Introduction
Carbocisteine (S-carboxymethyl-L-cysteine or SCMC), a mucolytic drug is used for the treatment of respiratory tract disorders associated with excessive mucus buildup. Carbocisteine reduces the viscosity of the mucus in the lungs, nose, and windpipe by the cleavage of disulfide crosslinking between the mucin monomers thereby making it less sticky and easier to cough out [1, 2]. It is used to relieve the symptoms in people suffering from bronchiectasis and chronic obstructive pulmonary disorder (COPD) by allowing the easy expulsion of mucus [3]. In addition to its mucoregulatory activity, carbocisteine also shows anti-oxidative and anti-inflammatory properties [4, 5]. Its side effects include skin rashes, headache, dizziness, gastrointestinal bleeding, although it rarely shows any severe adverse effect [6]. Carbocisteine, synthesized by the alkylation of cysteine with iodoacetic acid, is commercially available as oral liquid and capsules [7]. It is a thioether of tri-functional amino acid L-cysteine (containing carboxylic, amino, and thiol groups) in which one hydrogen of thiol has been replaced by carboxymethyl group Fig. 1 shows the structure of carbocisteine and N-R-salt.
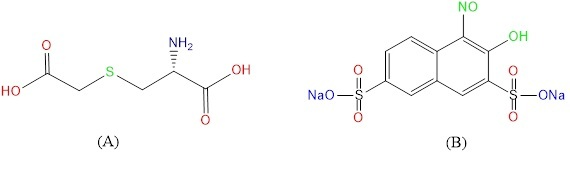
Fig. 1 (A) Structure of Carbocisteine [CCys] (B) Structure of 1-nitroso-2-naphthol-3,6-disulfonic acid disodium salt (N-R-salt)
Sulfur is a vital component of numerous drugs and biological molecules. Sulfur, which is present in the proteins and enzymes of the cell, plays an important role in distinct metabolic processes [8-11]. Therefore, the development of an effective method for determining the quantity of drugs and bioactive molecules containing sulfur in different samples is also very important and sought after by the pharmaceutical industry. The kinetic inspection and mechanistic description of oxidation/ligand substitution reaction of transition metal complexes in the aqueous environment are of paramount importance [12-15]. Such reactions attracted the attention of many chemists, biologists, and environmentalists due to their immediate applications in trace level determination of various biological molecules, drugs, and heavy metals [16-17]. In this regard, several kinetic studies on the exchange of integrated cyanide with ligand variants containing -O, -P, -S, and -N donor atoms from [Ru(CN)6]4- were examined by numerous authors [18-19].
Numerous reports are available for the micro-level determination of sulfur-containing drugs and molecules in biological samples and pharmaceutical preparations [20-23]. The determination methods includes potentiometry [24], NMR-spectrometry [25], colorimetry [20], chromatography [26-28], flow injection analysis [29], fluorimetry [30], voltammetry [31] and spectrophotometry [32, 33]. The high initial investment, time-consuming processes, high costs of sample analysis, and heavy instrumentation are major concerns of many reported methods. Very few kinetic studies have been performed for the determination of thio compounds using various determination methods [34-36].
Complexes of ruthenium with different drugs and bioactive molecules exhibit extensive applications as Immunosuppressant [37], Antileukemic [38], Antifungal [39]. DNA binder [40], Antiamebic [41], Antitumor [42], Anticancer [43] and Antimetastatic [44]. Hg2+ and Ag+ catalyzed substitution of cyanide with nitrogen-containing heterocyclic ligand form cyano complex of Fe(II) and Ru(II) has been reported by several authors [45-46]. The imitation of cyanide ligand form [Fe(CN)6]4- by pyrazine, catalyzed by mercury (II) has been used successfully in the determination of low Hg(II) levels [47]. Because of the formation of a stable complex between Hg2+ and organo-sulfur compounds, the added sulfur compounds inhibit the catalytic efficiency of Hg2+, thereby decreasing the catalyzed reaction rate to a considerable extent [34-36]. Such inhibitory property of thio compounds formed the basis for its micro-level determination using the kinetic method. The formation and stability of various metal-ligand formed during the substitution reaction can be better justified by HSAB (hard-soft acid-base) theory. Due to the strong interaction of CCys with Hg(II), the added CCys reduces the substitution rate of CN- with N-R-salt in hexacyanoruthenate(II), catalyzed by Hg(II). This CCys inhibitory feature motivated us to develop a simple and reproducible kinetic method for the rapid low-level determination of CCys using a UV-Visible spectrophotometer. The present ligand exchange reaction may generate more authentic results in the determination of CCys since the uncatalyzed exchange of CN- with N-R-salt does not proceed under the studied reaction condition [48]. The present communication is about the quantitative determination of carbocisteine by an inhibitory kinetic spectrophotometric method in distinct water samples. This technique can also be convincingly applied for the rapid quantitative estimation of CCys in the pharmaceutical samples.
Experimental
Reagent Used
Reagent-grade chemicals and double distilled water were used throughout the experiment. Standard solution of K4[Ru(CN)6].3H2O (Sigma-Aldrich) was prepared by its calculated amount and was black covered from the exterior to prevent any photo-degradation. Nitroso-R-salt (Merck) and carbocisteine (Sigma-Aldrich) solutions were prepared by dissolving their weighed amount in distilled water. HgCl2 (Merck) solution was prepared daily and the desired dilutions were done before performing the kinetic study as the effective concentration of Hg2+ may get reduced by its adsorption on the glass surface. Potassium hydrogen phthalate (Sigma-Aldrich) and NaOH / HCl procured from Sigma-Aldrich were used to manage the pH while ionic strength of the reaction was regulated by 0.1 N KNO3 (Merck) solution.
Instrumentation
Labman LMPH-10 digital pH meter, calibrated with predefined buffer solutions was used to check the pH of the reacting solutions. Systronics smart UV-Visible double beam spectrophotometer model-2203 was used for the kinetic study at 525 nm (absorption maximum of the substituted product) by measuring an increase in absorbance.
Kinetic Procedure
Since the catalyst and all reacting solutions do not exhibit any significant absorption at the studied wavelength, no modification in the recorded absorbance was carried out. The kinetic measurements were studied at 45 °C by sequential mixing of previously thermostatted solutions (at 45 °C for 30 min) in order: N-R-salt, mercuric chloride, buffer solution, and KNO3, [Ru(CN)6]4- was added at last. The slope of the absorbance versus time plot was used for the calculation of initial rate. Fixed time concept was used to address the dependency of substitution rate on [Hg2+] whereas the influence of temperature, pH, [Ru(CN)64-], [N-R-salt] and [electrolyte] on rate of reaction were discussed with the initial rate.
Determination of Carbocisteine
The exhaustive kinetic study of the ligand imitation reaction was wisely used to identify the optimum experimental condition, showing a substantial change in the absorbance. The kinetic measurements were studied at 45 °C by sequential mixing of previously thermostatted solutions (at 45 °C for 30 min) in order: N-R-salt, mercuric chloride, buffer solution, KNO3, and CCys, [Ru(CN)6]4- was added at last. After thorough mixing of reaction mixture, it was transferred swiftly to the spectrophotometric cell to record the absorbance value; the temperature of the spectrophotometric cell compartment was also fixed at 45 °C by circulating water arrangement. A graph plotted between the recorded absorbance with varying [CCys], considered as calibration curve and was used to quantitatively determine CCys.
Results and Discussion
A purple-red-colored complex of [Ru(CN)5 N-R-salt]3-, obtained during the reaction is due to the Hg2+ promoted imitation of coordinated CN- from [Ru(CN)6]4- with N-R-salt. The complex formed at 525 nm over wide range of concentration obeyed Lambert-Beer’s law and its molar extinction coefficient (ε) at this wavelength was found to be 3.1 × 103 M−1 cm−1, which is in good agreement with the earlier reported substituted complexes of pentacyanoaquoruthenate(II) with nitrogen heterocycles [46]. It was confirmed by the slope ratio and mole ratio studies of the final product that the reactants [Ru(CN)6]4- and N-R-salt reacts in a 1:1 mole ratio. Since the catalyst and all reacting solutions do not exhibit any significant absorption at the studied wavelength, the strong absorption band observed at 525 nm (due to Metal to ligand charge transfer) corresponds to the final product [Ru(CN)5 N-R-salt]3-.
Optimization of Reaction parameters
Dependency of initial rate on pH
Earlier literature on the imitation of cyanide from [Ru(CN)6]4- with heterocyclic compounds containing nitrogen clearly depicts the highly influential nature of pH on the substitution rate [18,19,48]. To identify the pH value for the optimal reaction rate, the substitution efficacy with respect to pH was exercised first in the range of 2.5 to 8.0 by determining the initial rate (Vi) at various pH.
The pH versus Vi plot (Fig. 2) illustrate clearly that the reaction rate was initially sluggish at lower pH value, increases sharply with pH up to 4.5 and ultimately became almost stagnant with further increment in pH up 8.0. Since Vl value is almost constant in the 4.5 to 8.0 range, so the biological pH=7 can be recommended as an optimal value for kinetic determination of carbocistiene. The reduced rate at low pH can be ascribed to the formation of protonated species, [H2O(CN)4Ru(HCN)]2-[48]. The species exhibits the decreased lability of H2O molecule. In conjugation with the less labile protonated species, the prevalent existence of less reactive protonated form of nitroso-R-salt and hexacyanoruthenate(II) is liable for the reduced rate at higher [H+] [48,50]. The deprotonated form of [Ru(CN)6]4−and nitroso-R-salt are the prevalent reactive species above 4.5 pH value The observed constancy in the substitution rate in between pH 4.5-8.0 is due to the deprotonated form of the reactants.
Dependency of initial rate on [N-R-salt]
To analyze the dependency of [N-R-salt] on the substitution rate under the above-optimized pH (7.0 ± 0.02) at 45 oC temperature, we varied the N-R-salt concentration from 1.25 × 10-4 to 6.3 × 10-4 M. The [N-R-salt] versus Vi plot (Fig. 3) illustrate the linear dependence of reaction rate up to [N-R-salt] = 3.5 × 10-4 M, with further increase in [N-R-salt] the decreasing trend in the initial rate is observed in the studied [N-R-salt] range [48]. The tribasic acid N-R-salt is a good complexing agent and is widely used for the detection of heavy metals. The first stability constant (log K1) of N-R-Salt with Pb(II), Cu(II), Zn(II), Cd(II) and Hg(II) at 30 oC are 5.62, 6.50, 5.68, 5.64 and 5.96 respectively [49]. At higher concentration, N-R-salt may reduce the effective concentration of catalyst (Hg2+) by forming a complex with it. The diminished catalytic efficacy at higher [N-R-salt] is the possible reason for the decrement in initial rate. Nitroso-R-salt is believed to exist in tautomeric (quinoneoxime to nitrosonaphthol) equilibria. In aqueous medium, due to hydrogen bonding the OH group of N-R-salt belongs both to the oxime = N OH and to the naphtha OH groups. The sudden decrease in initial rate (Vi) beyond a [nitroso-R-salt] = 3.5 × 10−4 M may be due to decease in absorbance on account of interconversions of a more absorbing form of nitroso-R-salt to a less absorbing form [48].
Dependency of initial rate on [Ru(CN)64-]
The varying concentration of [Ru(CN)64-] (2.7 × 10-5 to 2.5 × 10-4) under optimized condition of pH and [N-R-salt] were used to evaluate the initial rate as a function of [Ru(CN)64-]. The obtained Vi value with each [Ru(CN)64-] represented in Fig. 4 illustrate the variable order kinetics with respect to [Ru(CN)64-]. At lesser [Ru(CN)64-] the substitution reaction obeys first-order kinetics while at higher concentration (> 10-4 M) it exhibits fractional order kinetics [48].
Dependency of initial rate on [electrolyte] and temperature
To analyze the impact of ionic strength on the substitution kinetics, the concentration of neutral electrolyte (KNO3) was changed from 0.005-0.1 M while fixing [N-R-salt] = 3.5 × 10-4 M, Temp = 45.0 ± 0.2 o C, pH = 7.0 ± 0.03, [Hg2+] = 1.5 × 10-4 M and [Ru(CN)64-] = 8.75 × 10-5 M. The plot of Vi versus √µ / (1 + √µ) is in accordance with the negative salt effect (Fig. 5). The reduced electro density at ruthenium metal center due to the interaction of K+ (KNO3) with CN- requires extra energy to discharge water molecule from the coordination sphere of [Ru(CN)5OH2]3−. The reduction of electron density may be liable for the deceasing trend in initial rate with increasing electrolyte concentration [48].
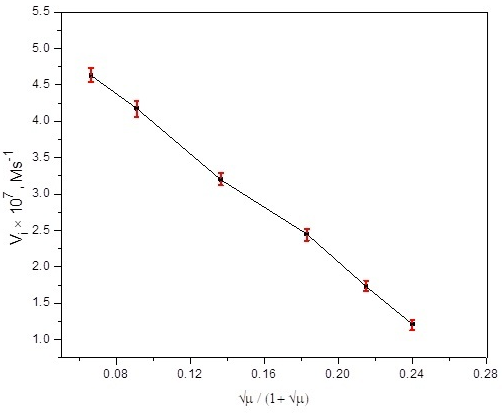
Fig. 5 Dependency of initial rate on [electrolyte] at [N-R-salt] = 3.0×10-4 M, Temp = 45.0 ± 0.2 o C, pH = 7.0 ± 0.03, [Ru(CN)64-] = 8.75×10-5 M and [Hg2+] = 1.5 ×10-4 M.
Under the optimized reaction condition, the temperature was varied from 303-323 K to evaluate the influence of temperature on the kinetics of substitution reaction. The possibility of degradation of the final product and no appreciable change in absorbance due to progress of reaction at very fast rate limit us to study the substitution kinetics at higher temperature [48]. As anticipated hike in temperature escalates the reaction rate. The smooth progress of reaction with modest rate at 318 K enable us to consider that temperature for further studies.
Dependency of initial rate on [Hg2+]
The ultimate application of Hg2+ catalyzed ligand exchange reaction in the trace level determination of various drugs and Hg2+ makes the study of varying impact of [Hg2+] on substitution rate highly important [16,17,47]. An optimized reaction condition with varying [Hg2+] was used to record the absorbance at a fixed time after the mixing of reactants (10 min). The plot of absorbance versus [Hg2+] (Fig. 6) displays a linear correlation at lower concentration of Hg2+. The absorbance/reaction rate increases non-linearly until the concentration of ruthenium complex and catalyst becomes comparative. The binuclear adduct formation between [Ru(CN)6]4- and Hg2+ may be liable for the increment in reaction rate up to 2.5 × 10-4 M [19]. The rapid isomerization of binuclear adduct in aqueous medium produces more labile [Ru(CN)5H2O]3-, with the liberation of catalyst as HgCN+. The fast reaction of incoming ligand N-R-salt with [Ru(CN)5H2O]3-, results in the formation of ultimate substitution product. At higher [Hg2+], the observed steep dwindle in the reaction rate is probably due to the increased complexation between HgCl2 and [Ru(CN)6]4−, which eventually reduces the effective [Hg2+] via abstraction of CN-[48].
Kinetic determination of Carbocisteine
The previous literature on sodium thiosulphate, mercaptoacetic acid, D- penicillamine, and methionine reveal that the added sulfur compound reduces the Hg2+ catalyzed exchange rate of cyanide by nitrogen heterocyclic ligands from [Ru(CN)6]4-[34-36, 51]. Carbocisteine, an organo-sulfur compound also reduces the rate of investigated reaction by forming a stable complex with Hg2+. CCys reduces the effective concentration of Hg2+ by forming a complex with it [Hg2+---- CCys]. The reduced catalytic activity is responsible for the decrease in the absorbance with the inclusion of CCys. An optimized reaction condition with varying [CCys] was used to record the absorbance at a fixed time after the mixing of reactants (10 and 15 min). The plot of absorbance versus [CCys] displays a linear correlation in the concentration range of 3.0 × 10-6 M to 5.25 ×10-5 M (Figure 7). The plot can be used as a calibration curve for the quantitative estimation of CCys. The linear regression expressions relating absorbance with varying [CCys] can be described as Eq.1 and 2.
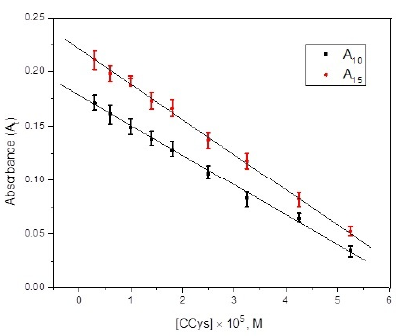
Fig. 7 Calibration curve for the determination of Carbocisteine at [N-R-salt] = 4.5×10-4 M, I = 0.05 M (KNO3), Temp = 45.0 ± 0.2 o C, pH = 7.0 ± 0.03, [Hg2+] = 8.0 ×10-5 M and [Ru(CN)64-] = 4.25×10-5 M.
Standard deviation and linear regression coefficient obtained from the A10 and A15 calibration curve (Fig. 7) are found to be 0.0035, 0.0007, and 0.9985, 0.9991 respectively. To verify the accuracy and reproducibility of the proposed method, a calculated amount of CCys was added to the reaction mixture, and recovered CCys was calculated by the calibration curve. The recovered CCys against the added CCys with percentage error and the standard deviation is compiled in Table 1. Results show that with the proposed method CCys can be determined quantitatively down to 3.0 × 10-6 M.
Table 1 CCYs determination against the added CCys.
| [CCys]×105 M (Taken) | A10 | A15 | ||
|---|---|---|---|---|
| [CCys]×105 M (Found) | % Error | [CCys]×105 M (Found) | % Error | |
| 0.40 | 0.41 ± 0.028 | + 0.032 | 0.42 ± 0.046 | + 0.051 |
| 0.60 | 0.60 ± 0.02 | 0.000 | 0.59 ± 0.05 | - 0.048 |
| 1.35 | 1.36 ± 0.036 | + 0.036 | 1.35 ± 0.00 | 0.000 |
| 1.75 | 1.73 ± 0.07 | - 0.046 | 1.74 ± 0.042 | - 0.037 |
| 2.25 | 2.25 ± 0.00 | 0.000 | 2.23 ± 0.06 | - 0.063 |
| 3.00 | 3.02 ± 0.038 | + 0.062 | 3.00 ± 0.03 | + 0.023 |
| 3.75 | 3.73 ± 0.08 | - 0.028 | 3.76 ± 0.068 | + 0.055 |
| 4.50 | 4.52 ± 0.055 | + 0.043 | 4.48 ± 0.042 | - 0.083 |
Reaction Condition: [N-R-salt] = 4.5×10-4 M, I = 0.05 M (KNO3), Temperature = 45.0 ± 0.2 o C, pH = 7.0 ± 0.03, [Hg2+] = 8.0 ×10-5 M, and [Ru(CN)64-] = 4.25×10-5 M.
The inhibition action of CCys towards the catalytic activity of Hg2+ for the CN- substitution from [Ru(CN)6]4- by N-R-salt can be schematically be represented by the modified mechanism (Eq. 3-7). The present ligand exchange reaction may generate more authentic results in the determination of CCys since the uncatalyzed exchange of CN- with N-R-salt is insignificant under the studied reaction condition [48].
Interference of co-existing components
The influence of excipients, which are usually present along with drugs in pharmaceutical preparations, was checked by performing the recovery experiments from the solution containing 4.0 µgml-1 CCys under the optimum reaction condition and a large amount of diverse species. The recovery results using the A10 calibration curve suggest that the addition of excipients even up to 1000 times with the [CCys] does not significantly interfere with the determination of CCys (Table 2).
Table 2 Recovery results of CCys (4.0 µg ml-1) in the presence of fillers.
| Additives | [Additives] / [D-PCN] | Recovery ± RSD (%) |
|---|---|---|
| Gelatin | 1000 | 100.2 ± 0.7 |
| Sodium Lauril sulphate | 500 | 99.7 ± 0.5 |
| Lactose | 500 | 100.3 ± 0.6 |
| Magnesium stearate | 1000 | 99.6 ± 0.3 |
| Sucrose | 500 | 100.9 ± 0.8 |
| Citrate | 1000 | 99.4 ± 0.2 |
| Sorbitol | 1000 | 100.7 ± 0.6 |
| Maltitol | 1000 | 99.6 ± 0.4 |
Experimental Conditions: [N-R-salt] = 4.5×10-4 M, I = 0.05 M (KNO3), Temperature = 45.0 ± 0.2 o C, pH = 7.0 ± 0.03, [Hg2+] = 8.0 ×10-5 M and [Ru(CN)64-] = 4.25×10-5 M.
Application in pharmaceutical preparations
The proposed inhibitory kinetic study was effectively used for the quantitative determination of CCys in pharmaceutical preparations. The content of CCys from 10 capsule/tablet was finally grounded and dissolved in 100 ml of de-ionized distilled water, which after sonication for 20 min was filtered off using Whatman filter paper. The solution was further diluted with de-ionized distilled water to bring [CCys] within the calibration range. Five different pharmaceutical samples of CCys (capsule/tablet) were subjected to the spectrophotometric determination of CCys. The statistical comparison of the result obtained by the designed method with the standard method indicates the accuracy and precision of the proposed method for CCys determination (Table 3) [52]. The mean recovery (99-101) demonstrates that the present kinetic method can be effectively used for the quick quantitative determination of CCys in the pharmaceutical samples with good accuracy and reproducibility.
Table 3 Determination of CCys in Pharmaceutical preparations and statistical comparison with the official method.
| Pharmaceutical Samples | Proposed Method Recovery ± RSD (%) | Official Method Recovery ± RSD (%) |
|---|---|---|
| Flemaliz 500 mg Capsule (Flamingo Pharmaceuticals Pvt. Ltd.) | 99.46 ± 0.52 | 99.72 ± 0.68 |
| Loviscol 500 mg Capsule (Pfizer) | 100.76 ± 0.72 | 99.54 ± 0.51 |
| Mucoflem 500 mg Capsule (Centurion Laboratories) | 99.94 ± 0.73 | 100.24 ± 0.38 |
| Mucosolv 375 mg Capsule (Cipla) | 101.08 ± 0.59 | 99.91 ± 0.49 |
| Mucodyne 375 mg Capsule (Elder Pharmaceuticals Pvt. Ltd.) | 99.69 ± 0.68 | 100.98 ± 0.75 |
Experimental Conditions: [N-R-salt] = 4.5×10-4 M, I = 0.05 M (KNO3), Temperature = 45.0 ± 0.2 o C, pH = 7.0 ± 0.03, [Hg2+] = 8.0 ×10-5 M and [Ru(CN)64-] = 4.25×10-5 M
Conclusion
Using the inhibition action of CCys towards the catalytic activity of Hg2+ for the CN- substitution from [Ru(CN)6]4- by N-R-salt, a sensitive and reproducible inhibitory kinetic method was proposed for the quick estimation of CCys. The present ligand exchange reaction may generate more authentic results in the determination of CCys since the uncatalyzed exchange of CN- with N-R-salt is insignificant under the studied reaction condition. Carbocisteine decreases the rate of substitution reaction by reducing the active concentration of Hg2+ through the formation of a stable complex with it. The addition of common excipients in pharmaceuticals even up to 1000 times with the [CCys] has no significant interference in the determination of CCys. With the proposed method, CCys can be determined quantitatively down to 3.0 × 10-6 M. The statistical comparison of the result obtained by the designed method with the standard method indicates that the proposed methodology can be convincingly adopted for the rapid quantitative estimation of CCys in the pharmaceutical samples with good accuracy and reproducibility. The methodology can be effectively used for the trace level determination of various drugs and biological molecules that can significantly inhibit the catalytic efficacy of Hg(II).











 nueva página del texto (beta)
nueva página del texto (beta)







