Introduction
The problem of marine corrosion resistance of steels is still urgent. A clear data of mechanical and physical properties of such materials, stability properties, the temperature ranges of use and the knowledge of their processing and exploitation in marine conditions is the key to significant economy. Being one of the largest corroded and abundant natural electrolytes, seawater has a corrosive behavior that shows that most common metals and alloys are attacked by that liquid. Corrosion resistance of metals used as building materials is essential for marine construction planning. Corrosion of metals and alloy in the marine environment was studied from the beginning of the century. The first date were reported by many authors [1-3]. Iron and low alloy cast iron are the primary cast alloy used for marine applications. Schumacher [4] reported that the corrosion of steels, grey cast iron, alloy cast iron and austenitic cast iron is proportional the concentration of oxygen. When metals are submerged in seawater, corrosion is faster. An important structural material and used for many years in marine applications was steel. The naval and petrochemical industry are affected by sea water corrosion in their structures used, such as ships, platforms, pipelines, distribution systems and chemical processeses. Due to the presence of ions dissolved in it, seawater produces a much faster corrosion of metals than freshwater. The sea waters containing chlorides and various salts, corrode the majority of metals and alloys. Corrosion of steel in marine environments can be quite extensive and normally proceeds very rapidly to beginwith and then levels off to a linear relationship [5]. As a result of spraying metals with sea water, there is a permanent reduction in the quality of metals, which are structurally disadvantageous but which can also cause degradation that can lead to real disasters. Based on the above facts the present work is undertaken in order to evaluate the corrosion influence of sea waters on the structure and magnetic properties of SR355JR and S355J2 steels.
Experimental
Materials and methods
The nominal chemical composition of test material of carbon steel SR355JR(SR EN1025-2/1994) and S355J2 (SR EN1025-2/2004) is given in Table 1.
Table 1 Chemical composition of mild steel used (wt.%).
| Element % | C | Si | Mn | P | S | N | Cu | Al | Fe |
| SR355JR | 0.24 | 0.55 | 1.6 | 0.35 | 0.35 | 0.012 | 0.55 | - | 96.98 |
| S355J2 | 0.20 | 0.55 | 1.6 | 0.25 | 0.25 | - | 0.02 | 0.02 | 96.58 |
The size of the steel samples were 50 mm × 25 mm × 5 mm. They were cleaned wet-polished with 2000# grade SiC paper, then cleaned in alcohol and acetone, after which they were dried, weighed and stored in a desiccator until use. The corrosive media used was natural sea water from Black, Aegean and Mediterranean Sea. Literature present that the salinity of those sea waters are: 17-18.5 g/L Black Sea, 38 g/L Aegean Sea and 37-39 g/L Mediterranean Sea [6].
The polarization curves were obtained using PARSTAT 2273 with a “Power Corr” software [7] with a classical three-electrode system: platinum plate (Radiometer) as the auxiliary electrode, Ag/AgCl (0.5M KCl Radiometer) electrode as the reference electrode, and steel samples (well-deffined working surface of 1cm2 area) as the working electrode. Natural aerated sea waters from Black, Aegean and Mediterranean seas were chosen as the electrolyte. The sample were immersed in the electrolyte before the tests start and were allowed to reach equilibrium, which usually took around 20 min. In order to obtain the linear polarization curves, the electrode potential was sweeping by ± 20 mV with respect to the OCP starting from the cathode pinace region, a constant value of the OCP was reached. Linear polarization curves were obtained by sweeping the electrode potential in the range of ±20 mV versus OCP starting from cathodic region, after a constant value of OCP was achieved. Tafel polarization experiments were performed with a constant scan rate of 0.166 mV⋅s-1, while the potential was shifted within ±250 mV vs. EOCP [8]. The Tafel plots were registered one year after from the introduction of the samples in the sea waters.
Characterization of the two carbon steels before and after the corrosion process in different seawaters was performed by X-ray analysis (XRD) and metallographic microscopy.
X-ray analysis of the studied samples was carried out on the modified and automatized apparatus “DRON-2” in CuK ( -radiation in the 20°≤2θ≤100° angle range at room temperature. X-ray patterns are received with automatic recording of the reflexes intensities with a 0.03° scanning step and the exposure time of 2-3s. For the purpose the crystal cell parameters determining were scanned the separate reflexes with a step of 0.01° with the exposure to 10 seconds. Using the PCPDFWIN version 2.0 we identified the phase composition of the samples. An optical metallurgical microscope (New York Microscope Comp.) with magnifications of □100 to □800 and with high digital camera was used to identify the macroscopic morphologies of the steel samples before and after the corrosion process in sea waters.
The study of magnetic properties of samples is carried out using automatized setting, which works on base of a method of the ponderomotive force measuring. The method makes it possible to investigate temperature dependences of magnetization and magnetic susceptibility taking small amounts of substance. This makes it possible to achieve relatively fast equilibrium temperature over the entire volume of the sample. It is obvious that the absence of the temperature gradient on the sample at the specific magnetization or susceptibility measurement moment provides the most accurate determination of their values. The specific magnetization temperature dependences were studied in the temperature range 77-1100 K by ponderomotive method in the 0.86 T field.
Results and Discussion
Electrochemical measurements
The corrosion test were performed in sea waters by the methods of time evolution potential without current (open circuit potential-OCP) and polarization curves (Tafel). The plot (curve) corrosion/time respects a logarithmic law [9,10]. The open circuit potential (OCP) of the studied steels is expected to be dependent on the characteristics of resulted oxide, such as oxide thickness, composition, conductivity, structure, etc. Immediately after the immersion, EOCP heads towards the noble direction, gradually approaching the value of the quasi-stable state (Ess) within 40 minutes. The OCP values range for SR355JR from -0.637 V to -0.662 V and for S355J2 from -0.598V to -0.636V as presented in Table 2. The time evolution of OCP indicates that the oxide film is thinning and consequent becomes vulnerable toward anion penetration of diferent ions presents in the sea waters. We noticed slight oscillations of OCP in time and we assume that these OCP oscillations may result from chemical interactions between chloride ions and the structured passive oxide film. Tafel plots were used in order to evaluate the corrosion resistance of the two steel in the studied seawaters. Fig. 1 show the Tafel plots obtained from the polarization curves of the SR355JR and S355J2 steel samples in the tree sea water in study. The corrosion plots vs.time respects a logarithmic law [11,12]. Corrosion parameters calculated from Tafel plots are summarized in Table 2 [13]. It can be observed from Fig. 1 that corrosion potentials (Ecorr) for both steel samples are very close each other in all three seawaters, simmilar to EOCP. The Tafel plots and corrosion data indicate that both steels have good corrosion resistance in seawaters with corrosion rates ranging from 0.221 - 0.503 mm/year. This good corrosion resistance express that the passive layer formed on its surface is stable and resistant.
Table 2 Corrosion parameter for electrochemical measurements of corrosion in sea waters.
| Sea water | Sample | EOCP / V | Rp / □ | Ecorr / V | Icorr / A | CR / mm/year |
|---|---|---|---|---|---|---|
| Black Sea | SR355JR | -0.662 | 354.73 | -679.484×10-3 | 43.01×10-6 | 0.503 |
| S355J2 | -0.598 | 366.98 | -599.341×10-3 | 33.92×10-6 | 0.418 | |
| Mediteraneean Sea | SR355JR | -0.657 | 955.15 | -890.767×10-3 | 26.10×10-6 | 0.329 |
| S355J2 | -0.636 | 10168 | -636.310×10-3 | 19.63×10-6 | 0.247 | |
| Aegean Sea | SR355JR | -0.637 | 14180.57 | -559.999×10-3 | 31.26×10-6 | 0.365 |
| S355J2 | -0.598 | 28604.70 | -568.535×10-3 | 18.82×10-6 | 0.221 |
EOCP=open circuit potential; Ecorr=corrosion potential; Icorr= corrosion current; CR=corrosion rate.
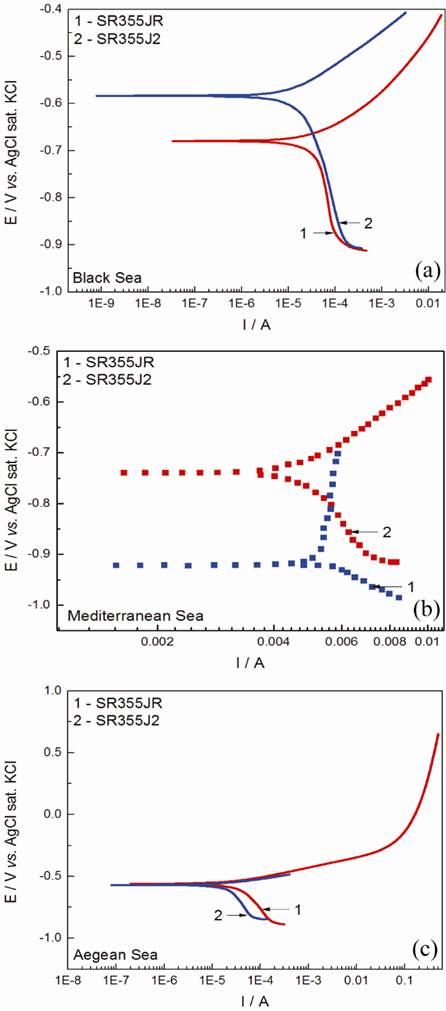
Fig. 1 Potentiodynamic polarization curves of SR355JR and S355J2 in: (a) Black Sea; (b) Mediterranean Sea; (c) Aegean Sea.
The results show a good corrosion resistance in all three seawaters in study for S355J2 if to compare to SR355JR steel and are in good agreement with the polarization resistance obtained by LP. It is obvious that the S355J2 impedes the attack of the aggressive ions (Cl-) on the electrode surface. The best corrosion rate was obtained for the S355J2 steel in Aegean seawater.
It was found that both SR355JR and S355J2 has the highest corrosion rate in Black seawater which has the lowest salinity and the lowest conductivity (□=24.41 mS) which is not very common. Most common the higher corrosion rate takes place due to the role of oxygen and chloride in electrochemical reactions [14]. It is already established that this media of seawater containing high amount of chlorine ions, promote the formation of FeOOH and Fe2O3•H2O which will facilitate the corrosion. Also we believe that the corrosion rate was found so high due to the presence of H2S in the region from where the seawater was collected. This region is well known with the presence of H2S and that is why here there are many spa sanatoriums. The pH was found to be 7.63 (determined by us in the laboratory).
The H2S accelerate the corrosion process. As for the fact that corrosion rate of both steels are lower in Mediterannean sea and in Aegean sea even if the salinity of these seawaters are much higher, we believe that the decrease in corrosion rate can be related to the formation of a passive film on the surface of the steel, which behaves as a protective layer against further corrosion. The XRD and micrographic data confirmed this assumption.
Data from Table 2 show that in all three seawaters in study S355J2 has a better corrosion resistance than SR355JR. We believe that even if the composition of the two stees are very appropriate, the presence of few amount of Al in S355J2 gives it better corrosion resistance.
XRD diffraction analysis
X-ray diffraction (XRD) pattern of initial and corroded samples of SR355JR and S355J2 steels in the 3 sea waters in study are presented in Fig. 2. Diffraction reflections on the X-ray diffraction pattern are given in Tables 3 and 4.
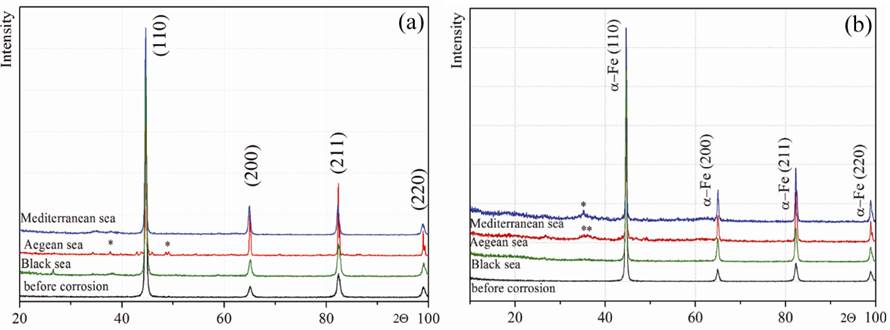
Fig. 2 XRD-Analysis of SR355JR (a) and S355J2(b) samples before and after corrosion in different sea waters.
Table 3 Phase composition of the SR355JR steel in initial stage and electrochemical corroded in the 3 sea waters studied.
| Sample/Sea water | 2θ / degree | (hkl) / Phase composition |
|---|---|---|
| Initial stage | ||
| SR355JR | 44.67 | (110) α-Fe |
| 65.07 | (200) α-Fe | |
| 82.27 | (211) α-Fe | |
| 98.91 | (220) α-Fe | |
| Corroded stage in | ||
| Black Sea | 26.59 | (001) Fe2O3( H2O |
| 44.71 | (110) α-Fe | |
| 65.07 | (200) α-Fe | |
| 82.35 | (211) α-Fe | |
| 99.07 | (220) α-Fe | |
| Aegean Sea | 37.71 | *(031) FeOOH |
| 44.63 | (110) α-Fe | |
| 48.67 | *(051) FeOOH | |
| 49.11 | *(220) FeOOH | |
| 64.99 | (200) α-Fe | |
| 82.35 | (211) α-Fe | |
| Mediterranean Sea | 98.95 | (220) α-Fe |
| 44.63 | (110) α-Fe | |
| 64.91 | (200) α-Fe | |
| 82.23 | (211) α-Fe | |
Table 4 Phase composition of the S355J2 steel in initial stage and electrochemical corroded in the 3 sea waters.
| Sample / Sea water | 2θ / degree | (hkl) / Phase composition |
|---|---|---|
| Initial stage | ||
| S355J2 | 44.63 | (110) α-Fe |
| 64.91 | (200) α-Fe | |
| 82.35 | (211) α-Fe | |
| 98.87 | (220) α-Fe | |
| Corroded stage in | ||
| Black Sea | 44.63 | (110) α-Fe |
| 64.95 | (200) α-Fe | |
| 82.27 | (211) α-Fe | |
| 98.91 | (220) α-Fe | |
| Aegean Sea | 35.31 | *iron oxide hydrate [e.g. FeO(OH)·nH2O] |
| 35.91 | *iron oxide hydrate | |
| 44.67 | (110) α-Fe | |
| 65.03 | (200) α-Fe | |
| 82.31 | (211) α-Fe | |
| 98.91 | (220) α-Fe | |
| Mediterranean Sea | 35.15 | *iron oxide hydrate |
| 44.63 | (110) α-Fe | |
| 65.03 | (200) α-Fe | |
| 82.27 | (211) α-Fe | |
| 98.91 | (220) α-Fe | |
The main phase for initial samples according chemical composition is α-phase of pure iron with unit cell of Im3m space group. Analysis of the patterns showed that after conducting studies of corrosion resistance, the main phase of the Im3m type of α-iron was preserved.
After electrochemical corrosion tests, the formation of a few amount of iron oxides and iron oxide hydrates is observed on the samples surface after corrosion action of sea waters.
In the case of the corrosive effect of Mediterranean water on steel SR355JR (Fig. 2a), weak diffuse scattering is observed in the X-ray pattern in the 35-40°angle range, which may indicate the presence of iron oxides on the surface of the samples. Due to their small amount, it is difficult to accurately determine the phase composition of corrosion products.
The processing of X-ray patterns is carried out using the FullProf Suite program, which is based on the Rietveld method to clarify the parameters of the crystal cell. The lattice parameter determination error was ± 0.0002 nm.
The average crystallite size is calculated by the Debye-Scherrer [15,16] formula:
where: d-average crystallite size, □-X-ray CuKα-radiation wavelength (in our case □=0.154056 nm); B-width of the diffraction peak at half-height, in radians (FWHM), □-diffraction peak position, in radians.
Dislocation density is determined by the formula:
Reflexes of X-ray patterns are indicated in accordance on base of PCPDFWIN database ID 06-0696 (a 0.2866 nm). The unit cells parameters, size of the crystallites and the dislocation density of the SR355JR and S355J2 steels before the sea waters corrosion are presented in Table 5.
Table 5 Unit cell parameters, the crystallite size and the dislocation density of the SR355JR and S355J2 alloys before and after the corrosive effects of sea waters.
| Sample | Corrosion media | a / nm | d / nm | δ 10-3 / nm-2 |
|---|---|---|---|---|
| SR355JR | before corrosion | 0.2868 | 15.77 | 4.02 |
| Mediterranean Sea | 0.2873 | 17.42 | 3.29 | |
| Black Sea | 0.2868 | 14.63 | 4.66 | |
| Aegean Sea | 0.2870 | 15.74 | 4.03 | |
| S355J2 | before corrosion | 0.2870 | 15.54 | 4.13 |
| Mediterranean Sea | 0.2871 | 18.54 | 2.91 | |
| Black Sea | 0.2871 | 17.14 | 3.40 | |
| Aegean Sea | 0.2871 | 17.88 | 3.12 |
Microscopic study
Microscopic images of the two studied steels before and after corrosion in the 3 sea waters are presentd in Figs. 3 and 4. The morphologies obtained provide us with information on the rust layer deposited during corrosion.It can be seen from Figs 3d, f and 4d, h that the rust layers have different colors, which mean different forms. Fig. 3 shows that SR355JR steel has a not very compact and homogeneous surface before corrosion and after corrosion the surface of that sample show some changes due to the corrosiveness of sea waters. In images from Fig. 3 we believe that the appearence of the colored phases is due to the formation of oxides or other coumpounds. In picture 3d and 3f we suppose the formation of the stable iron oxide hydrates (as presented by XRD data). On the whole, microscopic data are in perfect correlation with electrochemical results for SR355JR (showing the lowest corrosion in Mediterranean seawater).
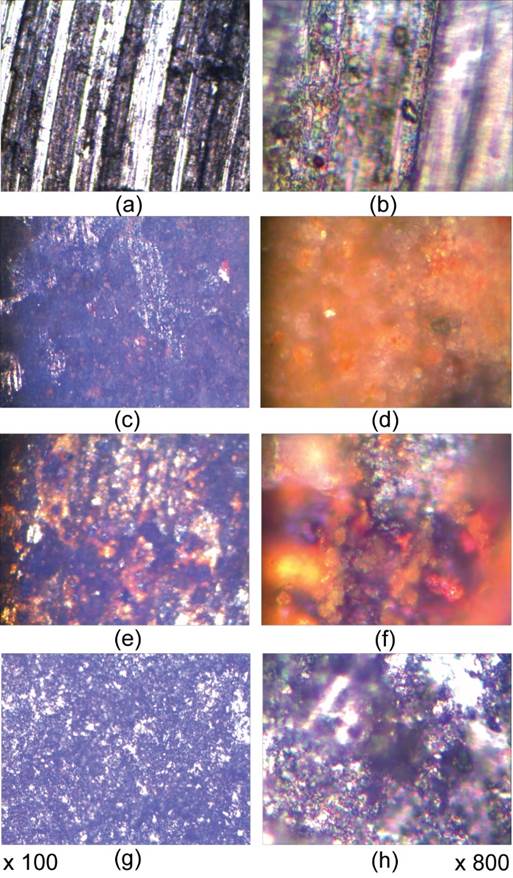
Fig. 3 Microscopic study for SR355JR with magnifiers of ×100 and ×800: (a), (b) Initial stage; and electrochemical corrosion in: (c), (d) Black Sea; (e), (f) Aegean Sea; (g), (h) Mediterranean Sea.
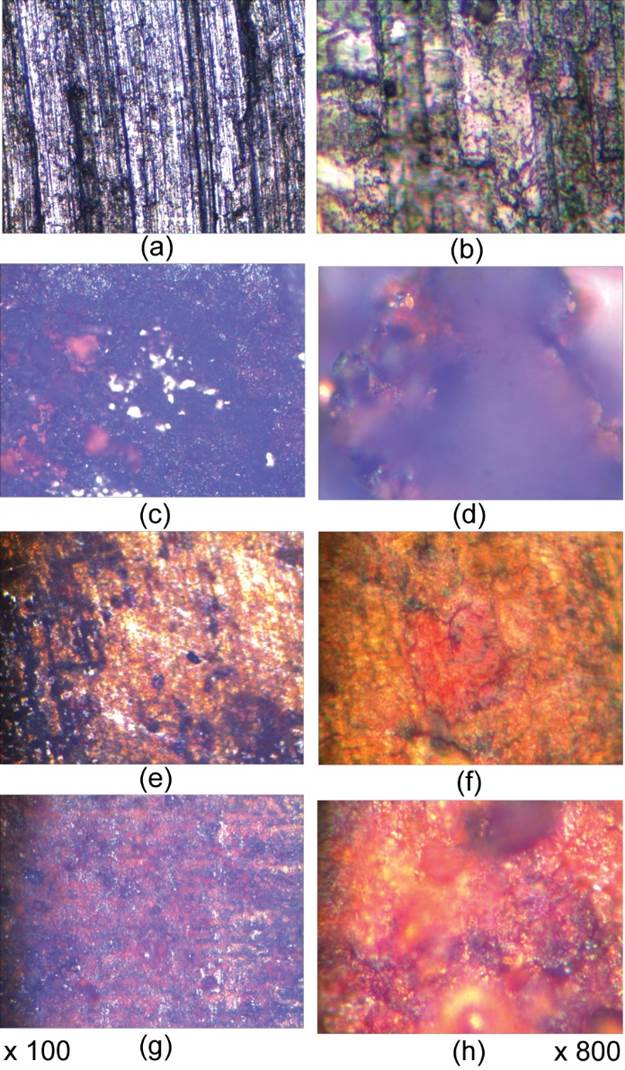
Fig. 4 Microscopic study for S355J2 with magnifiers of ×100 and×800: (a), (b) Initial stage; and electrochemical corroded in: (c), (d) Black Sea; (d), (e) Aegean Sea; (f), (g) Mediterranean Sea.
Microscopic images from Fig. 4 sustain the electrochemical results of corrosion for S355J2. Fig. 4c,d show the better resistance of S355J2 in Black Sea as compared with SR355JR. Fig. 4e-h demonstrate the presence big rust film formed on the surface of the corroded sample (hydrated iron oxides can be from red through dark-brown to black, depending on the degree of hydration, particle size and shape).
The micrographic study supports the results of electrochemical corrosion and XRD measurements for both researched steels.
Magnetic properties
One of the objectives of our study was to determine the corrosion resistance of the specific magnetic characteristics of SR355JR and S355J2 steels. The degradation of the functional characteristics of materials leads to the need to replace the products parts, and as a result, costly repairs. It was important to show that, from a practical point of view, corrosion does not affect the magnetic properties of a studied bulk sample. This is the reason for the choice of the experimental technique for studying the magnetic properties.
Fig. 5 presents the comparison of temperature dependence of specific magnetization for SR355JR and S355J2 on native state and electrochemical corroded in different sea waters.
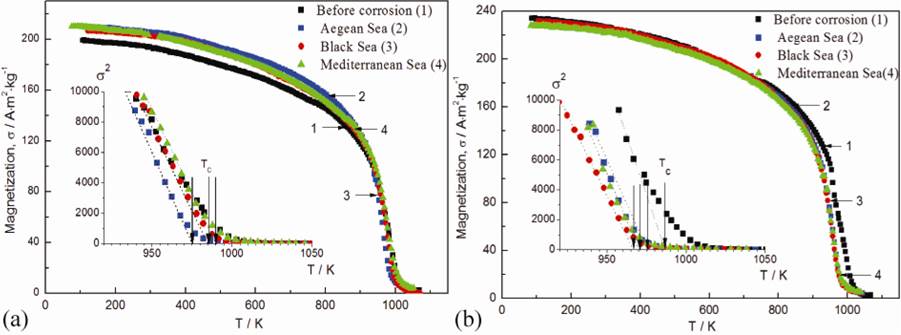
Fig. 5 The comparison of temperature dependence of specific magnetization for SR355JR and S355J2 on native state and electrochemical corroded in different sea waters.
The inserts of the Fig. 6(a, b) show the dependences σ2=f(T), allowing a more accurate determination of the Curie temperatures. Calculated magnetic parameters of the two steels in study before and after the electrochemical corrosion of the three studied sea waters are presented in Table 6. Steel has a similar nature of dependencies, confirming the presence of free iron. This fact is according with X-ray studies of the sample crystal structure. At nitrogen temperature the specific magnetization has a value of 200 A∙m2∙kg-1 for SR355JR steel. It should be noted that the dependences σ=f(T) of all samples on cooling coincide with the dependences σ=f(T) on heating. Curie temperatures determined from the dependences σ2=f(T) are near 970 K for the studied steel.
Table 6 Magnetic parameters of the SR355JR and S355J2 alloys before and after the electrochemical corrosive effects of sea waters.
| Sample | Corrosion media | Tc / K | σ77 / A∙m2∙kg-1 |
|---|---|---|---|
| SR355JR | before corrosion | 990 | 203.1 |
| Mediterranean Sea | 975 | 208.4 | |
| Black Sea | 975 | 204.1 | |
| Aegean Sea | 975 | 205.7 | |
| S355J2 | before corrosion | 985 | 233.9 |
| Mediterranean Sea | 985 | 227.2 | |
| Black Sea | 980 | 227.8 | |
| Aegean Sea | 975 | 226.2 |
A comparative analysis of the specific magnetization temperature dependences and data from Table 6 before and after corrosion action of three sea water types on SR355Jr and S355J2 one can conclude the high corrosion resistance of the stainless-steel magnetic characteristics.
There are literature data indicating that the corrosion rate depends on the remanent magnetized state of the material. The corrosion process in metals, as a rule, is electrochemical due to the fact that their surface consists of many micropairs (microcathodes and microanodes) and is a multi-electrode galvanic cell. This is inevitably accompanied by galvanic currents between areas (both at the macro- and microlevels) with different electrochemical potentials.
Therefore, we can conclude that the magnetic field of the sample can affect the corrosion process. In our case, it was important to show that the materials under study are functionally resistant to sea water, which provides practical information for engineers and designers of products operating in aggressive environments. Our results can be considered valid under the conditions of our experiments, and for an unambiguous answer about the negative and positive effects of the magnetic field on corrosion resistance, a systematic study is necessary but in future.
Conclusion
The conclusions of this study are:
The corrosion rates of S355J2 is better than of SR355JR for the immersion in Black, Mediterranean and Aegean Seawaters; value of corrosion rate for S355J2 in the three seawaters in study are in the range of 0.20-0.40mm/year.
The corrosion rate for SR355JR is also good (0.37-0.54mm/year) in all three seawaters in study.
X-ray diffraction measurements indicate the presence of iron oxide hydrates films formed on S355J2 in Mediterranean and Aegean Seawater. This film protects S355J2 from a higher corrosion in those salty seawaters.
Micrographic study sustain the electrochemical and XRD data.
Seawaters corrosion does not affect the magnetic properties of SR355JR and S355J2.











 nova página do texto(beta)
nova página do texto(beta)


