Introduction
Cereal grains are the most important staples and constitute a main source of energy, nutrient and roughages. Thus, storage of grains presents a vital component of food security. Crop losses due to insect pests varies between 10 and 30 % for major crops [1] representing major threats for the food security. In particular, the red flour beetle, Tribolium castaneum (Herbst) (Coleoptera: Tenebriodae), is the most important and destructive secondary pest in mills [2]. It attacks stored grains, flour, peas, beans, nuts, dried fruits and spices resulting in a significant loss in both weight and quality. T. castaneum infestation is troublesome because of its association with storage fungi particularly Aspergillus flavus which releases aflatoxin [3]. Furthermore, the beetles reduce stored product quality by contaminating foodstuffs with a nauseating secretion.
Currently, the measures to control pest infestations in grains and dry food products rely heavily upon the use of synthetic insecticides. Nevertheless, they have many side effects and cause damage like disturbances of the environment, insect resurgence, pest resistance, lethal effect on non-target organisms and toxicity. Therefore, there is an urgent need to find eco-friendly alternative methods.
Among the alternatives, plant secondary metabolites have exhibited their potential in killing or repelling target pests. Botanical pesticides have also the benefits of biodegradability and low mammalian toxicity. Monoterpenes are EO constituents, which exert a large spectrum of biological activities mainly insecticidal potential.
M. pulegium is a medicinal plant native from the Mediterranean area belonging to the Lamiacae family and growing widely in humid areas of the plains and mountains. Pennyroyal have biological, medical and gastronomical activities [4].
They are characterized by their antifeedant, ovicidal, repellent and fumigant action against the stored products beetles [5] as well as growth inhibitory activities. Aromatic plants and their monoterpenes constitute effective alternatives to synthetic pesticides without adverse effect [6]. Thus, Volatiles compounds especially monoterpenes possess insecticidal activity against several stored-product insects as fumigant and contact toxicants. They are repellants, feeding deterrents, and insect growth regulators. Generally, the target of EO was reported the insect nervous system. Most of the insecticides induce mortality in insects by inhibiting acetylcholinesterase (AChE), which is considered one of the most prominent enzymes in nerve connections in mammals as well as in insects [7].
Globally, we found that Mentha species is an important source of EO which exhibited significant insecticidal activity against Tribolium Spp. Previously, many researchers described Mentha EO insecticidal impacts versus Tribolium spp. In this context, Heydarzade et al., [8] reported the LC50 values were 18.422 and 7.939 µL/ml for M. spicata and M. pulegium respectively toward T. castaneum. Lougraimzi et al., [9] highlighted that EO and leaf powder of Pennyroyal exhibited insecticidal activity against T. castaneum. In addition, Amoura et al. [10] demonstrated a fumigant toxicity on T. confusum with an LC50 of 92.89 µL/L air. Then, M. piperita EO provided 50% of larval fumigant toxicity and 74 % of repellency of T. castaneum [11].
Our previous investigation has proven that Tunisian pennyroyal EO possess significant insecticidal activity on stored product pests: Lasioderma serricorne and Tribolium castaneum in vitro [8]. This is the first study in resaserching insecticidal effect of Pennyroyal EO in substrate under semi undustrial scale. So, EO mode of action and its effect on wheat flour quality didn’t report so far and in theses cases these are the novetly of our work. Accordingly, in order to continue our studies on bio-insecticidal compounds, this work investigates (i) the toxicity potential of pennyroyal EO against T. castaneum adults in wheat flour substrate during a storage period of 30 and 60 days, (ii) the effect of pennyroyal EO treatment on the quality of wheat flour in regard to physiochemical and head-space flour characteristics (iii) the possible mechanism of action of M. pulegium EO on in vivo acetylcholinesterase activity of Tribolium castaneum.
Material and methods
Sources of plant material and insect colonies
Mentha pulegium areal parts were collected in July 2016 from the region of Kef (El Kbouch (36°10′27″ N, 8°42′17″ E; altitude 627 m). All samples were identified by a botanist according to [9]. A colony was established and maintained in LB2A Laboratory in the National Agricultural Research Institute of Tunisia, INRAT (Tunis, Tunisia). Tribolium castaneum was reared on an artificial diet based on wheat flour at 28 ± 1°C, 65 ± 5% RH and complete darkness. Adults 0-24 d old were collected from the laboratory colony for the experiments.
Extraction and analysis of EO
All sample leaves of Pennyroyal were hydrodistillated for 3 h using a Clevenger type apparatus (Esel International, Ambala Cantt, Haryama, India; manufacturer city, state, country). The EO was stored in glass vials in darkness and at 4 °C until used in bioassays. EO Components were determined and quantified by GC-MS.
Extraction and analysis of volatile compounds retained by the Wheat flour
The Head Space method was applied to treated wheat flour with EO after storage in order to extract and identify volatile compounds retained by the substrate. This method consists in filling one third of a flask of 10ml by EO treated wheat flour which corresponds to 2.3 g of the weight. Then, these filled flasks were incubated in the oven of Headspace for 10 min at a temperature of 85 °C. The separation of the volatile constituents is made by means of type Teledyne Tekmar headspace HT3TM coupled with mass spectrometry. GC-MS was carried out by an Agilent 7890A GC system, coupled to an Agilent 5972C mass spectroscopy detector with electron impact ionization (70 eV). A HP-5 MS capillary column (30 m × 0.25 mm, coated with 5 % phenyl methyl silicone, 95 % dimethylpolysiloxane, 0.25 mm film thickness; Hewlett-Packard, City, CA) was used. The column temperature was programmed to rise from 40 to 240 °C at 5 °C/min, the carrier gas was Helium N60 with a 0.9 mL/min flow rate; split ratio was 100:1. Scan time and mass range were 1 s and 50-550 m/z, respectively. Mass spectra (compared with Wiley Registry 9th Edition/NIST 2011 edition mass spectral library), comparison of their retention indices (RI) with either those in the literature [10] or with those of authentic compounds available in our laboratories were taken into account for the compound identification.
Essential oil concentration toxicity bioassay
This test evaluated the fumigant toxicity of M. pulegium EO applied at three concentrations (75, 150 and 300 µL/L/ air). Sixty adults according a density of one insect per 10 g of wheat flour were introduced into a 2 L glass bottle containing 600 g of substrate (corresponding to 50 % container capacity). Essential oil droplets were applied to filter paper disks (8.5 cm diameter) using a micropipette at the three concentrations. Each treatment was repeated three times. Mortality was assessed by direct observation of insects in different treated wheat flour filled according to 50 % container capacity. When no leg or antennal movements were observed insects were considered dead. The number of dead and alive insects in each bottle was counted 10 days after initial exposure. Mortality data were corrected using [11] formula. Then, probit analysis [12] was used to estimate LC50 and LC95 values. Mortalities were analyzed with one-way analysis of variance and means were separated with Duncan test using SPSS Statistical Software 20.0.
Container capacity bioassay
A second experiment was designed to investigate fumigant toxicity in containers filled according to 50 % or 100 % capacity with wheat flour. Adults were treated with EO applied at 196.6 µL/L air as determined in previous assay. Filter paper (Whatman No. 1) of diameter 8.5 cm were impregnated with LC50 concentration of essential oil. The impregnated filter papers were attached to the screw caps of 2 L glass bottle Mortalities were evaluated after 30 and 60 days of storage. The mortality was corrected using Abbott’s formula [11]. Probit analysis described by [12] was used as previously.
Wheat flour quality
Wheat flour sample were generated by one of the Tunisian millings located in Tunis. The stored flours, under 50 % space occupation were analyzed at two different periods (30 and 60 days) for their physicochemical parameters according to the standard methods of analysis [13].
Acetylcholinesterase activity assay
The inhibitory action of M. pulegium EO on the AChE activity was evaluated in vivo. Treated T. castaneum adults with EO were homogenized in phosphate buffer (pH=7) using a Telfon glass tissue homogenizer. Homogenates were centrifuged (10000 rpm for 10 min at 4 °C) and supernatants were used as the enzyme source for determination of AChE activity according to the method of Ellman et al. [14]. Briefly, sodium phosphate buffer (pH 8.0), and enzyme solution (30 μL) were mixed and further, 60 μL of Ellman’s reagent/DTNB was added, and the reaction was initiated by the addition of substrate (60 μL of acetylthiocholine iodide). The hydrolysis of the ATCI can be measured by the formation of the coloured product 5-thio-2- nitrobenzoate anion, which is formed by the reaction of DTNB and thiocholine, released by the hydrolysis of the enzyme. The formation of the colored product was measured at 412 nm. Protein content in the homogenate was measured using bovine serum albumin (BSA) as the standard. Percentage of AChE inhibition was calculated by using the equation:
C is the activity of control, and I is the activity of treatment.
Results and discussion
Essential oil yield
Pennyroyal areal parts collected from Jebel Kbouch in the region of Kef (Tunisia) belonging to the semi-arid bioclimatic flour gave an EO with a yield reaching up to 3.1 % (w/w on dry weight basis). In fact, Salem et al., [8] have been reported that EO obtained by steam hydrodistillation from pennyroyal collected from Oued el abid (Tunisia) has a lower yield of 1.5 %.
EO Chemical composition
In Pennyroyal EO, 17 compounds representing 88.76 % of the total components were identified. (Table 1). Results showed that primary constituents present in the EO were menthone (23.64 ± 2.58 %) followed by pulegone (21.70 ± 3.22). Based on these results, M. pulegium EO from Kef site belonged to menthone chemotype. Pennyroyal can be considered as an important source of pulegone which is a monoterpene ketone representing approximatively 39.15 % of the overall studied M. pulegium EO.
Table 1 Chemical composition (%, w/w) of M. pulegium essential oil.
| Compound† | Formula | RI | % | Identification |
|---|---|---|---|---|
| α-Pinene | C10H16 | 1032 | 0.87 ± 0.07 | GC-MS, Co-GC |
| β-Pinene | C10H16 | 1118 | 0.77 ± 0.06 | GC-MS, Co-GC |
| Sabinene | C10H16 | 1132 | 0.32 ± 0.02 | GC-MS, Co-GC |
| Menthone | C10H18O | 1136 | 23.64 ± 2.58 | GC-MS, Co-GC |
| Isomenthone | C10H18O | 1146 | 16.70 ± 1.65 | GC-MS, Co-GC |
| β-Myrcene | C10H16 | 1176 | 0.49 ± 0.05 | GC-MS |
| Limonene | C10H16 | 1203 | 2.70 ± 0.22 | GC-MS, Co-GC |
| Piperitone | C10H16O | 1226 | 8.92 ± 0.77 | GC-MS |
| 8-Hydroxy-4-p-menthen-3-one | C10H18O2 | 1222 | 0.17 ± 0.02 | GC-MS |
| cis-Isopiperitenone | C10H14O | 1241 | 0.48 ± 0.05 | GC-MS |
| 3-Octanone | C8H16O | 1265 | 0.35 ± 0.04 | GC-MS, Co-GC |
| Menthol acetate | C12H22O2 | 1280 | 0.67 ± 0.08 | GC-MS |
| 3-Octanol | C8H18O | 1388 | 1.66 ± 0.13 | GC-MS |
| methyl isoeugenol | C11H14O | 1502 | 0.37 ± 0.05 | GC-MS, Co-GC |
| β-Caryophyllene | C15H24 | 1613 | 0.55 ± 0.06 | GC-MS |
| Pulegone | C10H16O | 1663 | 21.70 ± 3.22 | GC-MS, Co-GC |
| α-Humulene | C15H24 | 1688 | 1.28 ± 0.15 | GC-MS |
| α-Terpineol | C10H18O | 1705 | 0.37 ± 0.04 | GC-MS |
| Germacrene-D | C15H24 | 1716 | 0.45 ± 0.04 | GC-MS |
| Piperitenone | C10H14O | 1948 | 12.09 ± 0.34 | GC-MS, Co-GC |
| Isopulegone | C10H18O | 2224 | 2.70 ± 0.22 | GC-MS |
| p-Menth-3-ene | C10H18 | nd | 0.41 ± 0.03 | GC-MS |
| 8-hydroxy-p-menthan-3-one | C10H18O2 | nd | 0.11 ± 0.01 | GC-MS |
| 3-Octanyl acetate | C10H20O2 | nd | 0.77 ± 0.08 | GC-MS |
| Chemical classes | ||||
| Monoterpene hydrocarbons | 5.56 ± 0.61 | |||
| Oxygenated monoterpens | 89.04 ± 7.54 | |||
| Sesquiterpene hydrocarbons | 2.28 ± 0.23 | |||
| Others | 1.66 ± 0.18 | |||
| Total identified | 98.56 ± 8.52 | |||
*Compounds were listed in order of elution in HP-5 column. RI: relative retention index.
†Compounds in order of their elution on HP5-MS; values of volatile essential oil percentages are the average of three determinations (n=3).
Many researchers have been assessed the variability of M. pulegium EO from different regions. Our results are in accordance with those obtained by Salem et al. [8] already carried out in the region of Oued el Abid (Tunisia) where M. pulegium EO was characterized by the predominance of pulegone (55.58 %) and isomenthone (34.87 %). Hence, the fumigant toxicity potent of pennyroyal might be attributed to its chemical composition and particularly to its high content of monoterpenes especially pulegone and isomenthone (Table 1). In previous studies, pennyroyal oil and pulegone were screened for their insecticidal proprieties against stored product beetles [8]. In this regard, significant insecticidal proprieties were demonstrated.
Concentration bioassays
Results revealed that fumigant toxicity varied with different concentrations (Fig. 1). In fact, high mortalities were obtained for the concentration of 150 µL/L air of the tested oil under 50 % occupation space conditions. Moreover, results showed that there is a significant difference between the concentration of 150 µL/L air and the others (75 µL/L air, 300 µL/L air and control). For this concentration, fumigant impact was clearly more significant to T. castaneum adults. Fumigation with M. pulegium essential oil at the concentration of 150 µL/L air and under 50 % occupation spaces induces a mortality of 18.33 %. Probit analysis showed that T. castaneum was susceptible to M. pulegium essential oil with an LC50= 196.6 µL/L air.
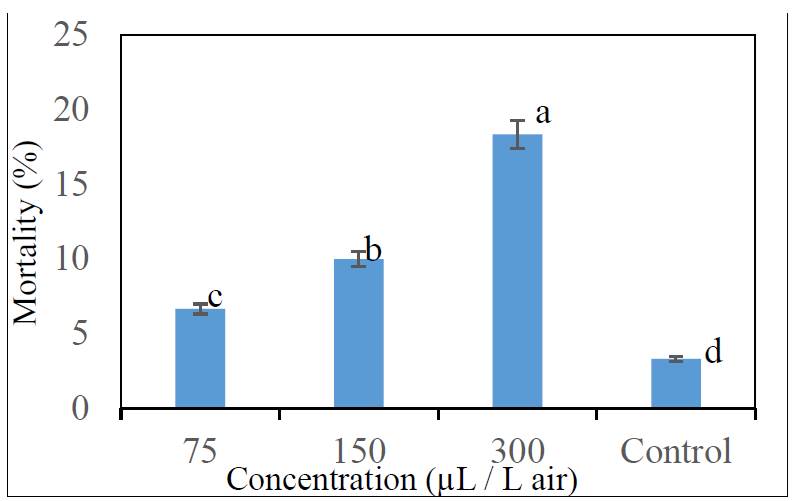
Fig. 1 Tribolium castaneum mortality under 50 % occupation space conditions after 10 days of storage period. (Diffrent letters indicate significant diffrences (at p< 0.05) among concentration. Each value is the mean ± SD of three replicate).
The fumigant activity of pennyroyal EO has been evaluated against stored product pests. Thereby, insecticidal activity of some aromatic plants such as M. pulegium has been highlighted by several studies. In this context, it appeared that M. pulegium EO at the concentration of 568.2 μL/L air achieved 100 % of mortality after 15 h of exposure on Lasioderma serricorne [8]. In fact, Zekri et al. [15] reported an important fumigant toxicity potential of Pennyroyal against Sitophilus orysae adults. Benayad et al. [16] proved that M. pulegium EO was toxic against Sitophilus orysae and Rhysopertha dominica in the first 24h. Moreover, some recent studies revealed insecticidal activity of volatile compounds when directly mixed with food. Saad et al. [17] reported that wheat treatment with 1 g/kg of eugenol for 7 days, induce 77.7 % mortality of S. oryzae. This monoterpene possesses insecticidal activity and was previously reported to have strongest contact and fumigant toxicities against S. oryzae in stored wheat [17]. It has been reported that the insecticidal activity of monoterpenes depends on the nature of compounds, such as hydrocarbon or oxygenated, cyclic or non-cyclic, aromatic or non-aromatic, degree of unsaturation, type of function groups, and location of functional groups. Structure-activity studies reported that the oxygenated monoterpenes including menthone, had higher contact toxicity than monoterpene hydrocarbons (p-cymene, α-pinene and α-terpinene) against some stored product insect [18]. Bulk M. pulegium EO exhibited a relevant fumigant potential against T. castaneum adults on wheat flour substrate and a low toxicity on non-target organisms. Nevertheless, there are many challenges and constraints to use EO due to their volatility and instability. To improve their proprieties and make them appropriate to be used as birational pesticide, nano-formulation paves the way to develop the applicability of EO in stored product management [19].
Container capacity bioassays
Fig. 2 revealed that high mortalities were obtained for less occupied spaces with wheat flour (50 %) whatever storage duration (30, 60 days). Furthermore, there is a significant difference between mortalities obtained for 50 % and 100 % occupation space percentage. In fact, fumigation with M. pulegium EO in spaces filled with 50 % and 100 % wheat flour led to 32.94 % and 14.57 % mortality after 30 days of storage. In addition, high mortalities were obtained after 60 days of storage period corresponding to 72.31 % and 43.43 % for 50% and 100 % occupation space respectively. Finally, for each storage period statistical analysis showed two significantly different groups 50 % and 100 % space occupation. Possible explanations include the rapid absorption to the surface of food (grains), degradation of active compounds by the metabolic process of grains or limited (or blocked) evaporation of active constituents [19].
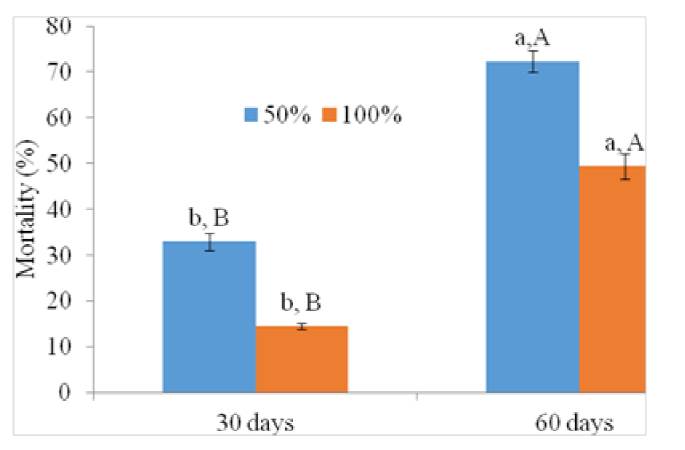
Fig.2. Tribolium castaneum mortality under occupation space conditions after 30 and 60 days of storage period (different letters indicate significant differences (at P < 0.05) among storage period for each occupation space (lowercase letters) and among occupation space for each storage period (uppercase letters). Each value is the mean ± SD of three replicate).
Effect of essential oil treatment on wheat flour quality
Physicochemical characteristics of wheat flour
The physicochemical characteristics of flour samples during storage period (30 days and 60 days) are given in Table 2. Statistical analysis showed that physicochemical characteristics of all studied wheat flour samples were affected by storage period.
Table 2 Impact of M. pulegium essential oil treatment on physicochemical characteristics of wheat flours.
| Storage period | Treatment | Moisture content (%) | Falling Number (s) | Protein content (%) | Ash content (%) | Gluten content (%) |
|---|---|---|---|---|---|---|
| 30 days | Control wheat flour | 13.77±0.06 a,A | 455.67±3.51b,A | 11.11±0.03b,A | 0.73±0.23c,B | 29.20±0.17c,B |
| Infested wheat flour | 13.87±0.06a,A | 457.00±4.58b,A | 9.73±0.03a,A | 0.69±0.12b,B | 27.20±0.17a,B | |
| Treated wheat flour | 13.83±0.06a,A | 451.00±4.58b,A | 11.81±0.03c,A | 0.66±0.21b,B | 28,80±0.00b,B | |
| 60 days | Control wheat flour | 13.80±0.06a,A | 453.33±18.45 c,A | 11.18±0.03b,A | 0.54±0.21b,A | 27,10± 0.17ab, A |
| Infested wheat flour | 13.97±0.00a,A | 455.00±10.82 c,A | 10.33±0.00a,B | 0.48±0.04a,A | 26,60±0.17a,A | |
| Treated wheat flour | 13.87±0.12a,A | 449.33±14.84 b,A | 12.28±0.04c,B | 0.56±0.03b,A | 27.5±0.17a,A |
For each parameter and each storage period, comparisons were made between different treatments (lowercase letters).
For each parameter, comparisons were made between storage periods for each treatment (uppercase letters). Means followed by the same letters are not significantly different at the 5 % threshold (Duncan).
Moisture content
Wheat flour usually experiences physical and chemical changes during storage [20]. In this study, during the first month of storage, results indicated that moisture content did not present a significant difference between infested and control wheat flour. The moisture content values in wheat flour samples were within the recommended moisture levels. The moisture contents in different wheat flour samples increased throughout the storage period (30 and 60 days).
Falling number
The Hagberg fall time test estimate the amylase activity of the flour, which corresponds to the degree of hydrolysis of the starch by the α-amylase enzyme. In general, falling number should be in the range of 250- 350 s to maintain functionality of wheat flour. Beyond this time, wheat flour will be intended to biscuit industry [21]. Results illustrated in Table 2 pointed out that during the first month of storage a significant difference was observed between control, infested and treated wheat flour samples. While, during the second month a significant difference was observed between control and infested wheat flour samples. The decrease in falling number suggests that the α-amylase activity increased over the storage period.
Protein content
The protein level during the first month of storage varies between 9.73 and 11.81 %. There is a significant difference between the control, the infested control and the treated wheat flour. Thus, results showed that the lowest protein level is recorded for the infested control while the highest rate is for the treated wheat flour.
Ash content
Regarding ash content, an indication of the mineral contents of food, values ranged between 0.48 % for the infested wheat flour (60 days of storage) and 0.73 % for the control wheat flour (30 days of storage).
Gluten content
Concerning gluten content, results pointed out that gluten content varied between 26.6 % for the infested wheat flour (60 days of storage) and 29.2 % for the control wheat flour (30 days of storage). During the second storage period, we note a slight drop in gluten levels for all samples. Result showed that the infested wheat flour has the lowest gluten content in both storage periods and was evaluated as lowest.
The maximum moisture levels for safe storage have been reported to be 13 % [22]. Moisture content increase was due to the ability of wheat flour to absorb moisture from the atmosphere. The presence of insects in wheat flour increased the relative humidity due to the transpiration metabolism of insects and then increased the moisture content of flour [23]. The increase in falling numbers also suggests that the α-amylase activity decreased over the storage period. This can be attributed to the increased fungal content of the wheat flour during storage due to the presence of insects [24]. Then, EO improves the falling number of wheat flour.
Concerning protein content, the standard norm varies between 9 and 14 % [25]. In fact, the protein levels for the first and second months of storage are in conformity with the standard and therefore are bread-making. The slight reduction in ash content could be attributed to biochemical activities of microorganisms and insects [25].
In addition, the observed decrease in gluten content was due to insect infestation which affects the quality of the wheat flour. In fact, insect consumes endosperm, rich in starch and gluten, and often leaves the wheat grain bran intact. According to the Tunisian standard (NT 51. 13, 1984), the baking flour requires a minimum wet gluten content equal to 24 %. Therefore, all studied wheat flour samples are in the standard and are therefore intended for bread making.
Characterization of volatile compounds retained by the treated substrate during the storage period
The extraction and the identification of Pennyroyal EO retained by the treated wheat flour during 30 and 60 days of storage were determined by Headspace method (Table 3). GC/MS analysis revealed that major compounds menthone and pulegone were detected in wheat flour after long time storage. The amount of these volatile compounds varied according to space occupation and time duration.
Table 3 Composition (µg/g) of essential oil volatile compounds retained by the treated wheat flour.
| Storage period | 30 days | 60 days | ||
|---|---|---|---|---|
| Space occupation | 50 % | 100 % | 50 % | 100 % |
| Menthone | 183.87a,B | 116.31b,B | 662.7a,A | 233.33b,A |
| Pulegone | 39.53a,B | 43.87b,B | 19.86a,A | 34.80b,A |
Each value is expressed as mean SD (n=3). Values with the letters (in capital letters for space occupation and in lowercase for period of storage) are significantly at p ≤ 0.05.
These results underline that during storage, treated wheat flour retained high quantities of menthone and pulegone especially in 50 % occupation space. In our study, long time of storage mainly 60 days allowed the retention of significant amounts of menthone. The accumulation of these ketones could be due to their low volatility. Persistence of insecticidal activity in the oils after long time treatment has been reported to be related to their chemical composition especially richness on oxygenated monoterpenes, molecules of relatively low volatility compared to monoterpenes hydrocarbons [26]. On the other hand, Demeter et al. [27] compared the toxicity of 25 essential oils on adult granary weevils and underlined that the increase of mortality after 7 days of treatment with three EO could be explained by the cumulative contamination effect during all the period including feeding which cause insect physiological disorders. Globally, to be a perfect candidate for a botanical pesticide, EO should exhibited high biocidal proprieties as well as a high volatility in order to limit residue accumulation in foodstuff or in the environment [28].
Inhibition of acetylcholinesterase (AChE) activity
The inhibitory effects of M. pulegium EO on acetylcholinesterase (AChE), is shown in Fig. 3. The results indicate that M. pulegium oil inhibited significantly the AChE activity in T. castaneum adults. After 30 days storage, the enzyme activity was inhibited by 34 and 29 % in space occupied with 50 and 100 % with wheat, respectively. After a longer period of storage of 60 days, the enzyme inhibition activity reached 85 % in 100% space occupied while the insects were totally killed in 50% occupied space. These results suggest that M. pulegium EO has neurotoxic effects on T. castaneum which was time-exposure dependent. The high fumigant toxicity of M. pulegium EO against T. castaneum can be attributed to high AChE inhibitory action. According to Saad et al. [17], some monoterpenes caused complete mortality (100 %) of S. oryzae adults at a concentration of 1 g/kg after 1 week of treatment of stored wheat. The mechanisms of action of plant derived EO is still not totally elucidated. It had been reported that at least some insecticidal EO are neurotoxic, with monoterpenes possibly acting as acetylcholinesterase inhibitors. Symptoms, such as hyperactivity followed by hyperextension of the legs and abdomen, then fast knockdown or immobilization were observed when insect are exposed to monoterpenes indicating that monoterpenes might act as neurotoxins [28].
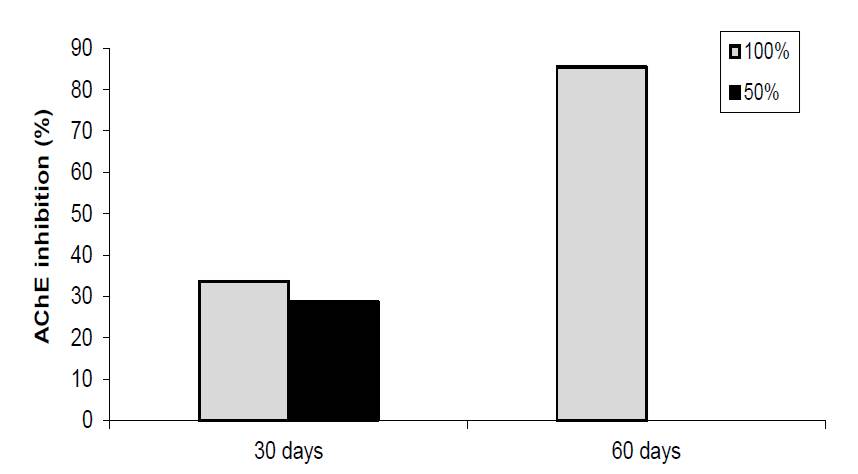
Fig. 3 In vivo acetylcholinesterase activity of M. pulegium essential oil under occupation space conditions after 30 and 60 days of storage period.
In our study, the observed anti-AChE activity of M. pulegium oil could be linked to its richness in oxygenated monoterpenes. Saad et al. [17] studied the inhibitory effects of monoterpenes and phenylpropenes on acetylcholinesterase (AChE) and showed that menthone caused strong inhibition of the enzyme. Pulegone is known for its insecticidal effect against numerous insect pests, destroying insects by a nervous system dysfunction or neuromuscular action. Tong and Coats [29] evaluated the pharmacological action of five monoterpenoids (α-terpineol, carvacrol, linalool, pulegone, and thymol) on native insect GABA receptors from house flies and American cockroaches and showed that pulegone is a positive allosteric modulator at insect GABA receptors. It binds to GABA receptors associated with Cl channels-located on the membrane of post- synaptic neurons-and therefore disrupt the function of GABA synapses, causing inhibitory effects on the nervous system of insects. Furthermore, pulegone has a neurotoxin effect on insects by inhibiting acetylcholinesterase activity [30].
In this context, Heydarzade et al. [31] reported the detoxifying enzyme activity of T. castaneum adult treated with Mentha spicata and Mentha pulegium EO through incorporation in insect food.
Conclusion
The finding revealed insecticidal potential of Pennyroyal (Mentha Pulegium L.) against T. castaneum. Mortality optimal response was recorded for 50 % occupation space during 2 months of storage period. Results provides evidence that pennyroyal might be a promising potential as an eco-friendly approach for stored food management against stored product pests biodegradation.











 nueva página del texto (beta)
nueva página del texto (beta)


