Introduction
The current research of drugs must be a satisfactory planning regarding the optimization of the molecules because they are going to be applied in human health to improve quality of life or to have a longer life expectancy. Within thousands of compounds that are developed, only a fraction has good absorption, distribution, metabolism, excretion, and toxicity (ADMET) / toxicology properties. By better understanding the properties-structure relationship, the necessary background is provided to interpret and apply the data for decisions to optimize in vivo pharmacokinetics, safety of biological tests, formulation, and therapy. Low solubility, chemical instability, low permeability can critically affect biological tests.
Several novel compounds have been developed for the treatment of different types of cancer. Casiopeinas are a family of copper (II) coordination compounds with the general formula:Cu (N-N)(O-N)NO3 or Cu (N-N)(O-N)NO3. These compounds were designed as a less toxic alternative to Cis-Platinum since this drug is used in the treatment of various types of cancer. [1] Within the family of Casiopeinas with antineoplastic activity, Casiopeina III-Ea, is a copper complex that shows cytostatic, cytotoxic, genotoxic and antineoplastic activities through different mechanisms of action, both in vitro and in vivo models [2 - 11]. Casiopeina III-Ea was recently reported to have results that possesses strong antileukemic activity in vitro. [12]
In the present work, the physicochemical and biopharmaceutical characterization of Casiopeina III-Ea, a new antineoplastic, was carried out; it has been found to be very efficient in pediatric leukemia and was designed as a less toxic alternative to Cis-Platinum. This basic research of the Faculty of Chemistry of the UNAM, is directed towards the maturation of a technology, which consists of the pharmaceutical development of an antineoplastic copper coordination compound Casiopeina III-Ea. In order to know what its behaviour in physiological media will be, it is necessary to perform the evaluation of physicochemical tests such as: pKa, intrinsic solubility, Log D distribution coefficient, and IC50 studies against MDCK cells, which will allow a better understanding of the ADMET process of the new molecule. In order to determine and quantify Casiopeina III-Ea in the different physicochemical and biopharmaceutical tests that were carried out, it was necessary to have precise and reliable methodologies. The analytical methodologies by HPLC-UV and spectrophotometry were validated taking into account some considerations and acceptance criteria of International guidelines for the Validation of Bioanalytical Methods, such as those established by the FDA, Guidance for Industry: Bioanalytical Method Validation (2001).
Experimental
Reagents and equipment
Casiopeina III-Ea (Fig. 1) was provided by the group of Dra. Lena Ruiz Azuara from the Department of Medicinal Inorganic Chemistry, Faculty of Chemistry, UNAM. Methanol grade HPLC X, deionized water Milli-Q, Monobasic sodium phosphate monohydrate J.T. Baker, anhydrous dibasic sodium phosphate J.T. Baker, HPLC grade water, ATCC fetal calf serum (FBS), ATCC dimethylsulfoxide (DMSO), Costar 96-well ELISA sterile plates, Gibco DMEM culture medium, Hank Gibco's solution, J.T. Baker ethyl alcohol, ELISA plate reader ELX800GDGR, sulforhodamine Aldrich Chemistry, Gibco Tris Base Solution, J.T. Baker Trichloroacetic Acid, ( 0.45 µm Millipore GVPL type filters. ), hydrochloric acid ( J.T. Baker), phosphoric acid (J.T. Baker), sodium hydroxide ( Meyer ), and n-octyl alcohol ( 1-octanol ), ( Sigma Aldrich ). A Shimadzu UV-1601 brand UV -Visible spectrophotometer was used for sample analysis. Shimadzu HPLC with pump model LC10ADVP (Tokyo, Japan), Shimadzu model SPD10AD UV variable wave detector, model SIL10ADVP autosampler with a 20 to 100 µL loop (Cotati, CA, USA), a Shimadzu brand controller system Model SCL10AVP (Tokyo, Japan), and a Shimadzu Class VP Version 5 Chromatographic Data Integrator 1999 were also used.
Spectrophotometric method validation
Linearity, repeatability and reproducibility for Casiopeina III-Ea
For the validation of the spectrophotometric method, some criteria of the FDA, Guidance for Industry: Bioanalytical Method Validation (2001) were taken into account.[13] The parameters of linearity, repeatability and reproducibility were determined in each of the pH's that were tested, using phosphate buffer solution as matrix.
From the Casiopeina III-Ea stock solution (100 µg / mL), 3 calibration curves were prepared in a range of 15 to 2.5 µg / mL, they were taken to the gauging with phosphate buffer solutions at the following pH: 1.2, 3.0, 4.6, 5.0, 6.8, 7.4 and 8.0. The procedure was repeated in 2 consecutive days. The samples were read in the UV-Visible spectrophotometer for pKa calculation, intrinsic solubility, and Log D to determine physicochemical properties of Casiopeina III-Ea.
Determination of pKa
The determination of the pKa of Casiopeina III- Ea by the spectrophotometric technique required the preparation of phosphate buffer solutions at different pH values, to promote ionization in such a way that the absorptive properties of the species generated were modified.
With the data obtained in the validation of the analytical method for each of the buffer solutions tested, the absorbance values of Casiopeina III-Ea were taken at 273 nm and the pKa was calculated with the following equation, where the pH value complies with the following relation:
A: Absorbance at which the compound is 50% in ionized form and 50% is in its non-ionized form, respectively, pH = pKa
Where,
A1: Absorbance of the acidic species
A2: Absorbance of the basic species.
Determination of log D at pH 6.8 and 7.4
The coefficient of determination for Casiopeina III-Ea (CasIIIEa) in the n-octanol-buffer solution system at different pH was carried out by the shake-flask technique [14]. Prior to carrying out Log D determination, the organic phase was saturated by placing solution buffer over n- octanol, the system was shaken for 5 min and it was allowed to stand to separate both phases and finally the aqueous phase was discarded. Buffer solution at a concentration of 15 µg / mL of Casiopeina III-Ea at each test pH and saturated n-octanol were tested. Once the phases were separated, the aqueous phase (Casiopeina III-Ea 15 ug/mL at test pH) and the organic phase were collected separately. After the extraction process was completed for three times, the aqueous phase was read at 273 nm in a UV-Vis spectrophotometer. The data were interpolated in the standard calibration curve ranging from 2.5 to 15 µg / mL at a pH 6.8 and at pH 7.4 solution. The concentration of Casiopeina III-Ea in the organic phase is obtained by difference when knowing its concentration in the aqueous phase. The value of the partition coefficient was[ calculated]using the following formula:
Intrinsic solubility in buffer solution at pH 6.8 and 7.4
Intrinsic solubility [15] was determined as follows: amounts of 10 to 1900 mg of Casiopeina III-Ea were added in 10 mL volumetric flasks and were brought to the level with each phosphate buffer at different pH. The flasks were placed in a water bath with shaking at 37 °C for 24 h. After the stirring time, it was observed if each flask presented a precipitate, samples were taken and filtered through a 0.45 µm nylon filter. The samples were analysed by spectrophotometry at 273 nm and interpolated in a standard curve (2.5 - 15 ug/mL) with a previously validated method, solubility was determined to the extent that it was observed that sample concentration no longer increased.
Quantification of Casiopeina III Ea
A liquid chromatographic method was developed and validated using Hank's solution, Based on previous chromatographic quantification conditions of unchanged compound Casiopeinas. [16, 17, 18]
An HPLC with a LC10ADVP pump model, a SPD10AD UV variable wave detector, a SIL10ADVP autosampler with a loop from 20 to 100 µL (Cotati, CA, USA), and a control system SCL10AVP VP version 5 chromatographic data integrator, 1999, all from Shimadzu (Tokyo, Japan) were used. Also, an Eclipse Plus C-8 column (4.6 x 150 mm), 3.5 µm (particle size) from Agilent Zorbax, and a guard column with C-18 cartridge from Phenomenex. Mobile phase in 70/30 ratio 0.01 M phosphate buffer pH = 7.6 / methanol, lambda = 273 nm, flow rate = 0.8 ml / min, injection volume 25 µL, and sample analysis temperature of 25 °C.
Calibration curve in Hank's solution
Casiopeina III -Ea (10 mg) was weighed and made up to a volume of 10 mL with Hank's solution (1000 µg / mL); consecutive dilutions were made at a volume of 1 mL to obtain a concentration range from 1.25 to 20 µg / mL. Samples were filtered through a 0.45 µm membrane before injecting into HPLC for analysis. For reproducibility, two days of analysis, 3 control points (high, medium and low) were evaluated in six times each day. The linearity was worked with the calibration curve from 1.25 to 20 µg / mL by triplicate in 1 day.
Cytotoxicity study in MDCK (Madin Darby Canine Kidney) cells
As a complement to the study of permeability in MDCK cells, the study of cytotoxicity in these cells was carried out to provide an idea about the adequate doses to work in these cells.
To evaluate cytotoxicity, the colorimetric method of Sulforhodamine B (SRB) was used in ELISA plates. The plate was divided into quadrants to test the following logarithmic concentrations (µM) in six-fold each: 0.22, 2.24, 22.4, 224, 2240, 11000 and 22000 for Casiopeina III-Ea. MDCK cells (2 x 104/well) were seeded in 100 μl of supplemented DMEM medium, incubated at 37 °C, 5% CO2 and 95% RH for 24 h. Then, the medium was removed and 90 μL of fresh medium and 10 μL of the solution to be evaluated were added. Cells were further incubated for 24 h. Once the treatment time had elapsed, the medium was removed and the cells were fixed with 100 μL of 10% TCA solution for 1 h, the TCA was discarded, and the cells were washed with water and left to dry. Once dry, the cells were stained with 50 µL of 0.4% SRB solution in acetic acid, the plate was read at a length of 564 nm. The viability or proliferation of the cells is determined by the degree of staining expressed by the absorbance of the wells, which were directly proportional, the greater the number of living cells, the greater the staining and therefore the greater the absorbance. [19, 20]
Results and discussion
Spectrophotometric method validation
The linearity and repeatability were determined by means of calibration curves in buffer solution at each test pH in a range of 2.5 to 15 µg / mL by triplicate, the results are presented in Table 1. (CV%, Percentage of coefficient of variability).
Table 1 Determination of the linearity and repeatability of Casiopeina III-Ea solutions at different pH′s.
| pH=1.2, Mean n=3 | pH=3.5, Mean n=3 | pH=4.6, Mean n=3 | pH=5.0, Mean n=3 | pH=6.8, Mean n=3 | pH=7.4, Mean n=3 | pH=8.0, Mean n=3 | |
| Concentration (2.5 - 15 µg/mL) r | CV% (0.2 - 2) | CV% (0.2 - 1.2) | CV% (0.1 - 1.7) | CV% (0.1 - 0.6) | CV% (0.1 - 1.6) | CV% (0.1 -0.4) | CV% (0.1 - 0.7) |
| r | 0.9995 | 0.9994 | 0.9997 | 0.9998 | 0.9998 | 0.9998 | 0.9998 |
| RSE (%) | 0.55 | 1.82 | 0.3223 | 0.01 | 0.01 | 0.9133 | 0.01 |
RSE (%), regression standard error (%)
Determination of pKa
The pKa of a molecule is a parameter related to the state of its charge, it is a descriptor of the acid-base balance. Casiopeina III-Ea was prepared at a concentration of 10 µg/ml, absorbance values were obtained at different pH′s.
To obtain the value of pKa, A = (0.9528 + 0.8227) / 2, A = 0.88775
By means of the regression obtained from the relationship pH vs absorbance value, the pH corresponding to pKa is obtained with the following formula pH = (A-B) / m = (0.88775- 1.0948) / - 0.0413 resulting in pH = 5.01. According to these results, Casiopeina III- Ea behaves as a weak acid conjugated with pKa = 5.01, in the form of a nitrate salt, the unionized compound will be the weak base. Therefore, based on these results, as the pH increases, it is expected to be better absorbed in the intestine. It is expected to be better absorbed as a unionized compound in the intestine. When considering an intravenous route of administration, there will be a pH of 7.4 in the blood, where it could be observed in terms of the Henderson-Hasselback equation; a higher concentration of the non-ionized compound considered as a weak base, a better distribution in the body will occur. This molecule compared to Casiopeina IIIia (pka = 5.3), for example of Cu (2,20 - bipyridine) (acetylacetonato), and Casiopeina IIgly (pka 5.95), for example of Cu (1,10- phenanthroline) (glycinato) has a similar pKa value. [8]
Determination of log D at pH 6.8 and 7.4
The determination of the distribution coefficient at different pH's was carried out using the shake -flask method. Once the water and n-octanol system reaches equilibrium, an aliquot of the aqueous phase was taken and the concentration of Casiopeina III- Ea was determined. Then by interpolating the absorbance in the previously validated calibration curve in a range of 2.5 to 15 µg / mL and by difference the concentration in the organic phase is established. Table 2 shows the results obtained in the determination of the distribution coefficient for Casiopeina III-Ea at the pH′s tested.
Table 2 Determination of the distribution coefficient.
| Mean | ||
| pH 7.4 | pH 6.8 | |
| Buffer phase concentration Casiopeina III-Ea µg/mL | 10.22 | 11.05 |
| Organic phase concentration Casiopeina III-Ea µg/mL | 4.77 | 3.95 |
| Organic phase/buffer phase relation (D) | 0.47 | 0.36 |
| log D | -0.33 | -0.44 |
Table 2 shows that according to the Log D values obtained at pH 6.8 and 7.4, Casiopeina III-Ea is a molecule with some hydrophilic characteristics (water soluble); thus, its lipophilicity is low. There is a good solubility but low absorption and brain penetration, owing to low passive diffusion permeability, these compounds tend to have high clearence by the kidney, owing to their polarity. May exhibits paracellular permeation if the molecular weight is low. This molecule compared with Casiopeina IIIia (log D = -0.95) has a less negative log D. [8, 21]
Intrinsic solubility
In the results obtained for the determination of the intrinsic solubility of Casiopeina III-Ea, this molecule presented a solubility of 180 mg/mL at pH 6.8 and 170 mg/ml at pH 7.4. The results have suggested that since it is a weak acid (conjugate), with pKa 5.01, it is soluble at these pH′s.
It was expected that its solubility and the behaviour of the non-ionized form to be higher in lipophilic medium than in aqueous medium (170 mg / mL, at pH 7.4), above the pKa of 5.01, but this situation did not happen, it means that Casiopeina III- Ea solubility remains hydrophilic. Good stability of the solution at those pH's, between 4 and 8, was reported, this indicates that the results at pH 7.4 are acceptable. [22]
Validation of the analytical method by HPLC-UV for Casiopeina III-Ea
Chromatograms shown in Fig. 2 confirm that there are no interferences from the components of Hank's solution for the retention time of Casiopeina III -Ea; therefore, the method is considered selective. The linearity of the method to quantify Casiopeina III-Ea in Hank's solution was obtained in a working range of 1.25 to 20 µg / mL, the average linearity was 0.9971 (n = 3), the analytical method complies with the linearity parameter, since the correlation coefficient was greater than 0.99.
The intra-day precision for Casiopeina III- Ea was estimated with Hank's solution calibration curve at the following concentrations: 1.25, 2.5, 5, 10 and 20 µg/mL. The following sextuplicate control points 2 (MCB), 8 (MCM) and 15 (MCA) µg/mL were used. Table 3 shows the results of precision (CV%) and accuracy (Abs Dev) for the analytical method.
Table 3 Precision and accuracy of the method for Casiopeina III-Ea in Hank's solution.
| 2 µg/mL | 8 µg/mL | 15 µg/mL | ||||
| CV% | Abs Dev % | CV% | Abs Dev % | CV% | Abs Dev % | |
| Mean n=6 | 10.58 | 5.34 | 5.26 | 4.63 | 5.91 | 4.99 |
The CV% and accuracy are less than 15% at each concentration level (2, 8 and 15 µg / mL), the accuracy values are less than 15% with respect to the nominal value, therefore, the results showed that the analytical method meets the parameters of precision and accuracy.
Cytotoxicity study in MDCK cells
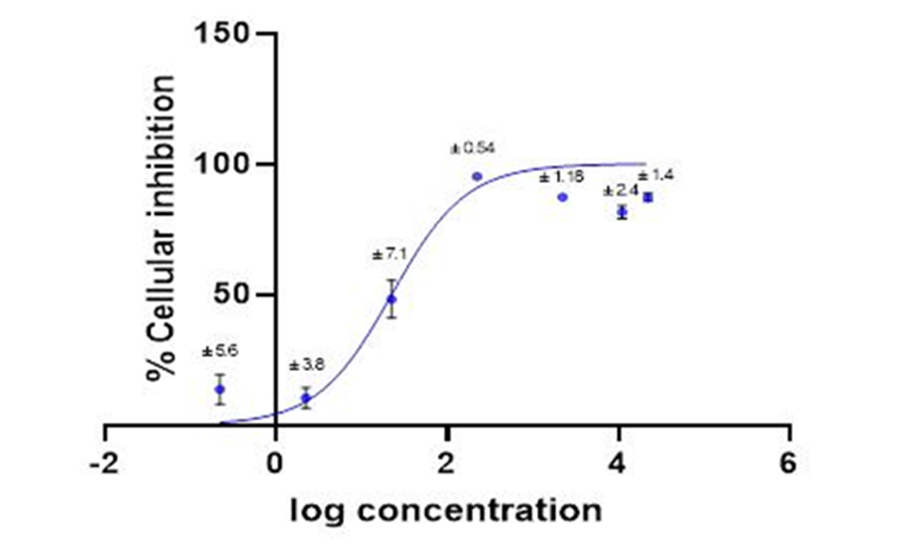
The cytotoxicity at different concentrations of Casiopeina III-Ea, was obtained in the MCDK cell line, it is shown in Fig. 3. The data for the IC50 determination was adjusted using a sigmoidal equation, the determination coefficient (r2) was 0.97, using GraphPad Prisma Statistical Program. The IC50 was 19.07 µM±7.1.

Fig. 3 % Inhibition of cell growth by Casiopeina III-Ea at different concentrations, with standard deviation.
According to the graph obtained in the cytotoxicity study (Fig. 4), it is observed that to be able to inhibit cell proliferation at 50% (IC50 = 19.07 µM ) higher concentrations are needed in MDCK cells in comparison with those reported with other tumor cell lines as HCT-15 ( IC 50 = 4.6 µM), Hela ( IC50 = 1.4 µM), SiHa ( IC50 = 0.96 µM) [8] y CHIP-212 (18.68 µM) [2]. For this reason, concentrations below the IC50 determined for Casiopeina III-Ea can be used without compromising cell proliferation for permeability studies [19, 20].
Within the studies of Casiopeinas, this study represents the first one in MDCK cells in order to apply them into permeability studies and within the coordination molecules with metals, they have not yet been reported.
Conclusion
Casiopeina III -Ea molecule behaves as a weak conjugated acid, ionized as a nitrate, bearing a pKa=5.01 and became hydrophilic. The physicochemical parameters of pKa, distribution coefficient and intrinsic solubility, are thermodynamic constants which help to understand the behaviour of Casiopeina III-Ea in the body, in addition to the fact that the molecule complies with some Lipinski rules: less than 5 hydrogen bond donors, molecular mass less than 500 Dalton and less than 10 hydrogen bond acceptors. Considering the IC50 for MDCK cells 19.07µM, and the molecular weight of Casiopeina III-Ea, (450.94 g / mol), MDCK cells remain alive to perform permeability studies with doses of 8.56 mg.
These findings help us, to qualify Casiopeina III-Ea as a new promising antileukemic activity, taking the advantages of their satisfying physicochemical properties that lie within the acceptable range of the 90% known drugs. However, in vitro permeability studies and preclinical pharmacokinetic studies are recommended to perform better understanding of the bioavailability of Casiopeina III-Ea. To date, coordination compounds with metals are gaining popularity, but studies based on physicochemical and biopharmaceutical properties for their optimization as therapeutic molecules have not been reported.











 nueva página del texto (beta)
nueva página del texto (beta)