Introduction
Organic polymers are the most applicable type of polymers needed for various applications and can be prepared by different means. Ring-opening polymerization (ROP) is a type of chain-controlled polymerization used recently for cyclic organic monomers. The main advantage of ROP is its living nature and the strict control of the polydispersity index (PDI). The livingness being a prominent feature of ROP; the number of active and dormant (reversibly terminated) chains is generated instantaneously and remains constant during the polymerization reaction, therefore, it is always offering additional cyclic monomers to extend polymer chains [1,2]. ROP is an important method of polymerization to make some interesting and valuable polymers to suit different demands and requests [3] and multi-arm can be prepared depending on the type of initiator used [4]. The ROP is the preferred route for preparing the high molecular weight PLLA in industries. Poly (lactic acid) PLA, and polylactide PLLA, show promise as a valuable alternative to petroleum-based polymers for uses as plastics, fibers, and coatings [5]. They are produced from renewable resources such as corn and sugar beets, and the material is biodegradable along these lines, making it perfect for modern uses in industry and medicine [6,7].
The most frequently reported controlled ROP of cyclic monomers in the literature [8,9] are coordination-insertion, anionic, cationic, and nucleophilic polymerization. The coordination-insertion and nucleophilic polymerization are undoubtedly the most efficient and general methods reported so far for the ROP of lactones, with cationic and anionic polymerization being much less investigated, while coordination-insertion polymerization uses metal-alkoxides and related complexes as catalysts. The successful ROP of lactide to make the biodegradable polymer polylactide was carried out by Robert and Aubrecht using tin (II) bis (2-ethyl hexanoate)/benzyl alcohol mediated ring-opening polymerization of lactide to form the biodegradable polylactide polymer. They found that the system of the polymerization is practically equivalent to that of a transesterification reaction. The influence of the water formation on the number-average molecular weight of the resulting polymer and the polymerization rate is also discussed [10].
An organocatalytic nucleophilic polymerization is a metal-free approach to ROP [11-13]. The proposed polymerization pathway differs fundamentally from the coordination-insertion mechanism involving metal complexes [14]. Metal-free catalysts, like enzymes and organocatalysts, are attracting growing interest as reported by Dubois et al. [11] since being considered to be the more economical and environmentally friendly alternatives. Indeed, the nucleophilic catalyst only activates the monomer toward ring-opening, whereas the metal complex activates the monomer, initiates the polymerization and remains bound to the growing chain. Dove et al. [15] reported the catalytic activity of a conjoined thiourea-tertiary amine molecule, in the ROP of lactide. Mechanics and theoretical studies support a supramolecular bifunctional mechanism involving activation of both the monomer and the alcohol nucleophile; polymerization is significantly slower with reaction times of a few days.
Rostami et al. [16] announced utilizing a tertiary aminosquaramide bifunctional organocatalyst, which is known as a catalyst-free of the metal producing a polymer having narrow PDI (≈1.05). The low PDI’s with uncommon control prominent is a result of specific transesterification of lactide comparative with the aliphatic esters. Practical and theoretical investigations enhance using such type of these functional components, increasing reactivity of alcohol nucleophile and the monomer. Screening studies demonstrated that a variety of thiourea-amine combinations are catalytically active for the ROP of lactide, and the structural flexibility and efficacy of thiourea-amine catalysts for the supramolecular activation and ROP of lactide are described again examined further by Pratt et al. [17] and Kazakov et al. [18]. They proposed that the polymerization was motivated by hydrogen bonding interaction, and these types of catalysts are regularly utilized for well-controlled ROP.
Mezzasalma et al. [19] reported the ring-opening polymerization (ROP) of L-lactide using different organocatalysts in solvent-free conditions at high temperatures (in bulk) has proven to be a significant challenge. Indeed, the high required temperature (180 °C) has led to poorly controlled polymerizations as a result of transesterification reactions of the polylactide backbone, racemization of the lactide monomers as well as the degradation and thus deactivation of the organocatalyst.
The 1,8-Diazabicyclo [5.4.0] undec-7-ene (DBU) catalyzed ROP of L-lactide was tested in CDCl3 at room temperature at a 100:1:1 monomer/catalyst/ initiator ratio [20], and the resulting polymer was found to have Mn 21,000 g/mol and polydispersity (PDI=Mw/Mn) 1.05. The molecular mass of the poly (lactide) could be controlled by varying the monomer/initiator ratio of 50:1 to 500:1 with a good correlation of the targeted and experimental Mn values with PDI less than 1.1. Qian et al. [21] used DBU as a known, effective, and convenient organocatalyst for the ring-opening polymerization of cyclic esters, to synthesize random copolymers of lactide and glycolide. Graft copolymerization of L-lactide onto chitosan was also carried out at room temperature by ring-opening polymerization using DBU under a nitrogen atmosphere to prepare chitosan-g-poly(N-lactide) graft copolymer. It was obtained in good yield and the success of the grafting process was checked by characterizing the grafted chitosan by FTIR [13].
Recently, it is observed an increased interest in star-shaped polylactide materials as they exhibit highly desirable rheological, mechanical, and biomedical properties that are inaccessible in the case of linear polymers. These branched polyesters have a higher concentration of functional end-groups that additionally influenced their properties and are expected to exhibit good degradation and controlled drug release properties [22,23].
The aim of the work reported in this paper was the synthesis of a series of high-molar-mass six-armed containing PLLA segments built on a core molecule of the dipentaerythritol (D-PLLA’s) in ring-opening polymerization. The elaborated method of the preparation of star polymers, using DBU as a non-metal catalyst, i.e. organocatalyst, at room temperature will be advanced the applications of these star polymers. Characterization of these star polymers using FTIR spectroscopy, 1H-NMR and 13C-NMR spectroscopy, gel permeation chromatography (GPC), and SEM allows us to observe the relationship between microstructure and macrostructure control in these systems. Nevertheless, this work is based on synthetic, novelty indicating that some polymer characteristics can be controlled by the sensible choice of using ROP in the presence of a DBU catalyst at room temperature. Significantly, this will possibly establish a useful starting point not only to improve the physical properties of the star poly (L-lactide) in comparison to linear poly (L-lactide) [24,25] but also to generate biodegradable nanoparticles with adjustable sizes may be useful for targeted drug release.
Experimental
Materials
N,N-Dimethylformamide (DMF) (Assay ≥99.8 %) and Dichloromethane (DCM) (Assay ≥ 99.5 %) were supplied by MACRON Company. Dipentaerythritol, L-Lactide, 1,8-Diazabicyclo [5.4.0] undec-7-ene (DBU), and Magnesium sulfate were supplied by Sigma-Aldrich Company.
Instruments
The 1H-NMR and 13C-NMR spectra were recorded using Agilent DDR2 500 MHz NMR spectrometers, FTIR spectra were recorded using Nicolet IR-42, Mid-IR spectrometer. The prepared polymers were examined under Scanning electron microscope type JEOL 7500F supplied by JEOL Company, USA. Available at Department of Chemistry/Michigan State University.
Polymers molecular weights and molecular weight distributions (Mw/Mn) were determined using a Waters 1515 gel permeation chromatography (GPC) equipped with a refractive index detector (Waters 2412), DMF was used as the eluent at a flow rate of 1.0 mL/min and calibrated with poly (methyl methacrylate) standard, which is available at Department of Chemistry/Michigan State University.
Preparation of Dipentaerythritol-lactide Polymer (D-PL10)
Dipentaerythritol (0.508 g, 0.002 mol) and L-lactide (8.6478 g, 0.06 mol) were dissolved in DMF at room temperature. After stirring for 5 minutes under Nitrogen atmosphere, DBU (90 µL) was added into the mixed solution. The reaction was kept under Nitrogen for 2 h. The reaction was stirred for a further 24 h at room temperature. Afterward, the reaction was worked up by the slow addition of the reactants to 500 mL distilled water to precipitate the polymer which was then filtered on Buchner funnel and washed with distilled water and then the filtrate was dissolved in DCM and dried over magnesium sulfate and filtered. The polymer was recovered through the column chromatography with silica gel, DCM was removed by rotary evaporator and the polymer was dried in a vacuum oven at 25 oC for 24 hours [26]. The product was viscous with a yield of 79%.
The same procedure was carried out to prepare polymers D-PL25, D-PL50 and D-PL100, Table 1 lists the quantities of reactants used in the preparation and the chemical equations can be represented by Scheme 1 of these polymers.
Table 1 The amounts of reactants utilized in the synthesis of D-PL (10-100) and the physical state of the resulting polymers.
| Polymer Code | Dipentaerythritol | L-lactide | DBU (µL) |
Yield (%) |
Physical State |
||
|---|---|---|---|---|---|---|---|
| Wt. (g) | Mol | Wt. (g) | Mol | ||||
| D-PLLA25 | 0.254 | 0.001 | 10.8 | 0.075 | 100 | 79 | Viscous |
| D-PLLA50 | 0.102 | 0.0004 | 8.6478 | 0.06 | 90 | 83 | Viscous |
| D-PLLA100 | 0.0508 | 0.0002 | 8.6478 | 0.06 | 90 | 87 | Solid |
Results and discussion
Synthesis of controlled six arms star-shaped poly(L-lactide)
Over the last ten years, there has been a huge increase in the development and applications of organocatalysis in which the catalyst acts as a nucleophile. Amidines and guanidines are often only thought of as strong organic bases, however, a number of small molecules containing basic functional groups have been shown to act as efficient nucleophilic catalysts. Interesting work on DBU catalyst has provided excellent control over the architecture of linear and stars PLLA polymer chains.
The main objective of the present work is to study ROP of L-lactide at room temperature using DBU as an organocatalyst to develop polymerization rate and narrow molecular weight distribution (MWD) of PLLA during the ROP of L-Lactide because most experiments mentioned in the literature were carried out at high temperatures ranging from temperature (150-190°C) using organocatalyst [12-14,23] or catalysts containing metals [27]. Using DBU in ROP at room temperature is provided conventional easy condition to develop a standardized synthetic procedure hopefully applicable to a wide range of catalyst systems. DBU as an organic base of low nucleophilicity that has found wide application as a catalyst for transesterification-like reactions at room temperature, with impressive effectiveness to afford polylactide that has defined structure, controlled molecular weight, and molecular weight distribution. To investigate systematically the effect of polymer architecture on a family of PLLA polymer stars, dipentaerythritol (DPE) was selected as the core initiator based on its inherent flexibility and successful use in catalyzed polymer star syntheses [28]. The proposed polymerization mechanism was steered by the hydrogen bonding interaction which is agreed well with Chuma et al [29]. This is following the fact that DBU does not cause extensive transesterification of PLLA on the time scale of lactide ROP [30].
The 6 primary hydroxyl groups of DPE are expected to initiate the polymerization of lactide. The resulting polymer is expected to have 6 arms terminated with secondary hydroxyl groups. Whether all 6 hydroxyl groups of DPE are reacted to form a six-armed polymer can be investigated by analyzing them using (FTIR, 1H-NMR and 13C-NMR, GPC) to prove the correctness of the expected structure and compositions.
Characterization of the new polymers by FT-IR
Two different techniques were utilized to examine the preparedness polymers by infrared spectroscopy depending on the physical state of the polymers. The viscous ones were cast directly on the NaCl disc, and the solid product was mixed with KBr.
Dipentaerythritol-lactide polymers, which were synthesized by ROP of L-lactide monomer with dipentaerythritol as a core molecule, gave strong bands around 3500-3513 cm-1, Fig. 1, which are corresponding to the stretching of hydroxyl groups of the terminal polymerized lactide monomer. Other bands that occurred at the region around 1750 cm-1 are characteristics of stretching frequency for the ester carbonyl groups that resulted from the ring-opening of the lactide monomer. Infrared bands occurred around 1190 cm-1 which are due to stretching frequencies of (C-O) group of ester as well. Other strong bands occurred around 2950 cm-1 are attributed to the stretching of different aliphatic carbon-hydrogen bonds present [31,32] as shown in Scheme 1. The intensity of the hydroxyl groups bands was decreased with increasing lactide repeated units chain length, Fig. 1, the same observation was made by Sweah et al. in the preparation of poly(ethylene glycol)-sebacate-poly(ethylene glycol) polymer chains using poly(ethylene glycol) having different molecular weight 2000, 10000 and 20000 g/mol [24].
Characterization of the new polymers by NMR
1H-NMR and 13C-NMR techniques were used to confirm the structure of the newly prepared polymers (D-PLLA10, D-PLLA25, D-PLLA50 and D-PLLA100). 1H-NMR spectra were recorded on Agilent DDR2 500 MHz NMR spectrometers, using CDCl3 (Sigma-Aldrich, 99.8% atom D) as a solvent.
Fig. 2 shows the representative 1H-NMR spectrum of D-PLLA10 polymer. The methylene protons of (CH2) group of dipentaerythritol appear at 3.25 ppm (a), while the signal at 5.16 ppm represented another methylene protons (CH2) group of dipentaerythritol (b), the signal at 5.29 ppm assigned to the methine protons (CH) of repeating unit of L-lactide segments (c), and at 1.48 ppm represented the methyl (CH3) protons of lactide repeated unit (d). The signal at 4.35 ppm assigned for the methine protons (CH) of the terminal molecule of L-lactide (e), and at 1.57 ppm represented the methyl (CH3) protons of the terminal molecule of L-lactide (f), and at 2.92 ppm for hydroxyl groups at the end chains of polymers (g). The chemical shift at 5.05 ppm is corresponding to the unreacted L-lactide monomer [33].
The 13C-NMR spectrum of the D-PLLA10 polymer as a representative of the prepared polymers spectra is shown in Fig. 3. The carbon atom of a methylene group (CH2) of dipentaerythritol appear at 36 ppm (a), the signal at 63 ppm represented the carbon atom of dipentaerythritol (b), while the signal at 67 ppm represented another methylene carbon atom in (CH2) group of dipentaerythritol (c), and at 170 ppm represented the carbon atom of the carbonyl group of repeating unit of L-lactide (d). The signal at 17 ppm represented the carbon atom of a methyl group (CH3) of L-lactide (e), and at 69 ppm for the carbon atom of methine group of repeating unit of L-lactide (f). The signal of the carbon atom of the carbonyl group of terminal L-lactide (g) appears at 175 ppm represented. The signal at 16 ppm represented the carbon atom of a methyl group (CH3) of terminal L-lactide (h), at 72 ppm for the carbon atom of methine group of repeating unit of terminal L-lactide (i).
All these results confirmed the expected structure and composition of the prepared polymers and promoted this matter with great accuracy of the GPC results, Table 2. They are close to those calculated from the [M]o/[I]o ratios, and their dispersity was in the range of typically observed for the ROP of lactides with the great polydispersity index (PDI) close to one. The same approached was made by Lohmeijer et al. [34] that DBU catalyst produced polymers displaying high-end group reliability, a good correlation between theoretical and observed molecular weight, and linear relationships between conversion and molecular weight. Although, GPC is often used to determine the relative molecular weight of polymer samples as well as the distribution of molecular weights. GPC truly measures the molecular volume and shape function as defined by the intrinsic viscosity. If comparable standards are used, as in this case PMMA standard was used, the relative data can be used to determine molecular weights within ± 5% accuracy [35].
Polymer Microstructures
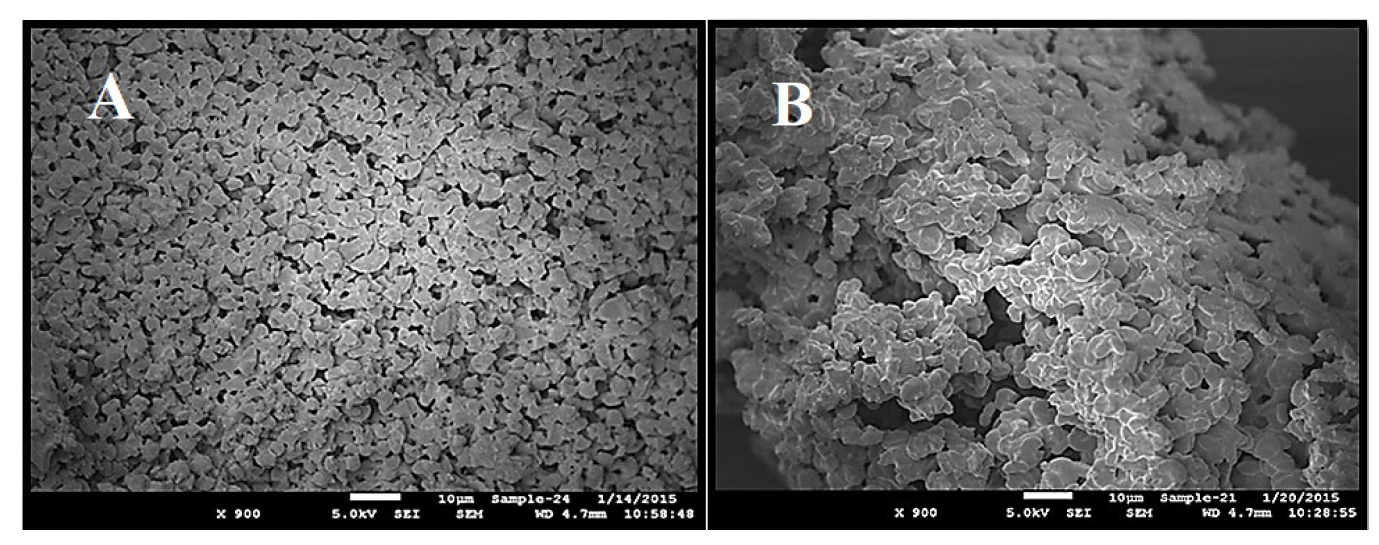
The examination of the morphology and the geometry of the nanoparticles of the prepared polymers was performed by using a JEOL 7500F scanning electron microscope, SEM. Fig. 4 shows the SEM micrographs of examined D-PLLA25 and D-PLLA100 polymers. The images obtained from scanning electron microscopy revealed the existence of nanoparticles in the microstructure of the prepared polymers due to the presence of the L-lactide array to give nanoparticles within the final compositions [24,25,36]. Analysis by Image-J program of the mean nanoparticles obtained from D-PLLA25 micrograph (Fig. 4 (A)) is in the range of (87-94) nm and from Fig. 4(B) for D-PLLA100 polymer is found in the range of (95-99) nm.
Conclusions
Star-shaped poly (L-lactide) with six arms having a different chain length were successfully synthesized via the ring-opening polymerization of L-lactide using a commercial dipentaerythritol as an initiator and DBU as a catalyst at room temperature. The advantage of this DBU catalyst, besides it, is free of metal, it retains high activity in the activation of multifunctional initiators and is effective in ROP of L-lactide monomer. This resulted in a convenient method for the preparation of the star-shaped D-PLLAx polymers. Moreover, the results revealed that there is a chance to be accurately predicted polymer molecular weight by the molar ratio of monomer to initiator implying the correctness of the expected polymer molar masses, and ensuing very low molecular weight distribution (PDIs ≤1.13), as measured by GPC and with the right structure as characterized by FT-IR, 1H-NMR and 13C-NMR spectroscopy. This combination of initiator-catalyst was found to give reproducible results, allowing the convenient preparation of different polymer and copolymer architectures that could be useful in different means. On the other hand, images obtained from Scanning Electron Microscopy (SEM) revealed the existence of nano-structures in the prepared copolymers due to the presence of the lactide array within the final compositions prepared. As a consequence of the controlled polymerization of lactide, molecular weight, composition, chain length, and distribution of different D-PLLA polymers could be easily manipulated for different applications.











 nueva página del texto (beta)
nueva página del texto (beta)