatán where introduced bullfrogs still occur. The lung fluke has, however, not been found in central and southwest Mexico, despite extensive sampling of appropriate hosts (León–Règagnon, 2003). Since infected dragonflies would have to pass over this area on their way to Costa Rica, it seems odd that infected leopard frogs are not found there. More importantly, parasite infection negatively affects the flight performance of various insects, decreasing their ability to migrate long distances (Bradley and Altizer 2005). It is thus more likely that infected dragonflies, which could not survive a migration of many hundred kilometers from North America to Costa Rica, could tolerate shorter movements within the country. Taken together, these data suggest that H. floedae was introduced initially with bullfrogs rather than with the snail or the dragonfly host, then spread northward a short distance with infected dragonflies.
Could H. floedae have been introduced with infected leopard frogs? The answer to this question is, of course, but we have no evidence that a population of leopard frogs large enough to contain enough infected individuals to establish a sustainable infection, has ever been introduced into Costa Rica. Leopard frogs are, after all, not a commercially important species. Overall, then, it is more plausible to propose that the presence of widespread, plesiomorphic resources at all stages of H. floedae's life cycle allowed it to become established in a novel environment even after the bullfrogs with which it was introduced were extirpated.
Discussion
Ecological Fitting and EIDs. We are only beginning to understand the role for introduced species in EIDs of endemic wildlife (Daszak et al., 2000; Altizer et al., 2003). Previous analyses have generally focused on the dangers of pathogens with simple transmission dynamics. This study shows that we can no longer assume that parasites with complex life cycles are less likely to be problematic because they require specific vectors in the novel ecosystem for transmission to be successful. Ecological fitting may allow a parasite to track widespread resources at all stages of its life cycle, increasing the probability of transmission. This means that parasites may persist in an area after efforts to mitigate the damage caused by their introduced host, via extirpation of that host, have been deemed successful. Moreover, we often assume that the spread of a pathogen with a complex life cycle is limited by the movement of its least vagile host (in this case the pulmonate snail). Our study suggests that this rate–limiting step can also be overridden if all stages of the pathogen's life cycle represent instances of ecological fitting to a widespread resource. In this case, the rate at which a pathogen can spread will be determined by the dispersing capabilities of the most vagile host (in this case the dragonfly) and the extent of resource distribution. Our example further indicates the complex interactions which may exist in dispersal or dissemination and successful establishment of pathogens. In this scenario the original or primary introduction with bullfrogs (hosts of low vagility) was driven by anthropogenic translocation, and secondary expansion and establishment was a function of natural processes and dispersal of dragonflies (hosts of high vagility) and colonization of novel anuran hosts.
In the past, researchers have generally assumed that the widespread susceptibility of different host species to a particular pathogen occurs because the pathogen has a high rate of genetic diversification and is thus capable of producing variants that can colonize new hosts (Woolhouse et al., 2001; Altizer et al., 2003). This, of course, presupposes that each new host represents a slightly new resource. Ecological fitting provides an alternative explanation for the phenomenon of widespread host susceptibility. If each new host represents "the same" resource, then pathogenic species may be able to expand their host repertoire and thus spread rapidly in new areas without high levels of genetic variability in capabilities for host utilization. More importantly, if the new hosts have no history of interaction with the pathogen or a close relative, then they may not be able to mount an effective immune response, further increasing the rate of pathogen spread (Jensen et al., 2002; Pearman and Garner, 2005). This means that models examining the dynamics of pathogen spread in novel environments (Dobson, 2004) need to include a subset of simulations that do not require a hiatus following introduction of the pathogen for the production of suitable genetic diversity in host requirements. In evolutionary terms, ecological fitting is the coevolutionary equivalent of preadaptation (Vrba and Gould, 1986); in other words, observed host "switches" or "jumps" are not driven by pathogen evolution in response to novel conditions, but rather by pre–existing pathogen biology (evolutionary history) and chance (Brooks and McLennan, 1993; Altizer et al., 2003).
Finally, this study highlights how little we know about the total impact of introduced species on endemic biodiversity. Rana catesbeiana has been imported worldwide in the quest for a sustainable source of culinary frog legs. Researchers initially focused their attention on the impact of these frogs on endemic anuran community structure, documenting a general negative interaction driven by bullfrog predation and superior competitive skills (Blaustein and Kiesecker, 2002). More recently came the discovery that bullfrogs serve as reservoir hosts for chytridiomycosis, and are thus a potential source of this devastating disease in some countries (Mazzoni et al., 2003; Hanselmann et al., 2004). Our study indicates that bullfrogs may also introduce parasites with complex life cycles to a novel ecosystem; parasites that may persist long after the bullfrogs have disappeared. Haematoloechus species damage and compress alveolar tissue (Shields, 1987), decrease lipids in the lungs and are often associated with an increase in lung size, presumably a compensatory reaction to the alveolar damage (Cain and French, 1975). All of these effects are costly on some level to the host. So, while the persistence of H. floedae may not appear to be a direct threat to the leopard frogs in Costa Rica at the moment, it is possible that changes in environmental stressors via changes in weather patterns due to global warming, increased UV radiation, etc., may tip the balance towards making H. floedae an EID (Pounds et al., 1999; Patz et al., 2000; Pounds 2001; Kiesecker et al., 2004) (ominously, Costa Rica is again considering introducing bullfrogs once again).
We suggest therefore that the concept of pathogen pollution (Daszak et al., 2000) be expanded to include parasites that have previously been treated as low risk for introduction because of their complex and presumably coevolved lifecycles. As a consequence of ecological fitting, such parasites may actually represent evolutionary accidents just waiting to happen. (Brooks and Ferrao, 2005; Brooks and Hoberg, 2006).
This study has documented the existence of ecological fitting in a parasite–host system; in this case, the ability of a parasite to colonize a species representing a plesiomorphic resource. Ecological fitting may be more widespread in parasite–host systems than currently recognized. For example, Perlman and Jaenike (2003) demonstrated that the potential host range of five nematode species was much larger than the reported host range; closely related hosts were more likely to be infected with the same parasite than distantly related hosts. Both of these studies indicate that the existence of "faux specialists" may confound our ability to predict the impact on endemic species of introducing infected hosts into novel environments or the impact of introducing novel hosts into an ecosystem containing potentially infectious pathogens.
If our knowledge of pathogen diversity is equivalent to our overall knowledge of general biological diversity, we have documented less than 10% of the world's pathogens (Wilson, 1988; Cotterill and Dangerfield, 1997). The remaining 90% represent the realm of potential EIDs. The realm of the EID crisis comprises the occurrence of susceptible hosts outside the area of origin for each pathogen, intersecting with our fundamental ignorance of their phylogeny, biogeography, host specificity, and transmission dynamics. It is not an overstatement to say that the crisis stems from the absence of comprehensive systematic inventories of the world's pathogens. Survey and inventory must also be married directly to a new emphasis on development of extensive archival collections as drivers for biodiversity research, and as historical baselines by which we can explore ecological perturbation on varying temporal and spatial scales (Hoberg et al., 2003; Cook et al. 2005). If we could be confident that EIDs were a rare phenomenon, perhaps it would be cost–effective to engage in the kind of a posteriori, management response we have seen globally to this point in time. Unfortunately, the evolutionary perspective provided by systematists leads us to assume that the potential number of EIDs is very large; there are many "accidents waiting to happen", involving both human beings and wildlife, as a result of continued anthropogenic activities (e.g., Kutz et al., 2004).
Our lack of a comprehensive taxonomic inventory of pathogens on this planet, and of phylogenetic assessments of their coevolutionary and biogeographic histories, are major hindrances to dealing with the problem (Brooks and Hoberg, 2000; Horwitz and Wilcox, 2005). The global society must therefore decide, and decide soon, whether to dedicate its increasingly scarce resources to managing the EID crisis or to solving it. Given that our attempts to manage the evolutionarily complex systems called biodiversity have generally not been resoundingly successful in the past (Fayer, 2000), we believe it is time to shift our attention to problem–solving. Our ability to be successful problem solvers is enhanced by incorporating phylogenetic (historical) information into our solutions. For example, given Haematoloechus floedae's life cycle and the phylogenetically conservative portions of that cycle (e.g., Fig. 1), we could have predicted that the parasite would back colonize leopard frogs in Costa Rica and thus represented a potential health threat to those amphibians. In the future, bullfrogs imported into any country with leopard frog populations must minimally be certified free of both lung flukes and chytrid fungus. In other words, we must move beyond retrodiction, as in the case study discussed herein, to prediction (Hoberg, 1997; Brooks and Hoberg, 2000; Hoberg et al., 2003; Brooks et al., 2004).
We thus wholeheartedly concur with Daszak et al. (2004), calling for the formation of multidisciplinary groups of scientists focused on "solution–oriented" approaches, and propose that experts in the integration of phylogenetic and ecological information be included in those groups. We have the tools and the personnel to move from being less uninformed–reactive to more informed–proactive. Why are we waiting?
Literature cited
Altizer, S., D. Harvell, and E. Friedle. 2003. Rapid evolutionary dynamics and disease threats to biodiversity. Trends in Ecology and Evolution 18: 589–596. [ Links ]
Artiss, T. 2004. Phylogeography of a facultatively migratory dragonfly, Libellula quadrimaculata (Odonata: Anisoptera). Hydrobiologia 515: 225–234. [ Links ]
Audy, J.R. 1958. The localization of disease with special reference to zoonoses. Transactions of the Royal Society of Tropical Medicine and Hygiene 52: 309–328. [ Links ]
Blaustein, A. R., and J. M. Kiesecker. 2002. Complexity in conservation: lessons from the global decline of amphibian populations. Ecology Letters 5: 597–608. [ Links ]
Bradley, C. A., and S. Altizer. 2005. Parasites hinder monarch butterfly flight: implications for disease spread in migratory hosts. Ecology Letters 8: 290–300. [ Links ]
Brooks, D. R. 2003. Lessons from a quiet classic. Journal of Parasitology 89 (Suppl): 878–885. [ Links ]
Brooks, D. R., and A. L. Ferrao. 2005. The historical biogeography of coevolution: emerging infectious diseases are evolutionary accidents waiting to happen. Journal of Biogeography 32: 1291–1299. [ Links ]
Brooks, D. R., and E. P. Hoberg. 2000. Triage for the biosphere: the need and rationale for taxonomic inventories and phylogenetic studies of parasites. Comparative Parasitology 67: 1–25. [ Links ]
Brooks, D. R., and E. P. Hoberg. 2001. Parasite systematics in the 21st century: Opportunities and obstacles. Trends in Parasitology 17: 273–275. [ Links ]
Brooks, D.R. and E.P. Hoberg. 2006. Systematics and emerging infectious diseases: from management to solution. Journal of Parasitology 92: 426–429. [ Links ]
Brooks, D. R., and D. A. McLennan 1993. Parascript: Parasites and the language of evolution. Smithsonian Institution University Press, Washington, D. C. 429 p. [ Links ]
Brooks, D. R., and D. A. McLennan 2002. The Nature of Diversity: An Evolutionary Voyage of Discovery. University of Chicago Press, Chicago. 668 p. [ Links ]
Brooks, T., G. A. B. da Fonseca, and A. S. L. Rodrigues. 2004. Species, data, and conservation planning. Conservation Biology 18: 1682–1688. [ Links ]
Cain, G. D., and J. A. French. 1975. Effects of parasitism by the lung fluke, Haematoloechus medioplexus, on lung fatty acid and sterol composition in the bullfrog, Rana catesbiana. International Journal for Parasitology 5: 159–164. [ Links ]
Cook, J.A., E.P. Hoberg, A. Koehler, H. Henttonen, L. Wickström, V. Haukisalmi, K. Galbreath, F. Chernyavski, N. Dokuchaev, A. Lahsuhtkin, S.O. Macdonald, A. Hope, E. Waltari, A. Runck, A. Veitch, R. Popko, E. Jenkins, S. Kutz and R. Eckerlin. 2005. Beringia: intercontinental exchange and diversification of high latitude mammals and their parasites during the Pliocene and Quaternary. Mammal Study 30: S33–S44. [ Links ]
Cotterill, F. P. D., and J. M. Dangerfield. 1997. The state of biological knowledge. Trends in Ecology and Evolution 12: 206. [ Links ]
Daszak, P., L. Berger, A. A. Cunningham, A. D. Hyatt, D. E. Green, and R. Speare. 1999. Emerging infectious diseases and amphibian population declines. Emerging Infectious Diseases 5: 735–748. [ Links ]
Daszak, P., A. A. Cunningham, and A. D. Hyatt. 2000. Emerging infectious diseases of wildlife – threats to biodiversity and human health. Science 287: 443–449. [ Links ]
Daszak, P., A. A. Cunningham, and A. D. Hyatt. 2003. Infectious disease and amphibian population declines. Diversity and Distributions 9: 141–150. [ Links ]
Daszak, P., G. M. Tabor, A. M. Kilpatrick, J. Epstein, and R. Plowright. 2004. Conservation medicine and a new agenda for emerging diseases. Annals of the New York Academy of Sciences 1026: 1–11. [ Links ]
Dobson, A. 2004. Population dynamics of pathogens with multiple host species. American Naturalist 164: S64–S78. [ Links ]
Dronen, N. O. 1975. Life–cycle of Haematoloechus coloradensis Cort 1915 (Digenea, Plagiorchiidae), with emphasis on host susceptibility to infection. Journal of Parasitology 61: 657–660. [ Links ]
Elton, C.S. 1958. The Ecology of Invasions by Animals and Plants. Methuen and Company, Ltd., London. 181 p. [ Links ]
Epstein, P. R., E. Chivian, and K. Frith. 2003. Emerging diseases threaten conservation. Environmental Health Perspectives 111: 506–507. [ Links ]
Fayer, R. 2000. Global change and emerging infectious diseases. Journal of Parasitology 86: 1174–1181. [ Links ]
Futuyma, D. J., M. C. McKeese, and D. J. Funk. 1995. Genetic constraints on macroevolution: the evolution of host affiliation in the leaf–beetle genus Ophraella. Evolution 49: 797–809. [ Links ]
Hanselmann, R., A. Rodríguez, M. Lampo, L. Fajardo–Ramos, A. A. Aguirre, A. M. Kilpatrick, J. P. Rodríguez, and P. Daszak. 2004. Presence of an emerging pathogen of amphibians in introduced bullfrogs Rana catesbeiana in Venezuela. Biological Conservation 120: 115–119. [ Links ]
Harvell, C. D., C. E. Mitchell, J. R. Ward, S. Altizer, A. P. Dobson, R. S. Ostfeld, and M. D. Samuel. 2002. Ecology – climate warming and disease risks for terrestrial and marine biota. Science 296: 2158–2162. [ Links ]
Hoberg, E. P. 1997. Parasite biodiversity and emerging pathogens: A role for systematics in limiting impacts on genetic resources. In Global genetic resources: Access, ownership and intellectual property rights. K. E. Hoagland, and A. Y. Rossman. (eds.). Association of Systematic Collections., Washington, D. C., p. 71–83. [ Links ]
Hoberg, E.P. 2005. Coevolution in marine systems. In Marine Parasitology. K. Rohde (ed). CSIRO Publishing, Collingwood, Australia. p. 327–339. [ Links ]
Hoberg, E.P., A. Jones, R.L. Rausch, K.S. Eom, and S.L. Gardner. 2000. A phylogenetic hypothesis for species of the genus Taenia (Eucestoda: Taeniidae). Journal of Parasitology 86: 89–98. [ Links ]
Hoberg, E.P., S.J. Kutz, K.E. Galbreath, and J. Cook. 2003. Arctic biodiversity: From discovery to faunal baselines–revealing the history of a dynamic ecosystem. Journal of Parasitology 89 (Suppl): S84–S95. [ Links ]
Horwitz, P., and B. A. Wilcox. 2005. Parasites, ecosystems and sustainability: an ecological and complex systems perspective. International Journal for Parasitology 35: 725–732. [ Links ]
Janz, N., and S. Nylin. 1998. Butterflies and plants: a phylogenetic study. Evolution 52: 486–502. [ Links ]
Janz, N., S. Nylin, and K. Bybom. 2001. Evolutionary dynamics of host plant specialization: a case study. Evolution 55: 783–796. [ Links ]
Janzen, D. H. 1985. On ecological fitting. Oikos 45: 308–310. [ Links ]
Jensen, T., M. van de Bildt, H. H. Dietz, T. H. Andersen, A. S. Hammer, T. Kuiken, and A. Osterhaus. 2002. Another phocine distemper outbreak in Europe. Science 297: 209. [ Links ]
Kiesecker, J. M., L. K. Belden, K. Shea, and M. J. Rubbo. 2004. Amphibian decline and emerging disease. American Scientist 92: 138–147. [ Links ]
Krull, W. H. 1933. Studies of on the life history of a frog lung fluke, Haematoloechus complexus (Seely, 1906) Krull, n. comb. Zeitschrift für Parasitenkunde 6: 192–206. [ Links ]
Kutz, S. J., E. P. Hoberg, J. Nagy, L. Polley, and B. Elkin. 2004. "Emerging" parasitic infections in arctic ungulates. Integrative and Comparative Biology 44: 109–118. [ Links ]
León–Règagnon, V. 2003. Incorporating morphological and molecular data in biodiversity inventories. Parasites of leopard frogs. Journal of Parasitology 89 (Suppl.): 141–148 [ Links ]
León–Règagnon, V., and D. R. Brooks. 2003. Molecular phylogeny of Haematoloechus Looss, 1899 (Digenea: Plagiorchiidae), with emphasis on North American species. Journal of Parasitology 89: 1206–1211. [ Links ]
León–Règagnon, V., S. Guillén–Hernández, and M. A. Arizmendi–Espinosa. 2005. Intraspecific variation of Haematoleochus floedae Harwood, 1932 (Digenea: Plagiorchiidae), from Rana spp. in North and Central America. Journal of Parasitology 91: 915–921. [ Links ]
Louda, S. M., A. E. Arnett, T. A. Rand, and F. L. Russell. 2003. Invasiveness of some biological control insects challenges adequacy of ecological risk assessment and regulation. Conservation Biology 17: 1–11. [ Links ]
Louda, S. M., and P. Stiling. 2004. The double–edged sword of biological control in conservation and restoration. Conservation Biology 18: 50–53. [ Links ]
Lozano, G. A. 1994. Carotenoids, parasites, and sexual selection. Oikos 70: 309–311. [ Links ]
Mazzoni, R., A. A. Cunningham, P. Daszak, A. Apolo, E. Perdomo, and G. Speranza. 2003. Emerging pathogen of wild amphibians in frogs (Rana catesbeiana) farmed for international trade. Emerging Infectious Diseases 9: 995–998. [ Links ]
Morgan, J. A. T., R. J. DeJong, Y. Jung, K. Khallaayoune, S. Kock, G. M. Mkoji, and E. S. Loker. 2002. A phylogeny of planorbid snails, with implications for the evolution of Schistosoma parasites. Molecular Phylogenetics and Evolution 25: 477–488. [ Links ]
Nylin, S., A. Bergström, and N. Janz. 2000. Butterfly host plant choice in the face of possible confusion. Journal of Insect Behaviour 13: 469–482. [ Links ]
Nylin, S., and N. Janz. 1999. Ecology and evolution of host plant range: butterflies as a model group. In H. Olff, V. K. Brown, and R. H. Drent (eds.). Herbivores: Between plants and predators. Blackwell, Oxford, p. 31–54. [ Links ]
Patz, J., T. K. Graczyk, N. Geller, and A. Y. Yitter. 2000. Effects of environmental change on emerging parasitic diseases. International Journal for Parasitology 30: 1395–1405. [ Links ]
Pearman, P. B., and W. J. Garner. 2005. Susceptibility of Italian agile frog populations to an emerging strain of Ranavirus parallels population genetic diversity. Ecology Letters 8: 401–408. [ Links ]
Perlman, S. J. and J. Jaenike. 2003. Evolution of multiple components of virulence in Drosophila–nematode associations. Evolution 57: 1543–1551 [ Links ]
Pounds, J. A. 2001. Climate and amphibian declines. Nature 410: 639–640. [ Links ]
Pounds, J. A., M. P. L. Fogden, and J. H. Campbell. 1999. Biological response to climate change on a tropical mountain. Nature 398: 611–615. [ Links ]
Prenter, J., C. MacNeil, J. T. A. Dick, and A. M. Dunn. 2004. Roles of parasites in animal invasions. Trends in Ecology and Evolution 19: 385–390. [ Links ]
Rausch, R.L. 1972. Tropical problems in the Arctic: infectious and parasitic diseases, a common denominator. Industry and Tropical Health 7: 63–70. [ Links ]
Russell, R. W., M. L. May, K. L. Soltesz, and J. W. Fitzpatrick. 1998. Massive swarm migrations of dragonflies (Odonata) in eastern North America. American Midland Naturalist 140: 325–342. [ Links ]
Shields, J. D. 1987. Pathology and mortality of the lung fluke Hematolechus longiplexus (Trematoda) in Rana catesbeiana. Journal of Parasitology 73: 1005–1013. [ Links ]
Snyder, S. D., and J. Janovy, J. 1994. Second intermediate host specificity of Haematoloechus complexus and Haematoloechus medioplexus (Digenea: Haematoloechidae). Journal of Parasitology 80: 1052–1055. [ Links ]
Snyder, S. D., and J. Janovy, J. 1996. Behavioral basis of second intermediate host specificity among four species of Haematoloechus (Digenea: Haematoloechidae). Journal of Parasitology 82: 94–99. [ Links ]
Torchin, M. E., K. D. Lafferty, A. P. Dobson, V. J. McKenzie, and A. M. Kuris. 2003. Introduced species and their missing parasites. Nature 421: 628–630. [ Links ]
Vrba, E. S., and S. J. Gould. 1986. The hierarchical expansion of sorting and selection: Sorting and selection cannot be equated. Paleobiology 12: 217–228. [ Links ]
Wetzel, E. J., and G. W. Esch. 1996. Influence of odonate intermediate host ecology on the infection dynamics of Halipegus spp, Haematoloechus longiplexus, and Haematoloechus complexus (Trematoda: Digenea). Journal of the Helminthological Society of Washington 63: 1–7. [ Links ]
Wilson, E. O. 1988. The current state of biological diversity. In E. O. Wilson (ed.). Biodiversity. National Academy of Science Press, Washington, D. C., p. 1–18. [ Links ]
Woolhouse, M. E. J. 2002. Population biology of emerging and re–emerging pathogens. Trends in Microbiology 10 (Suppl.): S3–S7. [ Links ]
Woolhouse, M. E. J., L. H. Taylor, and D. T. Haydon. 2001. Population biology of multihost pathogens. Science 292: 1109–1112. [ Links ]














