Servicios Personalizados
Revista
Articulo
Indicadores
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de biodiversidad
versión On-line ISSN 2007-8706versión impresa ISSN 1870-3453
Rev. Mex. Biodiv. vol.77 no.2 México dic. 2006
Taxonomía y sistemática
Contribution to the study of the mycobiota present in the natural habitats of Histoplasma capsulatum: an integrative study in Guerrero, Mexico
Contribución al conocimiento de la micobiota presente en los hábitats naturales de Histoplasma capsulatum: un estudio integral en Guerrero, México
Miguel Ulloa1*, Patricia Lappe1, Samuel Aguilar1, Houng Park2, Amelia Pérez–Mejía3, Conchita Toriello3, and Maria Lucia Taylor3
1 Departamento de Botánica, Instituto de Biología, Universidad Nacional Autónoma de México, México D.F. 04510, México.
2 American Type Culture Collection (ATCC), 10801 University Blvd, Manassas, VA 20110–2209, USA.
3 Departamento de Microbiología y Parasitología, Facultad de Medicina, Universidad Nacional Autónoma de México, México D. F. 04510, México.
*Correspondent:
mulloa@ibiologia.unam.mx
Recibido: 30 enero 2006
Aceptado: 26 junio 2006
Abstract
The mycobiota present in natural habitats of Histoplasma capsulatum was determined in samples of bat guano, poultry droppings, and intestinal contents of bats. The following fungi were isolated: 1) from bat guano: the ascomycetes Aphanoascus fulvescens, Gymnascella citrina, Gymnoascus dankaliensis, and Chaetomidium fimeti; the mitosporic fungi Aspergillus flavo–furcatis, A. terreus, A. terreus var. aureus, Penicillium spp., Malbranchea aurantiaca, and Sporothrix sp.; and the yeasts Candida catenulata, C. ciferrii, C. famata var. flareri, C. guilliermondii var. guilliermondii, and Rhodotorula spp. 2) from poultry droppings: the coelomycete Phoma sp.; and the yeasts C. albicans, C. catenulata, C. ciferrii, C. famata var. flareri, C. tropicalis, Cryptococcus albidus, Trichosporon moniliiforme, and Trichosporon spp. 3) from the intestinal contents of insectivorous, hematophagous, nectarivorous, and frugivorous bats: Ch. fimeti; the mitosporic fungi Aspergillus candidus, A. flavo–furcatis, A. sulphureus, A. sydowii, A. terreus, A. versicolor, Aspergillus sp., M. aurantiaca, Gliomastix murorum, and Scopulariopsis sp.; and C. famata var. flareri, C. lipolytica, Cr. albidus, and Trichosporon spp. Most of the species found are first records for these substrata and environments in Mexico. The coexistence with H. capsulatum was demonstrated by high specific antibody titers in ELISA serological method, using sera from BALB/c mice previously inoculated with the supernatant of the different samples studied.
Key words: mycobiota, Histoplasma capsulatum microenvironments, Guerrero, Mexico, Elisa, anti–H.capsulatum antibodies titres.
Resumen
Se determinó la micobiota presente en diferentes hábitats naturales de Histoplasma capsulatum, como guano de murciélago, excretas de aves de corral, y contenido intestinal de murciélagos. Se aislaron: 1) de guano: los ascomicetes Aphanoascus fulvescens, Gymnascella citrina, Gymnoascus dankaliensis, y Chaetomidium fimeti; los hongos mitospóricos Aspergillus flavo–furcatis, A. terreus, A. terreus var. aureus, Penicillium spp., Malbranchea aurantiaca, y Sporothrix sp.; y las levaduras Candida catenulata, C. ciferrii, C. famata var. flareri, C. guilliermondii var. guilliermondii, y Rhodotorula spp. 2) de excretas: el celomicete Phoma sp.; y las levaduras C. albicans, C. catenulata, C. ciferrii, C. famata var. flareri, C. tropicalis, Cryptococcus albidus, Trichosporon moniliiforme, and Trichosporon spp. 3) del contenido intestinal de murciélagos insectívoros, hematófagos, nectarívoros, y frugívoros : Ch. fimeti; Aspergillus candidus, A. flavo–furcatis, A. sulphureus, A. sydowii, A. terreus, A. versicolor, Aspergillus sp., M. aurantiaca, Gliomastix murorum, Scopulariopsis sp.; y C. famata var. flareri, C. lipolytica, Cr. albidus, y Trichosporon spp. La mayoría de las especies son primeros registros para estos sustratos y ambientes en México. Su coexistencia con H. capsulatum se demostró en la prueba serológica de ELISA, por la presencia de altos títulos de anticuerpos específicos en el suero de ratones BALB/c previamente inoculados con el sobrenadante de las muestras estudiadas.
Palabras clave: micobiota, hábitats de Histoplasma capsulatum, Guerrero, México, Elisa, títulos de anticuerpos anti–H. capsulatum.
Introduction
The causative agent of the deep mycosis histoplasmosis, Histoplasma capsulatum var. capsulatum, was first isolated by Emmons (1949). The description of its saprobic phase was a landmark for epidemiological and biological research concerning the ecological niche of this fungus and the environmental factors that favor its growth and survival (Zeidberg et al., 1955; Ajello, 1956; Goodman and Larsh, 1967; Mahvi, 1970; Domsh et al.,1993; Taylor et al., 1994).This fungus is a non–competitive soil saprobe whose development and survival depends on several conditions, such as its intrinsic characteristics (i.e., sporulation, growth rate) and its interaction with different factors that determine its ecological niches (Goodman and Larsh, 1967; Mahvi, 1970; Domsh et al., 1993; Taylor et al., 1994). Histoplasma capsulatum is able to grow in different soil types, from sandy loams to hard clays (Mahvi, 1970), whose chemical composition and physical characteristics influence the soil biota (Stotzky and Post, 1967). However, the competitive and non–competitive relationships necessary for the fungus establishment in nature have not been well described yet.
From the soil surface through the first centimeters, an abundant microbiota is found, represented by bacteria, actinobacteria, and fungi. To the advantage of H. capsulatum and other non–competitive saprobes, soil microorganisms are active organic matter decomposers, favouring availability of nutrient sources, and contributing to the maintenance of soil structure, aeration, and water content (Donahue et al., 1981). Bacteria and fungi actively grow in humid climates, at temperatures averaging from 25 to 35°C, and in neutral pH soils. Some fungi may resist more acidic (pH < 5.5) conditions and dry climates. Filamentous fungi and yeasts constitute the soil mycobiota, and their presence in this habitat depends on the species characteristics (competitive and reproductive abilities), as well as their interaction with abiotic and biotic factors (Phaff et al., 1966; Lachance and Starmer, 1998). Goodman and Larsh (1967) established that humidity and environmental temperature affect the survival of H. capsulatum in soil; Berliner and Biundo (1973) observed that darkness favors sporulation. Oxygen availability and pH are some soil factors that influence growth and morphology of this fungus. In particular, the mycelial phase requires Ca++ (Batanghari and Goldman, 1997) provided by lixiviation of calcareous rocks (Donahue et al., 1981).
Histoplasmosis is widely distributed in Mexico, and is especially common in certain geographic regions, such as the State of Guerrero. The disease is endemic and the infection is frequently associated with enclosed spaces such as caves and mines. Taylor and others (Taylor et al., 1994; Taylor et al., 1996; Tay lor et al., 1997a; Taylor et al., 1997b; Taylor et al., 1999) have made relevant contributions to the knowledge of this mycosis in this state, in the areas of Quechultenango, Olinalá, and Coyuca de Benítez, where H. capsulatum strains were isolated from guano, as well as from insectivorous bats, such as Mormoops megalophylla, Myotis californicus, and Pteronotus parnellii.
The environmental conditions, the phenotypic and the genotypic diversities of H. capsulatum strains isolated in Mexico have been described elsewhere (Taylor et al., 1997b; Reyes–Montes et al., 1998; Reyes–Montes et al., 1999; Taylor et al., 2000), but so far the mycobiota associated with the substrata from which this fungus was isolated has been scarcely studied. This research was aimed at identifying the mycobiota present in the natural substrata in which H. capsulatum has been detected or isolated from.
Material and Methods
Samples. The mycobiota present in natural habitats of H. capsulatum was isolated from guano and intestinal contents of bats, as well as poultry droppings, collected at different sites of the State of Guerrero. a) Bat guano: from the galleries El Beso Milenario and Ramal del Infierno, of the Juxtlahuaca cave, from the Chichicastle cave, and from the gallery El Sótano of the Zinacantla cave, as well as from soil fertilized with bat guano collected near the Colotlipa village (Quechultenango municipality); from the Diablo cave, Coapala cave, Chiautzingo cave, and La Mina cave (Olinalá municipality), and from the orchard La Chulada (Coyuca de Benítez municipality) (Fig. 1). All bat guano samples were obtained from the surface to a 10–cm depth. b) Poultry droppings collected from different orchards in the Olinalá village (Olinalá municipality): 11 samples with gamecock, and 1 with chicken droppings, c) Intestinal contents from the following bat species: Pteronotus parnellii, P. dayvi, Myotis californicus, Mormoops megalophylla (insectivorous), Desmodus rotundus (hematophagous), and Glossophaga soricina (nectarivorous/pollinivorous), captured in the same caves mentioned above, and Artibeus hirsutus (frugivorous), captured in the orchard La Chulada. During sampling the environmental temperature and relative humidity were measured, and the physical and chemical characteristics of the different samples were determined. Chemical analyses were performed by BSc Abel Ibáñez H., Laboratory of Edaphology, Facultad de Ciencias, UNAM. All bat species were identified as described in Chávez–Tapia et al. (1998), as well as in Taylor et al. (1994; 1999).

Fungal isolation from bat guano and poultry dropping samples. The methodology for the isolation of the mycobiota was the same as that described in Taylor et al. (1994) for H. capsulatum, although it also allows the isolation of other filamentous fungi and yeasts. Briefly, 1 g from each sample was mixed with 10 mL phosphate–saline buffer, pH 7.2, supplemented with 50 µL streptomycin and 100 U/mL penicillin (Lakeside Laboratory, Mexico City). The supernatant was centrifuged at 300 x g at 4°C, 10 min. Supernatant (0.1 mL) was plated on duplicate mycobiotic (MA) agar (Bioxon, Mexico City) and brain heart infusion (BHI) agar (Bioxon) plates, supplemented with 1% glucose, 0.1% L–cysteine, 0.05% cycloheximide, and 0.005% cloramphenicol (Sigma Chemical Co., St. Louis, MO). Additional duplicate plates were added with 0.02% of rose Bengal (Sigma). Plates were incubated six weeks at 28°C (MA), and at 37°C (BHI). All fungi different from H. capsulatum were transferred to Sabouraud agar (SA) (Merck) for colony purification, and further identification. Fungal isolation from bat intestinal contents. The methodology was previously described in Taylor et al. (1994) for H. capsulatum. Briefly, bats were killed within 1–2 days after capture, and intestinal samples were obtained. These were washed and homogenized with 5 mL of phosphate–saline buffer, pH 7.2, supplemented with streptomycin and penicillin, as mentioned above. The homogenate was centrifuged and processed as previously described.
Indirect evidence of H. capsulatum presence in bat guano and poultry droppings. This was performed by the detection of anti–H. capsulatum antibodies in BALB/c mice sera previously inoculated with the samples. This evidence was determined by indirect ELISA (Voller et al., 1979). BALB/c mice were inoculated intraperitoneally with 0.5 mL of each supernatant sample prepared as mentioned above. Mice were bled 25 days after and sera were tested by ELISA. Nunc plates with 96 wells (Nunc, Roskilde, Denmark) containing each 100 µg protein of histoplamin/100 µL of carbonate–bicarbonate buffer, pH 9.6, previously standardized in the laboratory (Toriello et al., 1991), were used. One hundred µL of serum samples to be tested were added per well in PBS/Tween dilutions. Afterwards, 100 µL of anti–mouse polyvalent immunoglobulin peroxidase–conjugate (Sigma) at 1/1000 dilution was used; and 100 µL of the substrate solution, o–phenylenediamine (Sigma) prepared at a concentration of 40 mg/100 mL with 40 µL of 30% hydrogen peroxide in citrate phosphate buffer, 0.05 M, pH 5.0, was added. Incubation was performed in darkness at room temperature during 20 min. The reaction was stopped by adding 50 µL of 2.5 M sulphuric acid to each well. Optical density (OD) values were read at 492 nm in a Multiskan MS Labsystems Apparatus (Helsinki, Finland). Serum titers > 1:640 with histoplasmin antigen provide indirect evidence of H. capsulatum in tested samples, based on previous serum titers cut–off from bat guano samples with positive H. capsulatum isolation (Taylor ML, personal communication).
Filamentous fungi identification. This was accomplished with the keys and descriptions by Domsh et al. (1993) and de Hoog and Guarro (1995) for Acremonium, Aphanoascus, Chrysosporium, Gymnascella, Gymnoascus, Chaetomidium, Gliomastix, Scopulariopsis, and Sporothrix; Raper and Fennell (1977) for Aspergillus species; Sigler and Carmichael (1976) and Currah (1985) for Malbranchea; Pitt (1985) for Penicillium; and Sutton (1980) for Phoma.
Yeast identification. This was done following the methodology of Yarrow (1998), and the keys and descriptions by Kurtzman and Fell (1998), Barnett et al. (2000), and the Centraalbureau voor Schimmelcultures (CBS) Database (2005). Molecular methodologies were only used for those isolates that were not clearly identified with morpho–physiological characteristics. Yeast protoplasts were prepared according to the method of Su and Meyer (1991), and genomic DNA's were isolated following a modification of Meyer and Phaff (1969). The DNA was used as template for PCR amplification and sequencing of the large subunit D1/D2 (LrDNA) region using the universal forward primer F63 (5' –GCATATCAATAAGCGGAGGAAAAG–3'), and the universal reverse primer R635 (5'–GGTCCGTGTTTCAAGACG–3'). PCR reaction was done in a 24–µL reaction volume containing: 10 mM Tris–HCl pH 8.4, 1 mM KCl, 1 mM MgCl2 0.02% gelatin, 1.25 mM deoxynucleoside triphosphates (dNTPs), 10 pmol of each primer, 10 ng genomic DNA, and 0.5 unit Taq DNA polymerase (Perkin–Elmer Cetus, Norwalk, CT). The reaction mixture was overlain with approximately 15 µL of mineral oil. Amplification, in a Perkin–Elmer thermocycler, was programmed for a 10 min initial activation at 95°C, followed by 30 cycles of: 60 s of denaturing at 95°C, 60 s of annealing at 50°C, 90 s of extension at 72°C, and a 7 min final extension at 72°C. After PCR reaction, the oil layer was removed from each tube by extraction with 60 µL of chloroform. The PCR products were cleaned with QIAEX II gel extraction kits (Qiagen Inc., Chatsworth, CA) according to the manufacturer's procedures. An agarose gel electrophoresis was performed to confirm the synthesis of amplicons. The eluted DNA was used as a template for automated sequencing (Applied Biosystem DNA sequencer, Foster City, CA), following the dideoxy chain termination reaction (Sanger et al., 1977). DNA sequences were edited and aligned with the BioEdit Program version 5.0.6 (Hall, 2001), and compared to sequences available in the GenBank (Altschul et al., 1997); the sequence similarity was expressed as percentage. All sequences obtained were deposited in the GenBank, and the accession numbers are indicated in Results and Discussion.
Results and Discussion
Physical and chemical characteristics of the different samples studied. Physical and chemical characteristics of bat guano, and environmental and sample temperatures, as well as some chemical analyses of poultry dropping samples, are given in Table 1. Environmental temperatures ranged from 25.0 to 32.2°C, and high relative humidities, from 75.2 to 100%, were normally registered in the different enclosed spaces where bat guano was collected. Sample temperatures varied from 24.1 to 30.2°C. The chemical analyses showed acidic (3.7–4.6) to basic (8.0) pH. Organic matter varied from 0.62 to 78.1%. High concentrations of Ca++ (155.7–280.9 mEq/100g) were detected in the Juxtlahuaca cave (El Beso Milenario and Ramal del Infierno galleries), and low concentrations (10.9–27.5 mEq/100g) in La Mina and Coapala caves, and in poultry droppings. Variable concentrations of Mg++ were regularly detected in most samples (32.9–88.6 mEq/100g), with the highest (104.4 mEq/100g) and the lowest (5.5 mEq/100g) in La Mina and Coapala caves, respectively.
Mycobiota present in H. capsulatum habitats: bat guano. The filamentous fungi and yeasts isolated from bat guano collected at different localities of the State of Guerrero are listed in Table 2; all of these species are first records in Mexico for these substrata and environments, except Penicillium sp. which was previously isolated by Hoffman et al. (1986) from the Aguacachil grotto, in Taxco, Guerrero. Acremonium sp. was isolated from a 2 cm guano depth. This genus has been described as entomopathogenic (Samson et al., 1988), and as an agent of human mycetoma (de Hoog and Guarro, 1995).
Four species of Onygenales, Aphanoascus fulvescens, Gymnascella citrina, Gymnoascus dankaliensis, and the mitosporic anamorph Malbranchea aurantiaca, were isolated from surface to 4 cm sample depth (Figs. 2–4) (2, 3, 4). The genus Chrysosporium, which was also isolated from a 4 cm guano depth, is a heterogeneous assemblage of various species. This genus, together with Renispora, should not be misidentified with the macroconidia of Histoplasma mycelial phase. Antigenic tests, histopathologic studies, and dimorphic conversion can distinguish them without misinterpretation. Histoplasma was found in some samples of bat guano processed in this study, as reported previously (Taylor et al., 1994; Chávez–Tapia et al., 1998; Taylor et al., 1999). The Onygenales share the same pattern, regarding their ecology and pathogenicity. All are keratinolytic and are found associated with warmblooded animals and their products, such as skin, hair, and nails. Pathogenic Onygenaceae can affect humans mainly by inhalation of dry propagules. In healthy persons the symptoms induced by these fungi are generally mild and infection is self–limited in most cases. However, sometimes the infectious agent can persist within the host and be reactivated endogenously, particularly, when the immune system is altered. These fungi are associated to systemic infections, for example in patients with AIDS (de Hoog and Guarro, 1995).



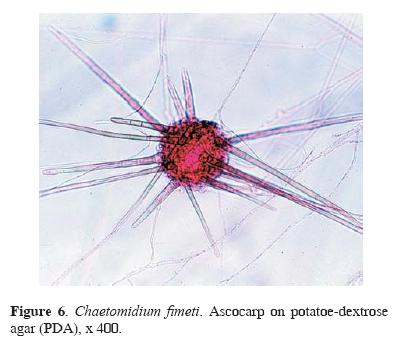
Four taxa of the genus Aspergillus, A. flavo–furcatis (Fig. 5), A. terreus, A. terreus var. aureus, and A. versicolor, were all isolated from surface to 4 cm sample depth. These species are common soil fungi, particularly, from tropical and subtropical geographic regions, where they grow on vegetable and animal residues. As other Aspergilli, these species may cause opportunistic infections in humans and animals (Raper and Fennell, 1977; de Hoog and Guarro, 1995). A cellulolytic fungus, Chaetomidium fimeti (Figs. 6, 7), was also identified from guano surface; its world distribution is not well known, although it has been recovered from arable soils in France, Russia, India, Japan, and USA, predominantly, between 0–5 cm soil depth, and from compost and burned timber, manure, plant detritus, leaves, paper, bird feathers, and nests (Domsch et al., 1993).


Two species of Penicillium were isolated from guano samples at 2 and 4 cm depth This genus includes some ubiquitous species (Pitt, 1985), others are mycotoxigenic, and some are opportunistic causing mycosis in humans and animals (de Hoog and Guarro, 1995). One nonidentified species of Sporothrix was also isolated from guano surface. This genus includes some species that are pathogenic to insects and humans (Samson et al., 1988; de Hoog and Guarro, 1995).
Of the filamentous fungi identified in the present study, some species have been previously isolated from bat guano collected in enclosed or open spaces. Orpurt (1964) isolated Acremonium vitis, Aspergillus versicolor and other Aspergilli, as well as different Penicillium species, from samples of the Eleutera Island cave, Bahamas. Other authors also reported the presence of Aspergillus and Penicillum species in guano samples collected in: an attic where a colony of bats (Eplesicus fuseus) lived (Brandesberg, 1968); in several caves from Mexico; southern, northern and southwestern Puerto Rico, including the Río Camuy system; and southern India (Hoffman et al., 1986; Carvajal–Zamora and Nieves–Rivera, 1998; Koilraj et al., 1999; Nieves–Rivera; 2003). Chrysosporium sp. was isolated from guano samples from an abandoned barn which had harbored a bat (Myotis velifer) nursery colony (Gaur and Lichtwardt, 1980), and C. indicum and C. tropicum from samples collected in bat habitats throughout Israel (Ajello et al., 1977). These findings suggest that some of the species identified in the present work belong to the natural mycobiota of bat guano either in cave or open environments.
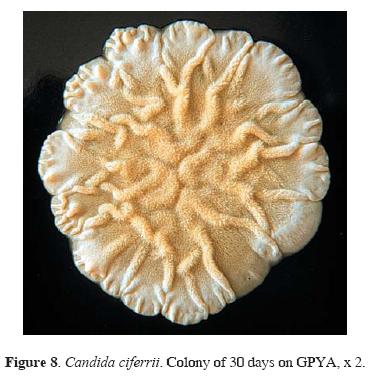
The different yeasts isolated from bat guano are listed alphabetically in Table 2. Candida ciferrii (Figs. 8, 9) was the most common yeast species, with 21 isolates obtained from surface to 10 cm depth guano, in 5 localities. This yeast is frequently considered as a soil inhabitant, although it has been recovered from vegetable and animal substrata (Kurtzman and Fell, 1998; Barnett et al., 2000). The second most common species, with 9 isolates from 4 localities, was C. famata var. flareri, that has been found in a variety of substrata (soil, tanning liquor, rice–vinegar–mash, spoiled sake, brine bath, beer, soy sauce, fruits, wine, and insects) and clinical samples (Nakase and Suzuki, 1985; Ellis, 1994; Kurtzman and Fell, 1998; Barnett et al., 2000). Candida guilliermondii var. guilliermondii was found in samples from surface to 4 cm depth, in 3 localities. This species has been isolated from air, soil, sediments, American elm frass, clinical samples, and insects and their feces, which explains its isolation from the studied samples (Kurtzman and Fell, 1998; Barnett et al., 2000). Candida catenulata (Fig. 10, 11) and Rhodotorula spp. were isolated in samples from 10 cm and from surface to 2 cm depth, respectively, in one locality. The former has been frequently found to be associated with different animal feces, soil, and water, while Rhodotorula spp. have a cosmopolitan distribution (Kurtzman and Fell, 1998; Barnett et al., 2000).



Few investigations have been devoted to study yeast ecology of caverns. Nevertheless, although present in low numbers, yeasts can be isolated from cave microenvironments. Among the yeast species isolated form guano in the present study, several Candida and Rhodotorula species were reported previously by Martini (1961, 1963), Orpurt (1964), and Vaughan–Martini et al. (2000) from caves in Italy and the Bahamas.
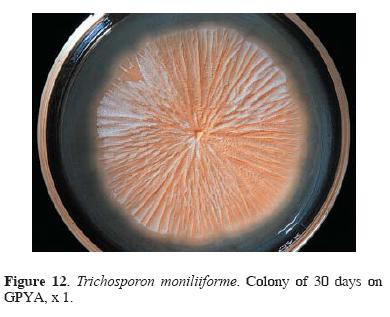
Mycobiota associated with H. capsulatum habitats: poultry droppings. Eleven poultry droppings samples were collected in Olinalá village. Filamentous fungi and yeasts isolates were only recovered from 9 (8 gamecock and 1 chicken) samples (Table 3). From gamecocks 18 isolates of C. catenulata, 2 of C. famata var. flareri, 5 of Cryptococcus albidus, 3 of Trichosporon spp., and 4 of T. moniliiforme (Fig. 12), were obtained.
All isolates of C. catenulata exhibited a wide variety of colonial morphologies and differed from the species description by Meyer et al. (1998) and Barnett et al. (2000), when identified by morpho–physiological characteristics. The strains isolated from bat guano differed in the non–assimilation of citric acid, whereas the ones isolated from gamecock droppings, besides the non–assimilation of citric acid, differed in their assimilation of ribose and inuline. The sequence of the large subunit D1/D2 (LrDNA) region of 4 isolates (2 from each substrate) showed a 100% similarity with C. catenulata (gb U45714), which is why all 4 isolates were identified as C. catenulata (gb AY288983, gb AY288984). This yeast has been frequently isolated from water, soil, chicken gut and clinical samples (Meyer et al., 1998; Barnett et al., 2000, CBS Database, 2005), which explains the 18 isolates obtained from gamecock droppings.
Most of the yeasts isolated from gamecock droppings are saprobes, although potentially pathogenic (de Hoog and Guarro, 1995; Kurtzman and Fell, 1998; Barnett et al., 2000). Cryptococcus albidus is a cosmopolitan yeast, frequently isolated from air, soil, fruits, and flowers, and is considered an opportunistic fungus (de Hoog and Guarro, 1995; Kurtzman and Fell,1998; Barnett et al., 2000). Trichosporon moniliiforme is a ubiquitous yeast restricted to natural sources such as soil, water, and bird and chicken droppings. Physiologically it cannot be distinguished easily from T. cutaneum, which is a human pathogen responsible for white piedra and other skin lesions, but this species is more sensitive to cycloheximide, and to elevated temperatures (de Hoog and Guarro, 1995; Kurtzman and Fell, 1998; Barnett et al., 2000; Gründer et al., 2004).
From the chicken dropping sample the filamentous fungus Phoma sp., which is soil borne (Domsch et al., 1993; Sutton, 1980), and the yeast species C. albicans, C. tropicalis, and C. ciferrii, were recovered. These yeasts are saprobes, with cosmopolitan distribution, and are frequently found as commensals in humans and animals. Candida albicans and C. tropicalis are opportunistic, and may cause from simple cutaneous infections to severe systemic mycoses, depending on the host's immune system status. Candida albicans has been recovered from different natural environments, such as soil, marine water, feces, leaves, flowers, mushrooms, and birds, and C. tropicalis from water, fruits, molasses, and baker yeast, and grows at temperatures as high as 42°C (de Hoog and Guarro, 1995; Ellis, 1994; Meyer et al., 1998; Barnett et al., 2000). Candida ciferrii has been frequently isolated from the gut of farm animals, mainly bovines (Kurtzman and Fell, 1998; Barnett et al., 2000). It is important to remark that Taylor et al. (1994) could only achieve the isolation of H. capsulatum (Table 3) from one out of 8 gamecock dropping samples.
Several fungal species identified in gamecock and chicken droppings have also been recovered from other bird droppings. Bransberg et al. (1969) reported Candida albicans, Candida sp., Trichosporon sp.,H. capsulatum and Phoma sp., in blackbird, starling, and grackle droppings from different roosts in Missouri. In pigeon droppings from rural and urban environments in Turin, a high number of opportunistic yeasts, C. albicans, C. tropicalis, Cr. albidus and Trichosporon spp. among others, were detected (Vidotto and Gallo, 1985; Gallo et al., 1989). In addition, in droppings from domestic parrots the same species mentioned above, as well as C. catenulata and C. famata, were also recovered (Mancianti et al., 2001). All these findings suggest that wild and domestic birds play a role as carriers for several Candida species and other opportunistic yeasts, which have been considered as transient mycobiota of the gastrointestinal tract.
Mycobiota from intestinal contents of bats captured in caves of the State of Guerrero. The filamentous fungi and yeasts isolated from intestinal contents of insectivorous (P. parnellii, Mormoops megalophylla, Myotis californicus), nectarivorous–pollinivorous (Glossophaga soricina), frugivorous (A. hirsutus) and hematophagous (D. rotundus), bats are listed in Table 4. Besides other Aspergillus species already cited, A. candidus (Fig. 13), A. sulphureus, and A. sydowii, were found; they may also be considered opportunistic fungi (Raper and Fennell, 1977). Chaetomidium fimeti and Malbranchea aurantiaca have already been commented on, when referring to the fungal species isolated from bat guano. Gliomastix murorum and Scopulariopsis sp. were also isolated; they are considered common soil saprobes (Domsch et al., 1993). Histoplasma capsulatum was only isolated from 2 adult insectivorous species, a female M. californicus, and a male P. parnelli, captured in El Beso Milenario (Taylor et al., 1994). However, H. capsulatum mammal infection acquired by inhalation of propagules from bat guano is irrespective of bat diet. Diseased bats could act as parasite dispersers by incorporating the fungus into new favorable environments, possibly through their carcasses once dead, or by their feces containing the fungal yeast phase that reach the gut lumen by a dissemination process in the infected bat (Taylor et al., 2005).

The frequency of H. capsulatum bat infection is related to small distances between guano deposits and the cave ceiling that increase the probability of inhaling the fungal infective propagules, and enhance the risk of man, bat, and other mammal infections (Taylor et al., 1999).
The yeast species found in bat intestinal contents have been already mentioned among the mycobiota isolated from bat guano and poultry droppings, with the exception of Candida lipolytica. All yeasts were isolated from insectivorous (M. megalophylla, P. davyi, P. parnellii) and nectarivorous–pollinivorous (G. soricina) bats, and in these cases are related to their feeding habits. From the first 3 bat species, only C. famata var. flareri was isolated; this yeast is commonly obtained from insects and fruits, which would explain its occurrence in bat intestinal contents. This variety has been recognized as the etiological agent of several pathological conditions of man. Another Candida species, C. chiropterorum, was isolated from the liver of M. megalophylla in Colombia (Grose and Marinkelle, 1968). However, it remains to be demonstrated if the relation of C. famata varflareri with the insectivorous bats is as saprobe or pathogen (de Hoog and Guarro, 1995; Kurtzman and Fell, 1998; Barnett et al., 2000).
Yeasts isolated from the bat G. soricina were C. famata var. flareri, C. lipolytica, Cr. albidus, and Trichosporon spp. The second one is a saprobe, which develops in high lipid content substrata, such as olives, vegetable fats, and milk. It has also been isolated from soil (Kurtzman and Fell, 1998; Barnett et al., 1990) and from the gummy secretions of Opuntia ficus–indica (prickly pear cacti) (Lappe P., personal communication), whose flowers and nectaries are visited and eaten by bats. Cryptococcus albidus is a cosmopolitan yeast that has also been recovered from fruits and nectaries of different plants, as well as from O. ficus–indica secretions (Lappe P., personal communication), and from pollinivorous bees (Kurtzman and Fell, 1998; Barnett et al., 2000). Probably, Cr. albidus may be found in the flower's mycobiota of different plants, from which G. soricina feeds.
Because the species of Trichosporon were not identified, it is not possible to explain its presence in the intestinal content of pollinivorous bats. This genus includes saprobic species with a cosmopolitan distribution, which have been isolated from soil; air; sea water and lakes; fruits and vegetables; tree exudates; deer, duck, swan and raccoon guts; human and animal feces; pigeon and chicken droppings. Other species are of clinical importance because they are opportunistic, and may be involved in localized, systemic and disseminated infections in immunocompromised patients, or are pathogenic species, particularly involved in superficial mycoses of man and other animals.
The isolation of saprobic and potential pathogenic yeasts from the intestinal content of bats without any clinical or histological pathology, suggests a commensal bat–fungus relationship (Mok et al., 1982).
Histoplasma capsulatum antibodies in different samples of guano and poultry droppings. Based on previous serum titers cut–off from bat guano samples with positive H. capsulatum isolation (Taylor ML, personal communication), the indirect coexistence of H. capsulatum with the mycobiota reported here was evidenced, in ELISA tests, by high titers, > 1:640, of anti–H. capsulatum antibodies detected in sera from mice previously inoculated with supernatants from the substrata (bat guano and poultry droppings) from which the mycobiota was isolated (Tables 2 and 3).
The filamentous fungi and yeast species listed in Tables 2 and 3 coexisted with H. capsulatum, with the exception of Acremonium sp., one Penicillium sp., and 2 C. ciferrii isolates; their corresponding supernatants had titers ranging from 1:20 to 1:40. The highest ELISA titers were found in 11 bat guano samples, which showed a titer of 1:20480. In 32 bat guano and 7 poultry dropping samples titers ranging from 1:640 to 1:10240 were detected. These data support the coexistence of H. capsulatum with the mycobiota isolated from the analyzed samples.
Cave environments offer ideal physical and chemical parameters (darkness, humidity, and constant temperature), as well as ideal nutritive substrata originated from animal droppings, mainly from bats, for the survival and growth of a diverse saprobic or pathogenic mycobiota, which affects the population dynamics of cave biota, and contribute to the decomposition of organic matter, making it available to the members of the cave community (Nieves–Rivera, 2003). A study of the yeast biota in three caverns in Italy suggests that the quantity and type of species comprising this biota are the result of outside pressures, including the influence of human and animal presence, imposed upon these delicate ecosystems (Vaughan–Martini et al., 2000).
In the present study the highest fungal species diversity was detected in the Juxtlahuaca cave, i.e., the gallery Ramal del Infierno (6 filamentous fungi and 4 yeast species) and the gallery El Beso Milenario (3 filamentous fungi and 3 yeast species). This mycobiota could be explained by the presence of ideal physical and chemical parameters, an average environmental temperature of 24–32°C, a relative humidity of 75–100%, and abundant bat guano deposits (> 20 cm to 1 m depth), which provide the necessary nutrients for fungal growth (Taylor et al., 1999).The acidic pH from 4.3 to 4.6 (Table 1), might have favored the development of some species. This acidic pH was associated with the high organic matter content, i.e., 39.6% in the Ramal del Infierno, and 30.1% in El Beso Milenario galleries (Table 1), in spite of the high Ca++ and Mg++ concentrations (Table 1), generally related to alkaline pH due to a carbonatation process (Duchaufour, 1984).
The distribution of the microorganisms was mainly located in the upper layer of the substrata, and only the yeasts C. catenulata, C. ciferrii, and C. famata var. flareri were recovered from 10 cm depth. All the mitosporic fungi isolated were present as spores or actively growing mycelium.
Isolation of H. capsulatum from the Ramal del Infierno and El Beso Milenario galleries was reported by Taylor et al. (1994). Climatic, biotic and abiotic conditions, and high Ca++ concentrations, that prevail in these ecological niches were adequate for the establishment and growth of the saprobic phase of this fungus, which coexists with the mycobiota found (Kwong–Chung and Bennett, 1992; Montagna et al., 2003). Histoplasma capsulatum isolation from nature is an unusual finding and, in general, a low frequency of isolation from bat guano has been reported by several authors (Menges et al., 1967; Bryles at al., 1969; Disalvo et al., 1970; Carvajal–Zamora, 1977; Ajello et al., 1977; McMurray and Russel, 1982; Nieves–Rivera, 2003), and could be explained by defi ciencies in the methods of fungus isolation from soil, described by Menges et al. (1967).
Candida ciferrii was the only isolate from La Mina and Coapala caves, and it is possible that environmental conditions, such as 31°C and 100% relative humidity, could influence the mycobiota development. In La Mina cave, the low organic matter content (0.62%), the alkaline pH (8.0), and the high concentrations of Mg++ and Ca++, which indicates that substrata come from another type of rock, different from limestone, were probably inhibitory for fungal growth, since these organisms require organic matter, and small numbers of species tolerate a pH of 8.0. In Coapala cave, where bat guano deposits were 6–20 cm depth, the pH sample was 3.8, and the organic matter was 14.2%, 7 isolates of C. ciferrii were found, and no other fungal species were recovered. The presence of C. ciferrii in both environments could be explained by its tolerance to a wide range of pH (3.5–10) and variable organic matter contents (CBS Database, 2005).
In gamecock and chicken dropping samples high organic matter contents (78.1 and 64.7%, respectively) and low ion concentrations were detected. These are rich nutrient substrata that support the development of a diverse mycobiota, predominated by saprobic yeasts, which are frequently found as commensals in humans and animals. The pathogen H. capsulatum was only isolated from a gamecock sample (Taylor et al., 1994).
The presence of H. capsulatum in bat guano and poultry dropping samples was unequivocally demonstrated by the high ELISA test, using a routinely specific H.capsulatum histoplamin antigen. High ELISA titers (> 1.640 to 1:20480) found in 41 bat samples and 7 poultry dropping samples, not necessarily indicating active infection. Sometimes the host eliminates the pathogen effi ciently, and as a consequence the fungal cells are scarcely isolated. During an acute infection high antibody titers could remain for a long time (> 6 months), although the pathogen had been eliminated. It is also important to emphasize that each host resolves the infection, according to a particular interaction with the pathogen, and depending on the type of this interaction, the infection has a favorable or unfavorable outcome.
As mentioned before, the caves and open spaces, which are habitats for H. capsulatum, may be also considered as the natural environments for several pathogenic, opportunistic, and saprobic fungal species that shear similar ecosystems.
It is important to remark that by the methodology used, only filamentous fungi and yeasts resistant to 0.05% cycloheximide were isolated. This concentration inhibits all sensitive and moderately resistant species, which could be present in the studied substrata, and did not produced a critical effect in H. capsulatum growth, which was the main purpose of the present research.
Most of the fungal species identified in the present study are first reports for these substrata and ecological niches in Mexico. It is probable that, among the components of the complex mycobiota of bat guano, bat intestinal contents, or poultry droppings, there are interesting biological phenomena, which remain to be investigated, such as antagonism and synergism, that could influence the biology of H. capsulatum in natural environments.
Massive inoculation with H. capsulatum propagules, as well as infections with other potential pathogenic filamentous and yeast fungi, such as those species detected in the present study, represent risks for people who spend a long time inside the cavern environments.
Acknowledgements
The authors thank Abel Ibáñez for the edaphological data of soil samples; Enrique Ortega Jiménez and Cayetano Morales Hernández for their help as cave guides; Lourdes Aguirre for the interpretation of the edaphological data; Sally Meyer for her laboratory facilities at Georgia State University; and Ingrid Mascher for editorial assistance.
Literature cited
Ajello, L. 1956. Soil as natural reservoir for human pathogenic fungi. Science 123: 876–879. [ Links ]
Ajello, L., E. S. Kuttin, A. M. Beemer, W. Kapla and A. Padhye. 1977. Occurrence of Histoplasma capsulatum Darling 1906 in Israel, with a review of the current status of histoplamosis in the Middle East. American Journal of Tropical Medicine and Hygiene 26: 140–147. [ Links ]
Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller and D. J. Lipman. 1997. Gapped BLAST and PSI–BLAST: a new generation of protein database search programs. Nucleic Acids Research 25: 3389–3402. [ Links ]
Barnett, J. A., R. W. Payne and D. Yarrow. 2000. Yeasts: Characteristics and Identification, 3rd ed. Cambridge University Press, Cambridge, 1139 p. [ Links ]
Batanghari, J. W. and W. E. Goldman. 1997. Calcium dependence and binding in cultures of Histoplasma capsulatum. Infection and Immunity 65: 5257–5261. [ Links ]
Berliner, M. D. and N. Biundo. 1973. Effects of continuous light and total darkness on cultures of Histoplasma capsulatum. Sabouraudia 11: 48–51. [ Links ]
Brandsberg, J. W. 1968. Fungi found in association with Histoplasma capsulatum in a natural contaminated site in Clarksburg, Maryland, U.S.A. Sabouradia 6: 246–254 [ Links ]
Bryles, M. C., G. C. Cozad and A. Robinson. 1969. Isolation of Histoplasma capsulatum from bats in Oklahoma. American Journal of Tropical Medicine and Hygiene 31: 399–400. [ Links ]
Carvajal–Zamora, J. R. 1977. Isolation of Histoplasma capsulatum from the air in the Aguas Buenas Caves, Aguas Buenas, Puerto Rico. Mycopathologia 60: 163–165. [ Links ]
Carvajal–Zamora, J. R. and A. Nieves–Rivera. 1998. Preliminary checklist of cave mycobiota of Puerto Rico with special reference to bat guano enriched soil hyphomycetes. Inoculum 49: 13. [ Links ]
CBS Centraalbureau voor Schimmelculture, Fungal Biodiversity Center, Database, 2005. http://www.cbs.knaw.nl/databases/index.htm [ Links ]
Chávez–Tapia, C. B., R. Vargas–Yáñez, G. Rodríguez–Arellanes, G. R. Peña–Sandoval, J. J. Flores–Estrada, M. R. Reyes–Montes and M.L. Taylor. 1998. El murciélago como reservorio y responsable de la dispersión de Histoplasma capsulatum en la naturaleza. II. Papel de los marcadores moleculares del hongo aislado de murciélagos infectados. Revista del Instituto Nacional de Enfermedades Respiratorias, México 11: 187–191. [ Links ]
Currah, R.S. 1985. Taxonomy of Onygenales: Arthrodermataceae, Gymnoascaceae, Myxotrichaceae and Onygenaceae. Mycotaxon 24: 12–16. [ Links ]
de Hoog, G. S. and J. Guarro. 1995. Atlas of Clinical Fungi. Centraalbureau voor Schimmelcultures/Universitat Rovira i Virgili, Baarn, 720 p. [ Links ]
Disalvo, A. F., W. J. Bigler, L. Ajello, J. E. Johnson and J. Palmer. 1970. Bat and soil studies for sources of Histoplasma capsulatum in Florida. Public Health Reports 85: 1063–1069 [ Links ]
Domsh, K. H., W. Gams and T. H. Anderson. 1993. Compendium of Soil Fungi. Vol 1. Academic Press, London, 859 p. [ Links ]
Donahue, L. R., W. R. Miller and C. J. Shickluna, 1981. Introducción a los suelos y al crecimiento de las plantas. Dossat, Madrid, 320 p. [ Links ]
Duchaufour, P. 1984. Edafología, edafogénesis y clasificación. Masson, Barcelona, 285 p. [ Links ]
Ellis, D.H. 1994. Clinical Mycology. The Human Opportunistic Mycoses. Pfizer, New York, 166 p. [ Links ]
Emmons, C.W. 1949. Isolation of Histoplasma capsulatum from soil. Public Health Report 64: 892–896. [ Links ]
Gallo, M. G., P. Cabeli and V. Vidotto. 1989. Sulla presenza di lieviti patogeni nelle feci di colombo torraiuolo (Columba livis, Gmelin 1789) nella citá di Torino. Parassitologia 31: 207–212. [ Links ]
Gaur, P. K. and R. W. Lichtwardt. 1980. Comparative study of a new Chrysosporium species with Histoplasma capsulatum. Sabouradia 18: 105–114. [ Links ]
Goodman, N. L. and H. W. Larsh. 1967. Environmental factors and growth of Histoplasma capsulatum in soil. Mycopathologica et Mycologia Applicata 33: 145–156. [ Links ]
Grose, E. and C. J. Marinkelle. 1968. A new species of Candida from Colombian bats. Mycopathologia 36:225–227. [ Links ]
Gründer, S., P. Mayser, T. Redmann and E. F. Kaleta. 2004. Mycological examinations of fungal flora of the chicken comb. Mycoses 48: 114–119. [ Links ]
Hall, T.2001. BioEdit Version 5.0.6 http://www.es.embnet.org/Services/ftp/software/Windows/BioEdit/BioDoc.pdf [ Links ]
Hoffman, A., J. G. Palacios–Vergara and J. B. Morales–Malacara. 1986. Manual de bioespeliología Universidad Nacional Autónoma de México, México D. F., 274 p. [ Links ]
Koilraj, J. A., G. Marimuthu, K. Natarajan, S. Saravanan, P. Maran and M. J. Hsu. 1999. Fungal diversity inside caves of southern India. http://144.16.79.155/currsci/oct25/articles25.htm [ Links ]
Kurtzman, C.P. and J. W. Fell. 1998. The Yeasts, A Taxonomic Study, 4th ed. Elsevier, Amsterdam, 1055 p. [ Links ]
Kwong–Chung, K. J. and J. E. Bennett. 1992. Histoplasmosis. Medical Mycology. Lea and Febiger, Philadelphia, p. 464–513. [ Links ]
Lachance, M. A. and W. Starmer. 1998. Chapter IV. Ecology and yeasts. In: The Yeasts, A Taxonomic Study, 4th ed., C. P. Kurtzman and J. W. Fell (eds). Elsevier, Amsterdam, p. 21–30. [ Links ]
Mahvi, A.T. 1970. Factors governing the epidemiology of Histoplasma capsulatum in soil. Mycopathologica et Mycologia Applicata 41: 167–176. [ Links ]
Mancianti, F., S. Nardoni and R. Ceccherelli. 2002. Occurrence of yeasts in psittacines droppings from captive birds in Italy. Mycopathologia 153: 121–124. [ Links ]
Martini, A. 1961. La microflora della caverna di Monte Cucco. Iº; Nota: I Blastomiceti. Annali della Facoltá di Agraria Perugia, XVI: 87–97 [ Links ]
Martini, A. 1963. Yeasts in cavern environments. Archiv für Mikrobiologie 45: 111–114 [ Links ]
McMurray, D. N. and L. H. Russell. 1982. Contribution of bats to the maintenance of Histoplasma capsulatun in a cave microfocus. American Journal of Tropical Medicine and Hygiene 31: 527–531 [ Links ]
Menges, R. V., M. L. Furcolow, L. A. Selby, R. T. Habermann and C. D. Smith. 1967. Ecologic studies of histoplasmosis. American Journal of Epidemiology 85: 108–118. [ Links ]
Meyer, S. A., R. W. Payne and D. Yarrow. 1998. Candida Berkhout. In The yeasts, a taxonomic study, C. P. Kurtzman and J. W. Fell (eds.). Elsevier, Amsterdam, p. 454–573. [ Links ]
Meyer, S. A. and H. J. Phaff. 1969. Desoxyribonucleic acid base composition in yeasts. Journal of Bacteriology 97: 56–57. [ Links ]
Mok, W. Y., R. C. C. Luizao and M. S. Barreto da Silva. 1982. Isolation of fungi from bats of the Amazon Basin. Applied and Environmental Microbiology 44: 570–575. [ Links ]
Montagna M.T., M.P. Santacroce, G. Caggiano, D. Tató y L. Ajello. 2003. Cavernicolous habitats harbouring Cryptococcus neoformans: results of a speleological survey in Apulia, Italy, 1999–2000. Mediacal Mycolgy 41:451–544. [ Links ]
Nakase, T. and M. Suzuki. 1985. Taxonomic studies on Debaryomyces hansenii (Zopf) Lodder et Kreger–van Rij and related species. II. Practical discrimination and nomenclature. Journal of General and Applied Microbiology 31: 71–86. [ Links ]
Nieves–Rivera, A. M. 2003. Mycological survey of Río Camuy Caves Park, Puerto Rico. Journal of Cave and Karst Studies 65: 23–28. [ Links ]
Orpurt, P. A. 1964. The microfungal flora of bat cave soils from Eleuthera Island, The Bahamas. Canadian Journal of Botany 42: 1629–1633. [ Links ]
Phaff, J. H., W. M. Miller and M. E. Mrak. 1966. The Life of Yeasts. Harvard University Press, Cambridge, 186 p. [ Links ]
Pitt, J. A. 1985. Laboratory Guide to Common Penicillium species. Commonwealth Scientific and Industrial Research Organization, Division of Food Research, North Ryde, 182 p. [ Links ]
Raper, K. B. and D. Fennell.1977. The Genus Aspergillus. The Williams & Wilkins Company, Baltimore, 686 p. [ Links ]
Reyes–Montes, M. R., M. Bobadilla–del Valle, M. A. Martínez–Rivera, G. Rodríguez–Arellanes, E. Flores–Robles, J. Sifuentes–Osornio and M. L. Taylor. 1998. Tipificación de aislados clínicos de Histoplasma capsulatum por métodos fenotípicos y genotípicos. Revista del Instituto Nacional de Enfermedades Respiratorias, México 11: 195–201. [ Links ]
Reyes–Montes, M. R., M. Bobadilla–del Valle, M. A. Martínez–Rivera, G. Rodríguez–Arellanes, E. Maravilla, J. Sifuentes–Osornio and M. L. Taylor. 1999. Relatedness analyses of Histoplasma capsulatum isolates from Mexican patients with AIDS–associated histoplasmosis by using histoplasmin electrophoretic profi les and amplified polymorphic DNA patterns. Journal of Clinical Microbiology 37: 1404–1408. [ Links ]
Samson, R. A., H. C. Evans and J. P. Latgé. 1988. Atlas of Entomopathogenic Fungi. Springer–Verlag, Berlin, 530 p. [ Links ]
Sanger, F., S. Nicklen and A. R. Coulson. 1977. DNA sequencing with chain–terminating inhibitors. Proceedings of the National Academy of Science, USA 74: 5463–5467. [ Links ]
Sigler, L. and J. W. Carmichael. 1976. Taxonomy of Malbranchea and some other Hyphomycetes with arthroconidia. Mycotaxon 4: 349–488. [ Links ]
Stotzky, G. and A. H. Post. 1967. Soil mineralogy as possible factor in geographic distribution of Histoplasma capsulatum. Canadian Journal of Microbiology 13: 1–7. [ Links ]
Su, C. S. and S. A. Meyer. 1991. Characterization of mitochondrial DNA in various Candida species: isolation, restriction endonucleases analysis, size and base composition. International Journal of Systematics and Bacteriology 41: 6–17. [ Links ]
Sutton, B. C. 1980. The Coelomycetes. Commonwealth Mycological Institute, Kew, 696 p. [ Links ]
Taylor, M. L., C. Toriello, A. Pérez–Mejía, M. A. Martínez, M. R Reyes–Montes, L. Espinosa–Ávila and C. Chávez–Tapia. 1994. Histoplasmosis in the State of Guerrero, Mexico: A biological approach. Revista Mexicana de Micología 10: 49–62. [ Links ]
Taylor, M. L., J. Granados and C. Toriello. 1996. Biological and sociocultural approaches of histoplasmosis in the state of Guerrero, Mexico. Mycoses 39: 375–379. [ Links ]
Taylor, M. L., A. Pérez–Mejía, J. K. Yamamoto–Furusho and J. Granados. 1997a. Immunology, genetic and social risk factors associated to histoplasmosis: studies in the State of Guerrero, Mexico. Mycopathologia 138: 1–5. [ Links ]
Taylor, M. L., M. R., Reyes–Montes, M. A Martínez–Rivera, G. Rodríguez–Arellanes, E. Duarte–Escalante and J. J. Flores–Estrada. 1997b. Histoplasmosis en México. Aportaciones inmunológicas y moleculares sobre su epidemiología. Ciencia y Desarrollo 136: 58–63. [ Links ]
Taylor, M. L., C. B. Chávez–Tapia, G. Vargas–Yáñez, G. Rodríguez–Arellanes, G. R. Peña–Sandoval, C. Toriello, A. Pérez and Reyes–Montes, M. R. 1999. Environmental conditions favoring bat infection with Histoplasma capsulatum, in Mexican shelters. Tropical Medicine 61: 914–919. [ Links ]
Taylor, M. L., C. B. Chávez–Tapia and M. R. Reyes–Montes. 2000. Molecular typing of Histoplasma capsulatum isolated from infected bats, captured in Mexico. Fungal Genetics and Biology 30: 207–212. [ Links ]
Taylor, M. L., C. B. Chávez–Tapia, A. Rojas–Martínez, M. R. Reyes–Montes, M. Bobadilla–del Valle and G. Zúñiga. 2005. Geographical distribution of genetic polymorphism of the pathogen Histoplasma capsulatum isolated from infected bats, captured in central zone of Mexico. FEMS Immunology and Medical Microbiology 45: 451–458. [ Links ]
Toriello, C., L. Arjona–Rosado and M. L. Taylor. 1991. Efficiency of crude and purified fungal antigens in serodiagnosis to discriminate mycotic from other respiratory diseases. Mycoses 34: 133–140. [ Links ]
Vaughan–Martini, A., P. Angelini and L. Zacchi. 2000. The influence of human and animal visitatio on the yeast ecology of three Italian caverns. Annals of Microbiology 30: 133–140. [ Links ]
Vidotto, V. and M. Gallo. 1985. Indagine sulla presenza de lieviti, nelle feci di colombo torraiuolo (Columba livis, Gmelin 1789) provenienti da contesti rurali. Parassitologia 27: 313–320. [ Links ]
Voller, A., D. E. Bidwell and A. Bartlett. 1979. The enzyme linked immunosorbent assay (ELISA). A guide with abstracts of microplate applications. Dynatech Europe Laboratories, London. 35–41 p. [ Links ]
Yarrow, 1998. Chapter 11. Methods for the isolation, maintenance and identification of yeasts. In The yeasts, a taxonomic study, C. P. Kurtzman and J. W. Fell (eds.). Elsevier, Amsterdam, p. 77–105. [ Links ]
Zeidberg, L. D., L Ajello and R. H. Webster. 1955. Physical and chemical factors in relation to Histoplasma capsulatum in soil. Science 122: 33–34. [ Links ]














