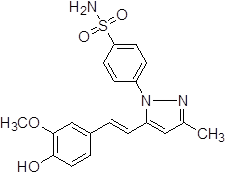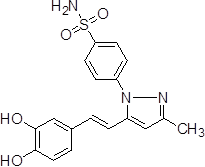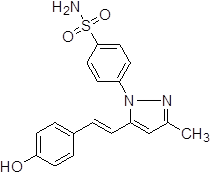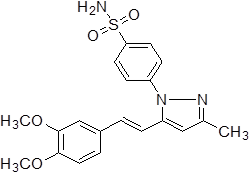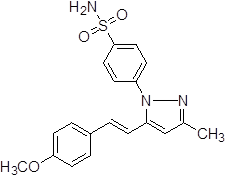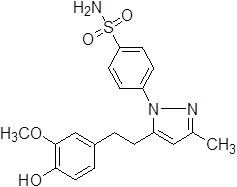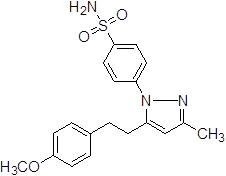Introduction
Hispolons are diketone compounds that show some structural similarity with curcumin and possess the essential pharmacophore features of curcumin [1]. These properties of hispolon attracted many researchers to explore the various biological activities of this molecule. Research publications reported that hispolons show antioxidant [2], anti-inflammatory [3,4], anti-estrogenic [5], anti-tubercular [6], anticancer [7,8,9,10,11,12], hepatoprotective [13] and antiviral [2] activities. We recently found a potential anti-TB activity for pyrazole derivatives that were synthesized from hispolons [14]. With a view to optimize the bioactivity further, we synthesized novel heterocyclic sulfonamides using hispolons.
On the other hand, sulfa drugs are very familiar in medicine to treat various bacterial infections in the urinary tract, respiratory tract, eyes, and ears, etc. These drugs contain sulfonamide group as the main moiety have shown antibacterial [15], anti-hyperglycemic [16,17], and anti-inflammatory [18] properties. Celecoxib, rofecoxib, and valdecoxib are sulfonamide derivatives which act as anti-inflammatory agents. As sulfonamides are known to interact with several proteins, we thought to interpret the functional group in the present work. Furthermore, the sulfonamides are also used to treat some bacterial infections effectively [19,20].
So, based on the above information and an extension to our prospective research towards the development of potent antitubercular agents [6,14,21], here we designed some hispolon molecules having sulfonamide moiety as shown in Schemes 1 and 2. Further, by varying the substitutions on hispolon and dihydrohispolon moieties various hispolon pyrazolesulfonamide derivatives were synthesized and screened for antimicrobial properties along with anti-TB activities.
Experimental
Chemistry
General
4-Sulfonamide-phenylhydrazine hydrochloride and remaining compounds were procured from Avra Chemicals Pvt. Ltd., Hyderabad, India. All reactions were monitored by TLC using silica gel-G (Merck grade) as the adsorbent. The synthesized compounds were characterized by 1H NMR using Bruker AMX 400 MHz NMR instrument using TMS as an internal standard (δ in ppm). The Mass spectra were recorded on LC-MS/ESI-MS Mass instrument using a positive/negative mode ionization method and the elemental analysis was done on a Perkin-Elmer 2400 series instrument. All the melting points were determined using the Meltemp apparatus in open capillaries, and expressed in °C, and are uncorrected to ±0.1 °C.
General method for the synthesis of hispolon pyrazolesulfonamide derivatives
4-Sulfonamide phenylhydrazine hydrochloride (2.5 Equiv.) and pyridine (1.5 Equiv.) were added to the stirred solution of hispolon (1a-f)/ dihydrohispolons (4a-d) (1.0 Equiv.) in ethanol (20 vol.). After complete addition, the reaction mass was heated to reflux and maintained at reflux condition for 6 h. After 6 h, the reaction mass was cooled to room temperature, and the solvent was removed under vacuum to obtain the crude. Ice cold water was added to the crude and stirred for 1 h. The precipitated material was filtered, dried to get the corresponding hispolon pyrazolesulfonamide.
4-{5-[2-(4-Hydroxy-3-methoxy-phenyl)-vinyl]-3-methyl-pyrazol-1-yl}-benzene sulfonamide (3a).
White solid: mp 220.2-223.6 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ 9.27 (s, 1H, -OH at C-4'), 7.97-7.95 (d, J = 8.0 Hz, 2H, H-2'', 6"), 7.71-7.69 (d, J = 8.0 Hz, 2H, H-3'', 5''), 7.47 (s, 2H, -SO2NH2 at C-1''), 7.15-7.11 (d, J = 16.0 Hz, 1H, H-8'), 6.97-6.95 (d, J = 8.0 Hz, 1H, H-5'), 6.84-6.80 (d, 1H, J = 16.0 Hz, H-7'), 6.77-6.75 (d, 1H, J = 8.0 Hz, H-6'), 6.61 (s, 1H, H-4), 3.78 (s, 3H, -OCH3 at C-3'), 2.26 (s, 3H, -CH3 at C-3). LC-MS: 386.0 (M+H)+. Analysis calcd for C19H19N3O4S (385.11): C, 59.21, H, 4.97, N, 10.90 %, found: C, 59.38, H, 4.89, N, 10.79 %.
4-{5-[2-(3,4-Dihydroxy-phenyl)-vinyl]-3-methyl-pyrazol-1-yl}-benzene sulfonamide (3b).
Grey solid: mp 231.1-233.4 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ 9.22 (s, 1H, -OH at C-3'), 9.03 (s, 1H, -OH at C-4'), 7.98-7.96 (d, J=8.0 Hz, 2H, H-2'',6''), 7.69-7.67 (d, J=8.0 Hz, 2H, H-3'',5''), 7.49 (s, 2H, -SO2NH2 at C-1''), 7.09-7.05 (d, J=16.0 Hz, 1H, H-8'), 6.90 (s, 1H, H-2'), 6.81-6.79 (d, J=8.0 Hz, 1H, H-6'), 6.72-6.70 (d, J=8.0 Hz, 1H, H-5'), 6.67-6.64 (d, J=12.0 Hz, 1H, H-8'), 6.64 (s, 1H, H-4), 2.25 (s, 3H, -CH3 at C-3). LC-MS: 372 (M+H)+. Analysis calcd for C18H17N3O4S (371.09): C, 58.21, H, 4.61, N, 11.31 %, found: C, 58.39, H, 4.80, N, 11.14 %.
4-{5-[2-(4-Dihydroxy-phenyl)-vinyl]-3-methyl-pyrazol-1-yl}-benzene sulfonamide (3c).
Off-white solid: mp 218.3-221.4 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ 9.71 (s, 1H, -OH at C-6'), 7.97-7.95 (d, 2H, J=8.0 Hz, H-2'',6''), 7.70-7.68 (d, 2H, J=8.0 Hz, H-3'',5''), 7.54-7.52 (d, J=8.0 Hz, 2H, H-2'',6''), 7.51 (s, 2H, -SO2NH2 at C-1''), 7.37-7.35 (d, J=8.0 Hz, 2H, H-2',6'), 7.16-7.12 (d, 1H, J=16.0 Hz, H-8'), 6.81-6.78 (d, 1H, J=12.0 Hz, H-7'), 6.76-6.74 (d, J=8.0 Hz, 2H, H-3',5'), 6.63 (s, 1H, H-4), 2.25 (s, 3H, -CH3 at C-3). LC-MS: 356.0 (M+H)+. Analysis calcd for C18H17N3O3S (355.10): C, 60.83, H, 4.82, N, 11.82 %, found: C, 60.97; H, 4.99; N, 11.69 %.
4-{5-[2-(3,4-Methoxy-phenyl)-vinyl]-3-methyl-pyrazol-1-yl}-benzene sulfonamide (3d).
Off-white solid: mp 198.3-202.5 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ 7.99-7.97 (d, J=8.0 Hz, 2H, H-2'',6''), 7.73-7.71 (d, J=8.0 Hz, 2H, H-3'',5''), 7.49 (s, -SO2NH2 at C-1'', 2H), 7.19-7.15 (d, J=16.0 Hz, 1H, H-8'), 7.10-7.09 (s, 1H, H-2'), 6.96-6.94 (d, J=8.0 Hz, 2H, H-5',6'), 6.92-6.88 (d, J=16.0 Hz, 1H, H-7'), 6.64 (s, 1H, H-4), 3.83 (s, 3H, -OCH3 at C-3'), 3.78 (s, 3H, -OCH3 at C-4'), 2.27 (s, 3H, -CH3 at C-3). LC-MS: 400.0 (M+H)+. Analysis calcd for C20H21N3O4S (399.13): C, 60.13, H, 5.30, N, 10.52 %. found: C, 60.29, H, 5.47, N, 10.41 %.
4-{5-[2-(4-Methoxy-phenyl)-vinyl]-3-methyl-pyrazol-1-yl}-benzene sulfonamide (3e).
Off-white solid: mp 212.2-214.6 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ 7.97-7.95 (d, J=8.0 Hz, 2H, H-2'',6''), 7.80-7.78 (d, J=8.0 Hz, 2H, H-3'',5''), 7.54 (s, 2H, -SO2NH2 at C-1''), 7.48-7.46 (d, J=8.0 Hz, 2H, H-2',6'), 7.21-7.17 (d, J=16.0 Hz, 1H, H-8'), 6.94-6.92 (d, J=8.0 Hz, 2H, H-3',5'), 6.86 (d, J=16.0 Hz, 1H, H-7'), 6.66 (s, 1H, H-4), 3.76 (s, 3H, -OCH3 at C-4'), 2.26 (s, 3H, -CH3 at C-3). LC-MS: 370.0 (M+H)+. Analysis calcd for C19H19N3O3S (369.11): C, 61.77, H, 5.18, N, 11.37 %. found: C, 61.91, H, 5.30, N, 11.21 %.
4-{5-[2-(4-Hydroxy-3,5-dimethoxy-phenyl)-vinyl]-3-methyl-pyrazol-1-yl}-benzene sulfonamide(3f).
Off-white solid: mp 214.1-218.5 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ 8.66 (s, 1H, -OH at C-6'), 7.99-7.97 (d, J=8.0 Hz, 2H, H-2'',6''), 7.73-7.71 (d, J=8.0 Hz, 2H, H-3'',5''), 7.48 (s, 2H, -SO2NH2 at C-1''), 7.15-7.11 (d, J=16.0 Hz, 1H, H-8'), 6.91-6.87 (d, J=16.0 Hz, 1H, H-7'), 6.84 (s, 1H, H-2',6'), 6.61 (s, 1H, H-4), 3.83 (s, 3H, -OCH3 at C-3'), 3.82 (s, 3H, -OCH3 at C-5'), 2.27 (s, 3H, -CH3 at C-3). LC-MS: 415.9 (M+H)+. Analysis calcd for C20H21N3O5S (415.12): C, 57.82, H, 5.09, N, 10.11 %. found: C, 57.98, H, 5.21, N, 10.06 %.
4-{5-[2-(4-Hydroxy-3-methoxy-phenyl)-ethyl]-3-methyl-pyrazol-1-yl}-benzene sulfonamide (5a).
Off-white solid: mp 186.4-190.1 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ 9.16 (s, -OH at C-4'), 7.95-7.93 (d, J=8.0 Hz, 2H, H-2'',6''), 7.75-7.73 (d, J=8.0 Hz, 2H, H-3'',5''), 7.66 (s, 2H, -SO2NH2 at C-1''), 7.44 (s, 1H, H-2'), 7.05-7.03 (d, J=8.0 Hz, 2H, H-2',6'), 6.67-6.65 (d, 2H, H-3',5'), 6.20 (s, 1H, H-4), 3.71 (s, 3H, -OCH3 at C-3'), 2.92 (m, 2H, H-8'), 2.73 (m, 2H, H-7'), 2.20 (s, -CH3 at C-3). LC-MS: 386.0 (M+H)+. Analysis calcd for C19H21N3O4S (387.13): C, 58.90, H, 5.46, N, 10.85 %. found: C, 59.06, H, 5.61, N, 10.77 %.
4-{5-[2-(4-Hydroxy-phenyl)-ethyl]-3-methyl-pyrazol-1-yl}-benzene sulfonamide (5b).
Off-white solid: mp 194.1-197.2 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ 9.10 (s, 1H, -OH at C-4'), 7.95-7.93 (d, 2H, H-2'',6''), 7.65-7.63 (d, 2H, H-3'',5''), 7.45 (s, 2H, -SO2NH2at C-1''), 6.97-6.95 (d, J=8.0 Hz, 2H, H-2',6'), 6.66-6.64 (d, J=8.0 Hz, 2H, H-3'',5''), 6.20 (s, 1H, H-4), 2.93 (m, 2H, H-8'), 2.75 (m, 2H, H-7'), 2.21 (s, 3H, -CH3 at C-3). LC-MS: 358.0 (M+H)+. Analysis calcd for C18H19N3O3S (357.11): C, 60.49, H, 5.36, N, 11.76 %. found: C, 60.60, H, 5.51, N, 11.67 %.
4-{5-[2-(4-Methoxy-phenyl)-ethyl]-3-methyl-pyrazol-1-yl}-benzene sulfonamide (5c).
White solid: mp 168.1-172.2 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ 7.92-7.90 (d, J=8.0 Hz, 2H, H-2'',6''), 7.65-7.63 (d, J=8.0 Hz, 2H, H-3'',5''), 7.44 (s, 2H, -SO2NH2 at C-1''), 7.09-7.07 (d, J=8.0 Hz, 2H, H-2',6'), 6.82-6.80 (d, J=8.0 Hz, 2H, H-3'',5''), 6.21 (s, 1H, H-4), 3.71 (s, 3H, -OCH3 at C-3'), 2.96 (m, 2H, H-8'), 2.82 (m, 2H, H-7'), 2.21 (s, 3H, -CH3 at C-3). LC-MS: 372.0 (M+H)+. Analysis calcd for C19H21N3O3S (371.13): C, 61.44, H, 5.70, N, 11.31 %. found: C, 61.61, H, 5.87, N, 11.23 %.
4-{5-[2-(3,4-Dimethoxy-phenyl)-ethyl]-3-methyl-pyrazol-1-yl}-benzenesulfonamide (5d).
Off-white solid: mp 195.2-198.3 °C; 1H NMR δ 7.94-7.92 (d, J=8.0 Hz, 2H, H-2'',6''), 7.65-7.63 (d, J = 8.0 Hz, 2H, H-3'',5''), 7.44 (s, 2H, -SO2NH2at C-1''), 6.82-6.80 (d, J=8.0 Hz, 1H, H-6'), 6.74-6.73 (s, J=8.0 Hz, 1H, H-2'), 6.67-6.65 (d, 1H, H-5'), 6.20 (s, 1H, H-4,), 3.72 (s, 3H, -OCH3 at C-3'), 3.69 (s, 3H, -OCH3 at C-3'), 2.97 (m, 2H, H-8'), 2.78 (m, 2H, H-7'), 2.21 (s, 3H, -CH3 at C-3). LC-MS 402.0 (M+H)+. Analysis calcd for C20H23N3O4S (401.14): C, 59.83, H, 5.77, N, 10.47 %. found: C, 59.98, H, 5.63, N, 10.29 %.
Results and discussion
Chemistry
A series of hispolon and dihydrohispolons were reacted with 4-sulfonamidephenyl hydrazine hydrochloride in the presence of ethanol/pyridine to provide their respective pyrazole sulfonamides as shown in Schemes 1 and 2. The yields of the products obtained were good and the details are provided in Table 1.
The compound 3e formed from p-methoxy substitution of hispolon was obtained in the highest yield (95%) followed by compounds 5d and 3a formed from dihydrohispolon with p-methoxy substitution and hispolon with p-hydroxy, m-methoxy substitutions respectively. The dihydrohispolon with p-hydroxy substitution generated the pyrazole product in the lowest yield (85%).
All the compounds were well characterized by NMR and Mass spectral data as shown below. The N-H proton signal of sulfonamides in NMR spectra was obtained at δ7.47 to 7.54 and dihydrohispolons were obtained at 7.44 to 7.66. The -OH proton signals in respect of p-hydroxy substituted compounds were found at δ 7.47 to 7.54. The proton signals of the trans olefinic group (7’ and 8’ of the styryl group) appeared between δ 6.5 to 7.2. The methoxy groups attached to hispolon moiety were observed at about 3.65 to 3.80 and methyl groups at C-3 position were observed between δ 2.20 to 2.30.
Biological activities
All these synthesized hispolon pyrazole analogs (3a-f; 5a-d) were screened for their antimicrobial activity using the agar well diffusion method [22] and anti-tubercular activity using the MABA assay method as reported [14].
The screening results of in vitro anti-tubercular studies of the compounds by Microplate Alamar Blue Assay (MABA) on MtbH37Rv (ATCC 27294) strain are included in Table 2. A study conducted by us [14] on similar type of compounds without sulfonamide group was also included for proper analysis of Structure Activity Relationship among the studied compounds. Further, to ascertain the lipophilicity effects of the compounds on activities a theoretical study was conducted to calculate logP values using Molinspiration property engine software (version- v2018.10), and the results are also included in Table 2 in respect of both the type hispolon pyrazoles.
Table 2 Results of antitubercular activity of novel hispolon pyrazolesulfonamides along with logP values.
| S. No. | Compound code | MIC (µg/mL) | Log P | MIC (µg/mL) (Compound without sulfonamide group) | Log P (without sulfonamide group) |
| 1 | 3a | 6.25 | 2.44 | 3.12 | 2.50 |
| 2 | 3b | 6.25 | 2.32 | 3.12 | 2.19 |
| 3 | 3c | 25 | 2.53 | 12.5 | 2.68 |
| 4 | 3d | 100 | 2.55 | 12.5 | 2.81 |
| 5 | 3e | 25 | 2.86 | 12.5 | 3.21 |
| 6 | 3f | 50 | 3.25 | 25 | 2.51 |
| 7 | 5a | 50 | 2.72 | 12.5 | 2.10 |
| 8 | 5b | 50 | 2.14 | 6.25 | 2.28 |
| 9 | 5c | 100 | 2.23 | 12.5 | 2.41 |
| 10 | 5d | 50 | 2.84 | 12.5 | 2.82 |
| Standards | Pyrazinamide | 3.12 | 3.12 | ||
| Streptomycin | 6.25 | 6.25 | |||
| Ciprofloxacin | 3.12 | 3.12 |
The hispolon pyrazolesulfonamides showed good antitubercular activity when compared to those sulfonamides obtained from dihydrohispolons. Further, compounds 3a and 3b were found to be more active with MIC of 6.25 µg/mL on H37Rv. However, these two compounds showed good anti TB activity in the absence of the sulfonamide group (MIC of 3.12 µg/mL) as reported in our previous study [14]. The compounds 3c and 3e were found to be moderately active with a MIC of 25 µg/mL; a similar observation was also found in these cases i.e. the absence of sulfonamide increased (MIC of 12.5 µg/mL) the activity [14].
Structure Activity Relationship
Sulfonamide has a very important function in many of the drug molecules including sulfamethoxazole-trimethoprim, erythromycin-sulfisoxazole, and glyburide, etc. Based on this importance of the sulfonamide group in medicinal (or) pharmaceutical chemistry we synthesized ten hispolon analogs with the sulfonamide group. However, these compounds without sulfonamide function have been reported for anti-tubercular activity [14]. Therefore, we have also tested these compounds for antitubercular activity and compared them with the previously reported activity. All the synthesized compounds were found to possess good to moderate anti-tubercular activity. Even though newly synthesized compounds (3a-f, 5a-d) have a sulfonamide group, these compounds have a slightly inferior activity when compared to respective non-sulfonamide compounds. Based on the obtained anti-tubercular activity results, we observed that the double bond is one of the essential key functions for the activity. So the compounds 3a, 3c-e showed good activity, when compared with that of compounds not have a double bond 5a-d.
Hydroxy group present in para-position also has major importance for the anti-tubercular activity, for example, compound ‘3a’ has an ‘-OH’ group at para-position and ‘3d’ has a methoxy group at para-position, while the rest of the structure remains the same, but 3a showed MIC of 6.25 µg/mL and 3d showed MIC of 100 µg/mL. Dramatically, when compare the activities of compounds 3c and 3e, no difference in the activity was observed. The compounds which have a p-hydroxy group and another hydroxyl or methoxy group adjacent to the p-hydroxy group showed very good activity [3a, 3b with MIC-6.25 µg/mL], but two methoxy groups neighboring to the p-hydroxy group (3f) showed inferior activity. Further, it was also observed that due to the two methoxy groups adjacent to p-hydroxy in 3f the activity was lower than the compound 3e which does not have a hydroxyl group at para- position.
Further, sulfonamide compounds having the only para- substitution that is with -OH (3c) or -OMe (3e), there is no difference in the activity but in respect compounds without sulfonamide group, the hydroxyl compound showed more activity compared to methoxy compound. If the compound has a para hydroxy and another methoxy or hydroxyl adjacent to hydroxy substituent the activity increases. Two methoxy groups adjacent to the hydroxy substituent, then the activity was a little bit decreased and the compound has two methoxy groups adjacent to each other showing very inferior activity.
The Molinspiration platform follows fragment-dependent contributions and rectification factors to estimate the parameters of the majority of the organic molecules. According to Lipinski’s rule of five, a potential drug candidate molecule should be orally active if its molecular weight is ≤ 500 Da, logP ≤ 5 [23]. In the present study, test compounds with and without sulfonamide exhibited logP below 5 and are also projected to be lipophilic. Even though all the compounds did not have an association between the logP vs MIC, but variation was observed among the compounds with or without sulfonamide. Subsequently, an antimycobacterial molecule has to permeable the waxy mycobacterial cell wall, its obvious to discover more lipophilic molecules that favor more activity as well as permeability.
Further, the antimicrobial activity of the present compounds was evaluated by using agar well diffusion method with 1 mg/mL concentration, and 100 µL volume was used for all measurements; the results are presented in Table 3.
Table 3 Antimicrobial results of the compounds 3a-f and 5a-d.
| Sample Code | Anti bacterial activity | Anti fungal activity | ||
| Zone of inhibition (mm) | Zone of inhibition(mm) | |||
| SA (100 µL/mL) | EC (100 µL/mL) | AN (100 µL/mL) | CA (100 µL/mL) | |
| 3a | 3.5 | - | - | - |
| 3b | 7.0 | 6.0 | - | - |
| 3c | 5.0 | 6.0 | - | - |
| 3d | 1 | - | - | - |
| 3e | - | - | - | - |
| 3f | - | - | - | - |
| 5a | 5.6 | 2.3 | - | - |
| 5b | 4 | 3.6 | - | 9 |
| 5c | - | - | - | - |
| 5d | 3.3 | 1.3 | - | - |
| Streptomycin | 8 | 7 | - | - |
| Fluconazole | - | - | 17 | 20 |
SA-Staphylococcus aureus (NCIM 2122), EC-Escherichia coli (NCIM 2137),
AN-Aspergillus niger (NCIM 652), CA- Candida albicans (NCIM 3102)
The compound 3b showed the highest inhibition on Staphylococcus aureus with a zone of inhibition of 7 mm. Further, the 3b compound also showed 6 mm inhibition zone on Escherichia coli bacteria but this was quite low when compared to the compound which has no sulfonamide group [14]. The compound 3c also found to be moderately active with a zone of inhibition 5 and 6 mm on Staphylococcus aureus and Escherichia coli bacteria respectively. It clearly observed that the dihydro compounds were found to be less potent compared to their respective non-dihydro analogs. Moreover, except for compound 5b on Candida albicans (NCIM 3102) with 9 mm zone no compound was showed zone of inhibition on both fungal strains.
SAR studies on antimicrobial activity
The synthesized compounds 3a-f, 5a-d were tested for their antibacterial activity against two strains i.e. Staphylococcus aureus, and Escherichia coli at 1 mg/mL concentration. Based on the observed activity results, most of the compounds showed similar activity when compared to the non- sulfonamide group analogs [14] except for 5d. In these, no other compound having the hydroxy group in the para- position and another hydroxy group present adjacent to the -OH group showed superior activity. When we increase the methoxy groups, the antimicrobial activity was decreased. The double bond also influenced the antibacterial activity. Trans double bond containing compounds showed better activity when compared to saturated compounds. Based on the results these compounds showed moderate antibacterial activity. Moreover, dramatically compound 5b does not have a trans double bond but has only one hydroxy group at para- position and still showed very good antifungal activity against Candida albicans.
Conclusion
In conclusion, novel hispolon derived heterocyclic sulfonamides (3a-f; 5a-d) were designed and synthesized. The compounds were further evaluated for their anti-TB and anti-microbial activity. Among them, 3a and 3b showed potent antitubercular activity (MIC 6.25 µg/mL). But, the presence of sulfonamide moiety decreased the activity. A comparative study among MIC and logP was also established. The synthesized compounds 3b and 3c showed antibacterial activity.











 nueva página del texto (beta)
nueva página del texto (beta)