Introduction
There is an increasingly frantic search for new nanomaterials and production methods. Nanotechnology is the most rapid growing field of manufacturing in the world today [1]. For the past decade, scientists have been dealing with reactions at the atomic or molecular level that have led to nanotechnology production [2]. An exciting and interesting area of scientific research includes the synthesis and applications of nanomaterials in the range of 1-100 nm [3,4]. Nanoparticles have received significant consideration due to their extensive usage in drug delivery systems, control, pollution, environmental, pharmaceuticals, biological tagging, optoelectronics, photonics, and catalysis [5,6]. Metal NPs have attracted much interest because of their antimicrobial agents [7,8]. Among the different types of metallic nanomaterials, AgNPs have attracted significant consideration because of their limit of applications like optoelectronics, medicinal, biosensing, catalytic, antioxidant, anticancer, antiviral, and antibacterial [9-11]. Silver, because of its toxicity versus most microorganisms containing viruses, bacteria, and fungi, is known as a potent antimicrobial agent. AgNPs, due to their lower particle size (less than 100 nm) and the higher specific area, which enhance the ratio of high-energy surface atoms, exhibit unusual antimicrobial and physicochemical properties [12-14].
For the synthesis of AgNPs, several physical and chemical preparation methods are available [15]. Chemical methods produce hazardous substances for human health and the environment due to the use of non-polar solvents and reducing chemicals. using high energy is also required for many physical methods to synthesize AgNPs [16]. Hence, researchers have been trying to develop environmentally friendly procedures for the synthesis of nanoparticles [17]. Recently, green methods have expanded as a substitute for usual physical and chemical methods to synthesize novel metal NPs [18,19]. Green methods have received significant concern because they do not produce dangerous by-products, do not consume high energy, and do not use toxic solvents [20]. An environmentally friendly method to synthesize AgNPs, biological systems including plants, bacteria, fungi, and yeast have been widely investigated [21]. AgNPs synthesized via green methods are widely used for many applications, such as antidiabetic, antimicrobial, antioxidant, and anticancer applications, and are also in industry [22,23]. Using plant extracts can also be advantageous over microorganisms for nanoparticle synthesis, by maintaining cell cultures, isolation, and elimination of the identification [24]. Plant-mediated way, due to various advantages, e.g., improved stability, rapid synthesis, and cost-effectiveness, is better than the microbial method [25]. Plants contain a wide range of secondary metabolites, such as terpenoids, polysaccharides, flavonoids, and phenolics, and redox. Therefore, nanoparticles are used for biosynthesis [26]. Parameters such as pH, reaction time and temperature affect the properties of these NPs extracted from plant extracts; however, the nature of biomolecules in plant extracts is the most critical factor in the biological process. Therefore, the quality of the selected extract has excellent importance [27,28]. Parsley is an herb. In beverages and foods, parsley is widely used as a flavouring, food, condiment, and garnish. In manufacturing, parsley seed oil is used as a fragrance in perfumes, cosmetics, and soaps. The root, seed, and leaf are used to make medicine. [20] The principal targets of the present article are: (i) synthesis AgNPs using Parsley leaf extract, (ii) screening method of synthesis of AgNPs among hydrothermal autoclave and heater starrier methods (iii) to study of the antimicrobial activities of the resulting nanoparticles.
Experimental
Material and methods
Materials
Silver nitrate salt (AgNO3) was provided by Sigma-Aldrich Company. Parsley (Petroselinum crispum) leaves were provided from Mahabad, West Azerbaijan province, Iran. Escherichia coli (PPTCC 1270) and Staphylococcus aureus (PTCC 1112) were purchased from the microbial Persian-type culture collection (PTCC, Tehran, Iran). Plate count agar (PCA) was obtained from Oxoid (Oxoid Ltd., Hampshire, England).
Preparation of the Parsley leaf extract
By using double-distilled water, Parsley leaves, after cutting, were washed and dried for four days under ambient conditions. 100 mL of double deionized distilled water was boiled, and 10 g of dried powder was added to it for 10 minutes and then filtered using filter paper (Whatman No. 1). The extract was kept at 4 °C.
AgNPs synthesis using Parsley leaf extract by two methods
According to previous studies, by adding 0.017 g of AgNO3 `s salt into 100 mL of distilled water, AgNO3 was prepared, and AgNO3 salt with a concentration of 1 mM was used [4,7]. Two different methods were used to synthesize AgNPs, namely, autoclave hydrothermal heating, and heater. In each method, 5 mL of the Parsley leaf extract is mixed with 25 mL of silver nitrate salt, and in the autoclave-accelerated synthesis method, by setting at a temperature of 121 °C and pressure of 1.5 bar for 15 min, the mixture solutions were placed into a laboratory autoclave. In the heater stirred synthesis method, by setting at a constant temperature (70 °C for 45 min), the mixture solution was heated.
Physico-chemical analysis
Infrared Fourier transform (FT-IR) was measured to identify the principal functional groups in the parsley leaf extract. By adjusting Nicolet Magna-IR 550, using KBr pellets in the 4000-400 cm−1 region, the FT-IR spectrum of parsley leaf extract was recorded.
Due to the resonance of the surface plasmon of AgNPs, the production of AgNPs can be easily observed using UV-vis spectrophotometry (U-2900 Hitachi spectrophotometer). The broad absorption peak (λmax) in the wavelength range of 400-450 nm in the mixed solution, including Se NPs was determined, due to the SPR signal of the NPs [2,18,29].
Particle size analyzer (Malvern Instruments, Zetasizer Nano ZS, Worcestershire, UK) and dynamic light scattering (DLS) were used to identify of zeta potential value, polydispersity index (PDI), and particle size of the synthesized AgNPs.
Antibacterial activity assay
By using a well diffusion method as a described technique by Ahmadi and Jafarizadeh-Malmiri, antimicrobial properties of the synthesized AgNPs were investigated [30]. According to the 0.5 McFarland standard, suspensions including bacteria E. coli (Gram-negative) and S. aureus (Gram-positive) (1.5×108 colony-forming units) were prepared and by adjusting the culture medium, 0.1 mL of it was spread on plates. After that, holes with a diameter of 5 mm were made in the culture medium. 10 microliters of synthesized AgNPs were poured into each hole. Then, the plates were placed in a laboratory incubator at 37 °C for 24 h. During incubation, the synthesized AgNPs diffuse into the agar and inhibit the growth and germination of the E. coli and S. aureus. To show the antibacterial activity of the samples, the diameter of the inhibitory growth zones was measured. Ola M. El-Borady et al. (2020), the antimicrobial attributes of gold NPs synthesized using parsley leaf extract were investigated, and good results were obtained [25].
Statistical analysis
Physico-chemical and antibacterial analyses of synthesized AgNPs were replicated three times. Data were interpreted using Minitab v.16 statistical packages via analysis of variance (Minitab Inc., PA, USA). To compare the mean values of achieved data with a significance level of 5 %, Turkey’s Comparison test was used (p ˂ 0.05).
Results and discussion
FT-IR spectra analysis
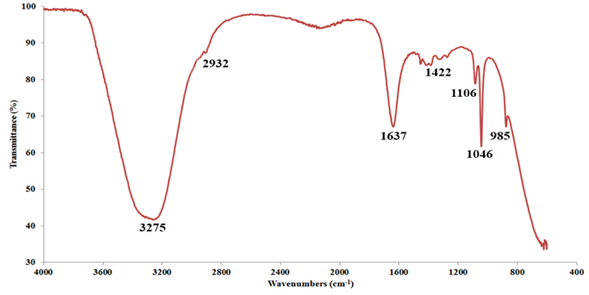
Functional groups in parsley leaf extract play an essential role in reducing silver metal ions. Therefore, to identify the functional groups, FT-IR analysis was utilized, as seen in Fig. 1. As shown in the figure, Parsley leaf extract has several functional groups and peaks, most of which are related to the wave number of 3275 cm-1 (-OH, hydroxyl group), 1637 cm-1 (C=O, carbonyl, and carboxylic groups) and 1046 cm-1 (C-O, esters group). Ulug et al., in synthesizing nanoparticles using Parsley leaf extract, identified the mentioned functional groups for Parsley leaf extract [31].
Formation of synthesized AgNPs
The synthesis of AgNPs was monitored at regular intervals due to their surface plasmon resonance (SPR) by scanning the reaction mixture under a spectrophotometer. Therefore, using a UV-Vis spectrophotometer, the absorption spectrum of the solutions was taken. The results of this analysis are shown in Fig. 2(a), (b). As mentioned, the highest adsorption of AgNPs occurred in the range of 400 to 450 nm, which is the result of all two methods. So that using a hydrothermal autoclave to synthesize AgNPs at the lowest wavelength (403 nm), 414 nm were obtained for the heater. Ahmadi et al., also synthesized AgNPs using Aloe vera leaf extract with λmax ranging from 403 to 415 nm [4]. Francis et al., synthesized AgNPs using Elephantopus scaber extract, and observed the peak at 420 nm [32].
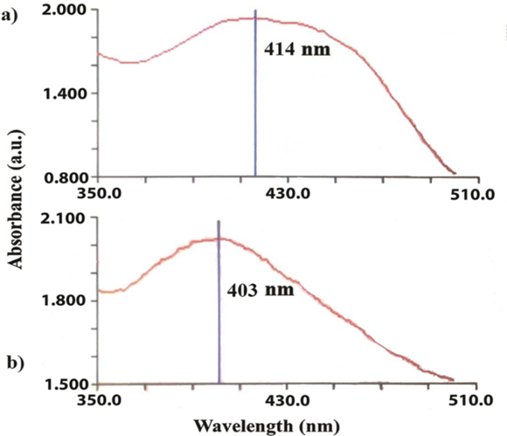
Fig 2. Absorption spectrum of the AgNPs solution. (a) Heater and (b) Hydrothermally autoclave technique.
Fig. 3(a) and (b) show the appearance of the synthesized AgNPs by two techniques. Fig. 3, relates the synthesized AgNPs by different techniques. As shown in this fig., with the production of AgNPs, a brownish color will be obtained. Jain et al., and Mohammadlou et al., obtained these results [24,33].
Zeta potential, PDI and particle size of the synthesized AgNPs
The properties of surface charge, polydispersity index (PDI) and mean particle size are shown in Table 1. According to Table 1, different techniques have acceptable results for the mean particle size of synthesized AgNPs. Synthesized AgNPs via the autoclave hydrothermal technique with the lowest particle size (68 nm), also 99 nm, were obtained for heater techniques. The uses of the autoclave technique are suitable methods for the synthesis of small mean particle size AgNPs. Due to the appropriate heat, these methods increase the activity of silver ions and reducing agents in the Parsley leaf extract, ultimately accelerating the process of reducing silver ions and the synthesis of low particle size AgNPs. Torabfam and Jafarizadeh-Malmiri use chitosan and microwave techniques for synthesis AgNPs to obtain acceptable results. Ahmadi et al., also using Aloe vera leaf extract via hydrothermal autoclave for synthesis AgNPs, indicated that suitable method for producing AgNPs [4, 7].
Table 1 PDI values of the synthesized AgNPs using two different methods.
| Properties | Zeta potential (mV) | PDI | Mean particle size (nm) | |
| Technique | ||||
| 1 | Heater | -6.2 | 0.659 | 99 |
| 2 | Autoclave | -14.3 | 0.196 | 68 |
Nanoparticles with uniform particle size distribution are highly desirable and will avoid undesirable phenomena such as agglomeration and aggregation. To evaluate these properties, the polydispersity index (PDI) parameter is used. This parameter has a value between 0 and 1, and the smaller the value, the uniformly synthesized nanoparticles. Table 1 shows the PDI values of the synthesized AgNPs using two different methods.
As can be seen, the synthesized AgNPs by the hydrothermal autoclave technique have good uniformity and PDI of 0.196 and concerning the received heat uniformity. However, the use of heather as a silver nanoparticle synthesis method has a high dispersion of nanoparticles and limits undesirable phenomena such as agglomeration and their application. Fig. 4(a), (b), indicates PSD (Particle size distribution) of the synthesized AgNPs.
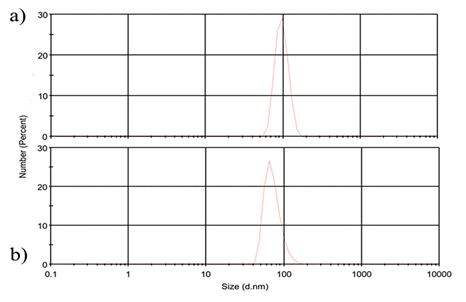
Fig. 4 PSD (Particle size distribution) of the synthesized AgNPs. (a) Hydrothermally autoclave technique, (b) Heater.
The synthesized nanoparticles lose their stability over time, and in some cases, they will grow in size. One of the stability measures of the nanoparticles produced in the zeta potential that calculate the surface charge content of the nanoparticles. Table 1 shows the zeta potential values of the fabricated AgNPs using two different methods. According to Table 1, all samples synthesized by different techniques have suitable zeta potential and acceptable stability. However, given the zeta potential of nanoparticles synthesized by autoclave (-14.3 mV), this method is suitable for producing AgNPs. Anandalakshmi et al., used Pedalium murex leaf extract to synthesize AgNPs and obtained -7.66 mV of zeta potential, which indicates the high stability of the nanoparticles [34]. In summary, and based on the results obtained, the autoclave is more a suitable method for synthesizing AgNPs due to the high surface charge, uniformity of the nanoparticles, and smaller particle size.
Antibacterial activity of the synthesized AgNPs by two different methods
Antibacterial activity of the synthesized AgNPs against both Gram-positive and Gram-negative bacteria strains shows in Table 2.
Table 2 Antibacterial activity of the synthesized AgNPs against both Gram-positive and Gram-negative bacteria strains.
| Diameter of created clear zones around the wholes (mm) | ||||
| - | AgNPs synthesized by Autoclave | AgNPs synthesized by Heater | Parsley leaf extract | |
| 1 | E. Coli | 16 | 12 | 5 |
| 2 | S. Aureus | 17 | 14 | 5 |
As can be seen in this fig., the antibacterial effect of Parsley leaf extract was compared with AgNPs synthesized in different methods. The results showed that Parsley leaf extract had no antibacterial effect on Gram-positive and Gram-negative strains. In Table 2, comparing the antibacterial properties of the synthesized AgNPs by different techniques (autoclave and heater) on Gram-negative bacteria (E. coli) were obtained 16±1, and12±1, respectively. However, in the plate containing PCA amended with S. aureus, the diameter of created clear zones around the wholes containing synthesized AgNPs with different methods (autoclave and heater) observed in this Table 2, were 17±1, and 14±1 mm, respectively. As anticipated, the antibacterial effect of AgNPs produced by the autoclave technique due to smaller particle size, more excellent stability and mono-dispersed particles, has the more extraordinary bacterial inhibitory ability, and will work better on cell wall degradation. Also, the antibacterial effect of the nanoparticles produced on Gram-positive bacteria was more than Gram-negative. Access to the bacterial cell wall was not allowed due to the presence of proteins and lipopolysaccharide layers on the cell wall of gram-negative bacteria and digested it can be explained by the fact that Gram-negative bacteria are more resistant to antibiotics than gram-positive bacteria [35]. Furthermore, other studies indicated that the antibacterial properties of various nanoparticles such as gold, selenium, and zinc oxide on Gram-positive bacteria are higher than those of Gram-negative bacteria [36-38].
Conclusion
The green synthesis of nanoparticles is one of the optimal and suitable methods for the synthesis of colloidal nanoparticles due to its low cost, reasonable time consumption, and uniformity of the produced nanoparticles. Among the various methods of green synthesis of nanoparticles, there are various techniques and methods to accelerate the reaction, and there is a faster synthesis, each of which has different advantages and disadvantages. In this research, the use of the hydrothermal method (autoclave device) due to the uniformity of the applied heat and also the temperature increases in the form of a step that is proportional to the pressure, causes the synthesis of nanoparticles with uniform dispersion and the average size of the obtained particles is low. Also, one of the essential and fundamental principles in the synthesis of nanoparticles is their stability during the storage period, and in order to understand this feature, measuring the zeta potential is a great help in understanding this problem, which synthesis with uniform autoclave heat leads to nanoparticles with the proper surface charge that will ultimately make the nanoparticles more stable.











 nueva página del texto (beta)
nueva página del texto (beta)