Introduction
The link between oxidative stress and inflammation has been properly documented [1-3]. Oxidative stress is established once the activity of reactive oxygen species (ROS) in living systems overwhelms the ability of the system to eliminate the ROS efficiently. It has been reported to be a major promotor of numerous diseases, one of which is inflammation [4]. The mechanism of oxidative stress promoted inflammation involves the activation of some genes involved in inflammatory process [3]. Similarly, inflammatory process has also been strongly linked to the development of oxidative stress and diminution of antioxidant status [1]. Inflammation may also occur as a response to a variety of signals such as infection, disease condition or tissue injury [5]. Living systems produce certain inflammatory mediators such as cytokines, bioactive amines, eicosanoids, chemokines, bradykinin (a product of proteolytic reactions), which arises from the interactions of the innate and adaptive immune system, endothelial cells and other inflammatory stimulus linked to fibroblasts [6]. These mediators are known to promote inflammation and target specific tissues, manipulating their homeostatic properties. In combating, inflammation driven by oxidative stress and vice versa or those promoted by other factors (infection, tissues injury or inflammatory mediator activities), anti-inflammatory agents are employed which may act by suppressing the production of inflammatory mediators, or inhibition of enzymes linked with the inflammatory signals. Such agents range from steroids, non-steroidal anti-inflammatory drugs (NSAIDs), intravenous immunoglobulins, immunosuppressor cells, and neutralizing monoclonal antibodies to inflammatory cytokines [6]. However, these agents specifically the immunosuppressor cells and neutralizing monoclonal antibodies to inflammatory cytokines are expensive and rarely available in developing countries. Furthermore, the toxicity associated with the use of most anti-inflammatory agents has driven the search for the use of plants or natural products from plants as alternative and complementary therapies for the management of oxidative stress and inflammation. One key component from plants that have gained increasing interests are essential oils. Essential oils are derived from plants as secondary metabolites perfused with non-polar liquids that contains volatile ingredients. Generally, they are made up of terpenes and phenylpropanes [7]. Their therapeutic activities have been widely reported ranging from antibacterial, antifungal, antioxidant, antidiabetic, anti-obesity, anti-lipid peroxidation, anti-cancer etc. [8-13].
The plant Stachytarpheta jamaicensis belongs to the family Verbenaceae [14]. It is a spontaneous plant occurring naturally in cultivated areas with spontaneous growth [15]. Phytochemical screening of Stachytarpheta jamaicensis leaves from Nigeria have shown the presence of saponins, tannins and flavonoids, with strong antimicrobial activity [16]. Ataman et al. [17], reported the ability of the plant to treat malaria, diabetes, and serve also as antipyretic agent. Hot decoction from the aerial parts of the plant taken orally has been reported to promote the epithelial duct tract's operation, manage fever and chronic liver issues [18]. The antioxidant, anti-inflammatory, anti-arthritic and anti-bacterial activities of the methanol extract of the leaves have been reported by Ololade et al. [19]. Stachytarpheta jamaicensis has been reported to have anti-inflammatory, antinociceptive, anti-ulcerogenic [20], anti-diarrheal, sedative, and hypotensive activities [21]. However, despite the therapeutic potentials of this wonder plant, little or no scientific information has been reported on the therapeutic potentials of its essential oil fraction or components. Therefore, the study sought to report for the first time, the essential oil components of the leaves of Stachytarpheta jamaicensis, and its potential antioxidant and anti-inflammatory activities. The total phenolics and total flavonoids of the EO were also determined.
Experimental
Collection and preparation of plant sample
Fresh leaves of Stachytarpheta jamaicensis were collected from a garden in Umuariaga Village, Ikwuano Local Government Area of Abia State. The sample was identified and authenticated by Dr. Ibe K. Ndukwe, a taxonomist from the Department of Forestry, College of Natural Resources and Environment Management, Michael Okpara University of Agriculture, Umudike. Voucher specimen was prepared and kept at the herbarium of the department. The leaves were air-dried under shade for two (2) weeks at room temperature (20-25 °C) to attain a constant weight and pulverized into powder using a mechanical grinder.
Extraction of essential oil
The extraction of EO from leaves of Stachytarpheta jamaicensis was performed using the method described by Oboh et al. [22]. Briefly, EO was extracted from the milled samples (100 g), using hydro distillation techniques in a Clevenger apparatus. The essential oil layer obtained from the Clevenger apparatus was collected via the outlet and passed over anhydrous sodium sulphate to remove any water molecule. The EO was stored in sterile amber vial and stored at 4 °C for future analysis.
Phytochemical analysis of EO extracted from leaves of Stachytarpheta jamaicensis
Total phenolics and total flavonoid quantification
The total phenols (TP) quantification was performed using spectrophotometric techniques as described by Paśko et al. [23], using Folin-Ciocalteu reagent. Briefly, 2.7 mL of de-ionized water, 0.3 mL of extracts, 0.3 mL 7 % Na2CO3and 0.15 mL Folin-Ciocalteu reagent were properly mixed together, and the absorbance of mixture measured at 725 nm using a spectrophotometer (Jasco UV-530 Medson, Paczkowo, Poland). A standard curve was prepared with gallic acid. Final results were given as gallic acid equivalents (GAE). The total flavonoids content (TFC) in the sample was estimated by the method of Benzie and Strain [24]. Briefly, 1.25 mL of distilled water was added to 0.25 mL of the extract. Exactly 75 μL of 5 % sodium nitrite and 0.15 mL of aluminum chloride solution was added to the solution. 0.5 mL of 0.1 M NaOH was added after 5 min and made up to 2.5 mL with distilled water. The solution was mixed well, and the absorbance was read at 510 nm in comparison with standard quercetin at 5-25 μg/mL concentration. The results are expressed as mg of flavonoids as quercetin equivalent/mg of extract.
Gas chromatography mass spectrometry (GC-MS) analysis of EO
The components of the essential oil from leaves of Stachytarpheta Jamaicensis was analyzed using a gas chromatograph (Hewlett Packard Agilent 6890N, Palo Alto, CA, USA) coupled with an Agilent mass selective detector, driven by Agilent Chemstation software (Agilent Technologies, Palo Alto, CA, USA). A DB-5SIL MS capillary column was used (30 m × 0.25 mm × 0.25 µm). The carrier gas was ultra-pure helium at a flow rate of 1.0 mL/min and a linear velocity of 37 cm/s. The injector temperature was set at 250 °C, and the oven temperature programmed at 60 °C to 280 °C at a rate of 10 °C with a hold time of 3 min. Injections of 1 mL were made in the splitless mode with a manually split ratio of 1:0. The mass spectrometer operated in the electron ionization mode at 70 eV and electron multiplier voltage at 1859 V. Other MS operating parameters were as follows: ion source temperature 230 °C, quadrupole temperature 150 °C, solvent delay 4 min and scan range 50-700 m/z values. Total GC running time was 32 min, and the compounds were identified by direct comparison of the retention times (RT) and mass fragmentation pattern with those from the National Institute of Standards and Technology (NIST) library [25], and by comparing the retention indices (RI exp) of components with those available in literature (RI lit).
In vitro antioxidant assay
2, 2-Diphenyl-1-Picrylhydrazyl (DPPH) inhibition photometric assay
The free radical scavenging activity of the essential oil from leaves of Stachytarpheta jamaicensis was investigated by the DPPH assay [26]. The extract at concentrations (25, 50, 100, 200 and 400) µg/mL each was mixed with 1 mL of 0.5 mM DPPH (in methanol) in a cuvette. The absorbance (517 nm) was taken after 30 min of incubation in the dark at room temperature. The experiment was done in triplicate. The percentage antioxidant activities were calculated as follows.
One (1) mL of methanol plus 2 mL of the test extract was used as the blank while 1 mL of the 0.5 mM DPPH solution plus 2 mL of methanol was used as the negative control. Ascorbic acid (vitamin C) was used as reference standard.
Ferric reducing antioxidant power
The ferric reducing antioxidant power was carried out as described by Benzie and Strain [24]. The protocol involved is as follows: Reagents:
Acetate buffer (300 mM), pH 3.6 (3.1 g sodium actate•3H2O and 16 mL glacial acetic acid in 1000 mL buffer solution).
2, 4, 6-triphridyl-s-triazine (TPTZ) (10 mM) in 40 mM HCl.
FeCl3 6H2O (20 mM) in distilled water.
FRAP working solution was prepared by mixing solution 1, 2 and 3 in the ratio of 10׃1׃1, respectively. The working solution was freshly prepared. The FRAP reagent (3 mL) and 100 μL sample solution at concentrations of 25, 50, 100, 200 and 400 µg/mL was mixed and allowed to stand for 4 min. The absorbance was recorded at 593 nm, at 37 °C. The ascorbic acid was tested in a parallel process. The absorbance of each test tube was taken at 0 and 4 min after addition of sample.
Where, abs 4 min is the absorbance taken at 4 min and abs 0 min is the absorbance take just after initiating reaction.
Determination of anti-inflammatory activity
The effect of the essential oil on the hemolysis of human red blood cell (HRBC) in hypotonic saline solution was evaluated as described by Anosike et al. [27]. Blood sample (5 mL) was collected from a healthy male donor (that has not received anti-inflammatory drug in the past 10 days) into EDTA sample bottle. The HRBC was repeatedly washed with normal saline by centrifugation as described by Anosike et al., [27] until the supernatant was clear. Thereafter, 0.5 mL of 10 % suspension of the HRBC was added to test tubes containing different concentrations (25 - 400 µg/mL) of essential oil dissolved in hypotonic solution in triplicate. The mixtures were incubated for 30 min at 37 °C and later centrifuged at 3000 rpm for 5 min. The absorbance of the supernatants was recorded at 560 nm with spectrophotometer. Hypotonic solution was used as control while diclofenac (200 µg/mL) was used as reference standard.
Where: AA = absorbance of control, BB = absorbance of test substance
Heat-induced hemolysis assay
The effect of the essential oil on heat-induced hemolysis of HRBC was evaluated as described by Anosike et al. [27]. The blood collection and preparation were as stated in the previous section. Thereafter, 0.5 mL of 10 % suspension of the HRBC was added to test tubes containing different concentrations (25 - 400 µg/mL) of EO dissolved in phosphate buffer saline in triplicate. The mixtures were incubated for 30 min at 54 °C and later centrifuged at 3000 rpm for 5 min. The absorbance (ABS) of the supernatant was determined at 560 nm with spectrophotometer. Hypotonic solution was used as control while diclofenac (200 µg/mL) was used as reference standard.
Where: abo = absorbance of control, abu = absorbance of test
Ethical clearance with number (CLU/FS/ETC/2021/047) was obtained from the Ethical Committee of the Department of Chemical Sciences, Faculty of Science, Clifford University, Owerrinta, Abia State, Nigeria.
Statistical analysis
The present data were statistically analysed using SPSS software Ver. 22. Mean values separated by the Duncan multiple tests. The different levels of significance within the groups were examined using one-way analysis of variance (ANOVA). For data involving two means, student T -test was employed. The data were expressed as mean ± SD and considered significant at p < 0.05.
Results
Phytochemical analysis of essential oil from leaves of Stachytarpheta jamaicensis
Total phenol and flavonoid contents of essential oil from leaves of Stachytarpheta jamaicensis
The total phenol and flavonoid contents of essential oil of Stachytarpheta jamaicensis leaves is presented in Table 1. The total phenol content was 25.78 ± 0.68 GAEequ.mg/g, while the total flavonoid was 6.92 ± 0.22 mgQE/100mg.
Gas chromatography mass spectrometry quantification of essential oil obtained from leaves of Stachytarpheta jamaicensis
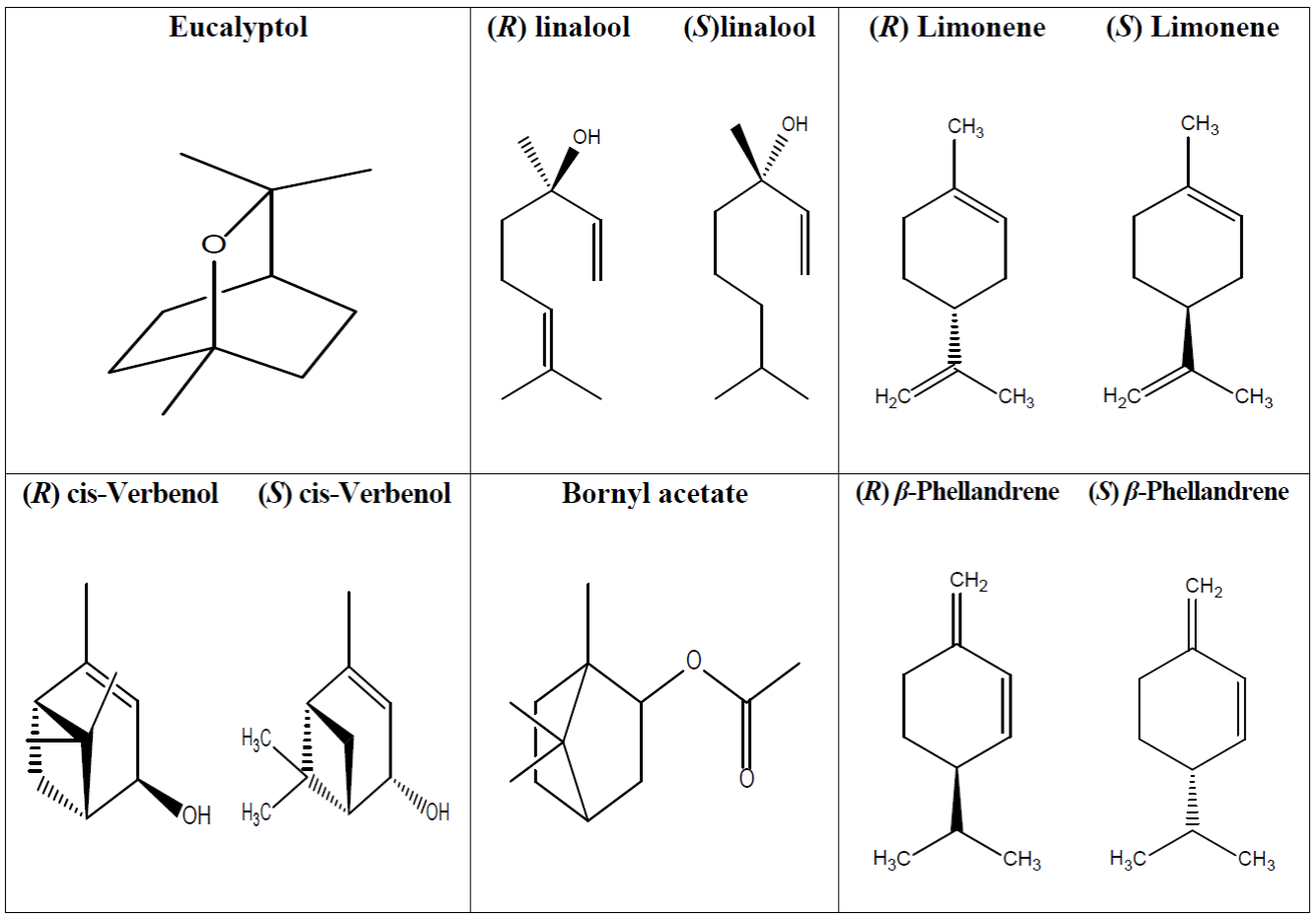
The identified components in the essential oil obtained from the leaves of Stachytarpheta jamaicensis using GC-MS is presented in Table 2. below. The identified components were majorly limonene (13.85 %), β-phellandrene (5.59 %), eucalyptol (10.73 %), linalool (5.36 %), cis-Verbenol (19.54 %) and bornyl acetate (12.65 %).
Table 2 GC-MS identification of major components in essential oil obtained from Stachytarpheta jamaicensis.
| Peak No. | Name | RT (min) | M.W. (g) | Conc. (%) | RI (EXP) | RI (LIT) | Formula | Class of comp. | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | α-Pinene | 4.56 | 136 | 0.56 | 937 | 939 [28] | C10H16 | Bicyclic monoterpene | |
| 4 | β-Pinene | 5.61 | 136 | 0.60 | 979 | 981 [29] | C10H16 | Bicyclic monoterpene | |
| 5 | Limonene | 6.11 | 136 | 13.85 | 1030 | 1031 [30] | C10H16 | Cyclic monoterpene | |
| 6 | β-Phellandrene | 6.70 | 136 | 5.59 | 1031 | 1031 [28] | C10H16 | Cyclic monoterpene | |
| 7 | Eucalyptol | 6.80 | 154 | 10.73 | 1032 | 1026 [31] | C10H18O | Monoterpene | |
| 8 | γ-Terpinene | 7.07 | 136 | 0.55 | 1060 | 1054 [31] | C10H16 | Monoterpene | |
| 12 | Linalool | 8.25 | 154 | 5.36 | 1099 | 1095 [31] | C10H18O | Cyclic monoterpene | |
| 13 | endo-Borneol | 8.39 | 154 | 0.40 | 1167 | 1165 [30] | C10H18O | Bicyclic monoterpene | |
| 16 | Geijerene | 12.15 | 162 | 0.42 | 1143 | 1139 [32] | C12H18 | Monoterpene | |
| 17 | cis-Verbenol | 15.36 | 152 | 19.54 | 1142 | 1142 [33] | C10H16O | Bicyclic monoterpene | |
| 18 | trans-Verbenol | 16.05 | 152 | 1.26 | 1144 | 1150 [33] | C10H16O | Bicyclic monoterpene | |
| 19 | Pinocarvone | 17.47 | 150 | 0.45 | 1164 | 1163 [34] | C10CH14O | Bicyclic monoterpene | |
| 21 | D-Verbenone | 17.83 | 150 | 0.94 | 1228 | 1218 [35] | C10H14O | Monoterpene | |
| 23 | Bornyl acetate | 18.04 | 196 | 12.65 | 1285 | 1285 [30] | C12H20O2 | Bicyclic monoterpene |
RT- retention time; MW- molecular weight; Conc- concentration; RI (EXP)- retention index (experimental); RI (LIT)- retention index (literature).
In vitro antioxidant activity of essential oil from leaves of Stachytarpheta jamaicensis
The in vitro antioxidant activity of the essential oil from leaves of Stachytarpheta jamaicensis showed a dose dependent activity, with the highest dose (400 μg/mL) having the highest activity. At all studied concentration, the activity of the essential oil was significantly (p<0.05) lower than the standard agent used (vitamin C).
Table 3 In vitro antioxidant activity of essential oils from leaves of Stachytarpheta jamaicensis.
| Conc. (μg/mL) | DPPH (% inhibition) | FRAP (µM) | ||
|---|---|---|---|---|
| EOSJ | Ascorbic acid | EOSJ | Ascorbic acid | |
| 25 | NA* | 66.42 ± 2.99 | 0.003 ± 0.002* | 0.029 ± 0.002 |
| 50 | 8.89 ± 0.27* | 84.95 ± 1.94 | 0.006 ± 0.004* | 0.035 ± 0.006 |
| 100 | 10.30 ± 1.32* | 88.28 ± 1.49 | 0.012 ± 0.001* | 0.049 ± 0.004 |
| 200 | 14.27 ± 2.03* | 90.26 ± 0.23 | 0.016 ± 0.002* | 0.067 ± 0.002 |
| 400 | 35.20 ± 0.39* | 92.41 ± 0.09 | 0.027 ± 0.001* | 0.106 ± 0.011 |
Values are Mean ± SD of triplicate determinations. Values with an asterisk (*) are significantly (p<0.05) different across the rows. NA- No activity, EOSJ = Essential oil of Stachytarpheta jamaicensis leaves.
In vitro anti-inflammatory activity of essential oil from leaves of Stachytarpheta jamaicensis
The human red blood cell (HRBC) membrane stabilization activity showed a dose dependent, with the highest dose (400 µg/mL) having the highest activity. At 200 µg/mL, the essential oil from the leaves of Stachytarpheta jamaicensis was more potent than the standard agent used (diclofenac).
Table 4 Human red blood cell (HRBC) membrane stabilization activity.
| Conc. (μg/mL) | Hypotonicity (% inhibition) | Heat (% inhibition) | ||
|---|---|---|---|---|
| EOSJ | Diclofenac | EOSJ | Diclofenac | |
| 25 | 0.76 ± 2.26 | 4.62 ± 0.97 | ||
| 50 | 4.04 ± 1.19 | 6.26 ± 1.79 | ||
| 100 | 5.39 ± 1.70 | 8.21 ± 1.79 | ||
| 200 | 20.29 ± 2.29* | 13.09 ± 2.05 | 28.58 ± 1.60* | 25.49 ± 0.70 |
| 400 | 25.59 ± 1.63 | 29.68 ±3.50 | ||
Values are mean ± SD of triplicate determinations. Values with an asterisk (*) are significantly (p<0.05) different across the rows. EOSJ- Essential oil of Stachytarpheta jamaicensis leaves.
Discussion
This study investigated the in vitro antioxidant and anti-inflammatory potentials of essential oil (EO) from leaves of Stachytarpheta jamaicensis. The EO demonstrated fair antioxidant activity, but showed very strong anti-inflammatory potentials, which can be linked to the components detected in the EOs such as limonene, eucalyptol, cis-Verbenol, bornyl acetate linalool and β-Phellandrene. The total phenolics and flavonoids content of the essential of Stachytarpheta jamaicensis is presented in Table 1. The total phenolic concentration (TPC) was 25.78 ± 0.68 GAEequ.mg/g and total flavonoid content (TFC) 6.92 ± 0.22 mgQE/100mg. The presence of flavonoid corresponds with the report of Idu et al. [16], who identified flavonoids in leaves of the Stachytarpheta jamaicensis. Similarly, phenolics were identified via phytochemistry analysis of the hydro alcohol extract of leaves of Stachytarpheta jamaicensis [37-38]. The values reported in this study for total phenolics and total flavonoids was higher than the values reported by Sivaranjani et al. [39] and Ololade et al. [19] for leaves extract of Stachytarpheta jamaicensis grown in India and Nigeria respectively. The difference observed in the TFC and TPC and those reported in literature could be attributed to differences in environmental conditions, planting season, soil type, species and climatic conditions [40]. The antioxidant and anti-inflammatory activities of phenolics and flavonoids have been reported [41]. The structural features of phenolics and flavonoids promotes them to act as antioxidant compounds. Phenolics ability to donate hydrogen make it serve as an antioxidant [42], while flavonoid’s OH- in its structure has suggested it to be an antioxidant [43,44]. Collectively, these molecules are able to interact with free radicals, neutralizing them [45].
GC-MS analysis of essential oil from leaves Stachytarpheta jamaicensis to identify and quantify the essential oil components is presented in Table 2. Result showed the presence of cis-Verbenol, limonene, bornyl acetate, eucalyptol, β-Phellandrene and linalool as major components of the EO with concentrations of 19.54 %, 13.85 %, 12.65 %, 10.73 %, 5.59 % and 5.36 % respectively, representing a total of 67.72 % of the volatile fractions. The concentration of EO components in this study was higher than the values reported for Stachytarpheta indica, a similar specie in the same family [46]. The components of EO identified in this study have been reported to have antioxidant and anti-inflammatory properties. cis-Verbenol have been reported to inhibit the expression of pro-inflammatory cytokines in ischemic brain, and eradicated peroxyl radicals [47]. Moreso, cis-Verbenol and limonene have been reported to be a good inhibitor of leukotriene production, therefore managing inflammatory issues [48]. The antioxidant activity of limonene has been reported via its ability to inhibit free radicals [49]. Limonene anti-inflammatory ability has also been confirmed via the inhibition of cytokines, ROS and eosinophil migration in bronchial asthma. Studies by Kim et al. [50] reported the antioxidant potentials of bornyl acetate via the inhibition of DPPH. Bornyl acetate showed anti-inflammatory via the inhibition of nitric oxide and prostaglandin E2 production in lipopolysaccharide activated macrophages [51]. Furthermore, in animal model, bornyl acetate inhibiting mice writhing reaction and alleviated pain caused by hot plate on mice [52]. Eucalyptol showed anti-inflammatory effects through the inhibition of the development of edema, neutrophil migration and vascular permeability in rats induced by carrageenan, dextran and histamine [53]. Moreso, in vitro studies have established that eucalyptol regulates inflammatory processes and production of inflammatory mediators [54]. The antioxidant properties of eucalyptol have been investigated using aldehyde/carboxylic acid conversion assay and malonaldehyde formation from lipid oxidation. Eucalyptol from eucalyptus species successfully inhibited the oxidation of hexanal and malondialdehyde formation [55]. Treatment of Carp with eucalyptol was reported to down play inflammatory gene expression while upregulating genes associated with antioxidant activities. Further, eucalyptol reduced MDA levels and improved the activities of catalase in fishes exposed to copper [56]. Siqueira et al. (57), reported that phellandrene anti-inflammatory potentials lies on its ability to modulate neutrophil migration and stabilization of mast cells. Linalool has been reported to inhibit DPPH [58].
The in vitro antioxidant assay using DPPH inhibition and FRAP assay was used to ascertain the antioxidant capacity of the essential oil from Stachytarpheta jamaicensis. Result for DPPH and FRAP assays presented in Tab. 3 followed a dose dependent approach. At 400 µg/mL the essential oil showed highest DPPH inhibition (35.20 ± 0.39 % inhibition) and FRAP (0.027 ± 0.001 µM) activities. However, this activity was lower than that of ascorbic acid, the standard antioxidant agent used. The DPPH inhibition ability of the EO in this study suggest the ability of the EO to contain compounds that can act as hydrogen donors, scavenging free radicals or inhibiting generators of free radicals. The components of EOs in this study have previously been reported to be potent antioxidant agents. Furthermore, the presence of phenolics and flavonoids contributes to antioxidant potentials [59], and this was evident in this study. Result for DPPH inhibition at 400 µg/mL was similar to the report by Sivaranjani et al. [39] who reported the DPPH inhibition of chloroform extract of leaves of Stachytarpheta jamaicensis as 35.51 ± 0.09 % inhibition. However, our result was higher than the acetone leaves extract and lower than petroleum ether, ethanol and methanol extracts. The antioxidant potentials of ethyl acetate extract of leaves of Stachytarpheta jamaicensis showed that it sufficiently suppressed the production of reactive oxygen species [60], and this was implicated in our study. This suggests the antioxidant potentials of the essential oil, and its potential application of the EO from Stachytarpheta jamaicensis in managing oxidative stress and its promoted diseases.
To ascertain the anti-inflammatory potentials of the essential oil of leaves of Stachytarpheta jamaicensis, we employed the human red blood cell (RBC) membrane stabilization assay, and results are presented in Tab. 4. Similarly, the result for the anti-inflammatory assay employed showed a dose dependent approach, with 400 µg/mL of the essential oil having the most potent inhibition activity for hypotonicity induced hemolysis (25.59 ± 1.63 % inhibition), and heat induced (29.68 ± 3.50 % inhibition). Interestingly, the essential oil of leaves of Stachytarpheta jamaicensis showed better % inhibition activity than the standard anti-inflammatory agent used (diclofenac) at dosage of 200 µg/mL for the performed assays. The values reported for RBC membrane stabilization assay was low compared to the reports of Siju et al. [61] for aqueous and ethanol extracts of whole plant of Stachytarpheta cayennensis grown in India. The strong anti-inflammatory activities can be linked to the activities of the components of the essential oil from the leaves of Stachytarpheta jamaicensis. Their individual anti-inflammatory activities have been previously mentioned. The essential oil sufficiently preserved the human red blood cell membrane from hypotonic saline solution and heat stress induced lysis. Inflammation is known to promote the lysing of the RBC membranes, leading to loss of cellular activity and leakage of cellular components [27]. Furthermore, inflammatory mediators such as cytokines has been reported to increase membrane permeability [62], and since the RBC membrane is similar to lysosomal membrane, the inhibition of RBC membrane destabilization and hemolysis by heat and hypotonic saline solution by the essential oil of Stachytarpheta jamaicensis can justify its use in managing inflammatory conditions. The components of the EO in this study may have inhibited inflammatory mediators and the release and activity of lytic enzymes, stabilizing the RBC membrane. Phenolics and flavonoids are known to inhibit the activities of enzymes involved in the production of certain inflammatory mediators [63-64]. Collectively, this substantiates the use of the plant in managing inflammation and its related diseases.
Conclusion
In conclusion, the study has shown that the essential oil from leaves of Stachytarpheta jamaicensis essential oil (EOS) contained bioactive compounds such as cis-Verbenol, limonene, bornyl acetate, eucalyptol, linalool and β-phellandrene with antioxidant and anti-inflammatory properties. Therefore, the study suggests that the essential oil from the leaves can be a potential source of antioxidant and anti-inflammatory agents that can be used in medicine. Further studies are warranted to knock out the possibility of toxicity, purify and isolate these compounds of interest for medical application.











 nueva página del texto (beta)
nueva página del texto (beta)