Introduction
As a result of increasing industrial activity, contamination of water resources with toxic heavy metals is becoming a serious problem. Chromium is commonly employed in leather tanning, electroplating, metal finish, paints and textile industries. Toxic metals as chromium should be removed before coming in contact with the environment. Chromium exists in various oxidation states but in aqueous solutions the most stable are the Cr(VI) and Cr(III) states. Cr(VI) is significantly more toxic than trivalent, even at low concentrations with a potential carcinogenic and mutagenic effect to living organism; the limit value of chromium in drinking water is 0.05 mg L-1. Several methods are used to remove Cr(VI) from aqueous solutions, such as electrolysis, reverse osmosis, ion exchange, solvent extraction and absorption among others [1-5]. The absorption method is considered the most cost effective and efficient method of removing Cr(VI) from aqueous solutions.
Chitosan (CS) is a natural polysaccharide, obtained from deacetylation of chitin, which is the second most abundant natural biopolymer. CS is commonly used to remove metal ions and dyes from wastewater because of amino and hydroxyl functional groups [6]. Moreover, it is possible to modify the inherent properties of CS to improve its performance introducing different functional groups into structure. Chemical modification can be made by several methods, but radiation grafting offers advantages such as the reaction could be carried out without any additives, at any temperature, and different monomers can be grafted onto substrate [7-9].
The objective of this work was to study the possibility of improving the Cr(VI) adsorption capacity of net-CS, modifying it with a binary grafting of N-vinylcaprolactam and N,N-dimethylacrylamide by gamma radiation. NVCL is a nonionic, nontoxic, thermal sensitive monomer with a lower critical solution temperature (LCST) of ∼ 32oC, while DMAAm is a hydrophilic monomer, able to improve the mechanical properties of materials. Both monomers contain carboxylic and amide groups, suitable for the retention of heavy ions.
Experimental
Materials
N-vinylcaprolactam and N,N-dimethylacrylamide from Sigma-Aldrich, Mexico was vacuum distilled before use; chitosan of low molecular weight (1x106), K2CrO4 and formaldehyde from Sigma-Aldrich Mexico, and acetic acid from T. Baker were used as received. Distilled water was used in all experiments.
Crosslinking of CS
Deacetylation of 70.8 % was determined by titration methods [10]. Two different solutions of CS were prepared (1 % and 3 % w/v) in acetic acid solution 10 % (v/v), and subsequently crosslinked. Briefly, 10 mL of formaldehyde (37 % wt in H2O) was added into 100 mL of CS solution; it was stirred for 2 h and then vacuum dried for 96 h. The net-CS at different concentrations (nCS1 and nCS3) were washed with acetic acid solution (1 % v/v) to eliminate un-crosslinked CS, and later with water until reached neutral pH. The experiments were carried out by triplicate. The crosslinking reaction yield was calculated with the equation (1).
where, Wf and Wo are the weight of insoluble crosslinked CS and the initial CS, respectively.
Binary grafting of NVCL/DMAAm onto crosslinked CS
The nCS was grafted by direct irradiation method, in one step, according to Perez-Calixto et al [11]; 0.2 g of nCS was placed in glass ampoules with 7 mL of NVCL/DMAAm (15/5 % v/v) methanol solution. The system was swelled during 24 h, the excess of solution was separated, and the ampoules were bubbled with argon for 20 min to eliminate oxygen and sealed. The samples were irradiated at 10 kGy with a dose rate of 9.5 kGy h-1. The binary grafting system (BGnCS) was washed with acetic acid solution (1 % v/v) and water to remove residual monomer and homopolymer. The samples were filtered and vacuum dried, and the grafting percentage calculated with equation (2).
where, Wg and Wo are the weights of the binary graft copolymer and initial crosslinked CS, respectively.
Batch Cr(VI) retention experiments
To determine the kinetic of Cr(VI) ions removal, batch type experiments were carried out by triplicate at 25°C. 100 mg of nCS or BGnCS were shaken in closed vials with 10 mL of Cr(VI) aqueous solution, with a pH value of 5.5. The change in chromium concentration was monitored by ultraviolet spectroscopy at 275 nm (UV-Vis spectrometer SPECORD200 Plus, Analytik Jena, Germany). The absorption capacity of the systems was quantified from calibration curve using the absorbance changes in function of concentration. The used linear equation is Abs = 0.0237[Cr(VI)] + 0.0251 (R2 = 0.9984), which is valid between 2 and 100 mg Cr(VI) L-1. The precision range of the spectrophotometer was ±0.008 absorbance.
Adsorption isotherms of Cr(VI)
The equilibrium sorption of CrO4 2- was carried out onto nCS and BGnCS as mentioned earlier. Solutions with different concentrations of K2CrO4 were prepared by dilutions of a stock solution of 1.0 x 10-3 mol L-1 in distilled water. Samples were placed into solutions and the concentration was analyzed by UV spectroscopy. The experiments were performed at 25°C by triplicate. The data was fitted into Freundlich and Langmuir isotherms.
Results and Discussion
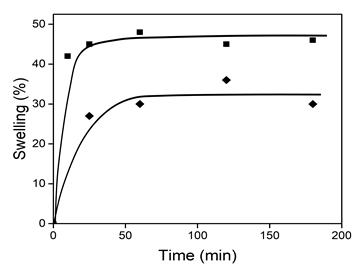
The crosslinking yield of nCS1 was 56.8±0.52 % while for nCS3 was 90.7±1.6 %. In order to choose an appropriate solvent to graft the monomers, the swelling behavior was studied in methanol and dimethyl-formamide (Fig. 1). Water was not considered due to high swelling of net-CS in this solvent (~400 %), that causes a diminishing in mechanical properties. nCS3 reached the equilibrium swelling at 60 min, and methanol was a better solvent with a ~45 % swelling, compared to the ~30 % swelling in DMF. A similar behavior was shown in nCS1 (43 % and 28 % in methanol and DMF, respectively). Therefore, net-CS swelling degree in methanol is enough for carrying out the grafting reaction.
Fig. 2 shows the binary graft of the monomers onto net-CS as a function of dose. In the first case, the yield of grafting onto nCS3 increased with the dose and a maximum grafting percentage of 35 % was obtained at 15 kGy (Fig. 2(a)). In the case of nCS1, the binary graft of the monomers decreased with the dose, but higher grafting percentages were obtained (Fig. 2(b)). This behavior is due to an easier diffusion of monomers into nCS1 than nCS3, causing a higher swelling and a big grafting yield. By other side, it is well known that chitosan undergoes degradation with gamma radiation [12], but the nCS1 was degraded faster than nCS3.

Fig. 2 Binary graft onto net-CS at different CS initial concentration, as a function of dose; (a) nCS3 (b) nCS1. [NVCL/DMAAm]: 15/5 % v/v, in methanol.
Determination of contact time for the maximum Cr(VI) retention
In spite to determinate the optimal time contact for Cr(VI) retention study, three grafted samples with different grafting percentages were chosen: BGnCS1 (184%), BGnCS1 (200%) and BGnCS1 (270%). The results can be observed in Fig. 3, it is clear that retention increased rapidly, and then leveled off at the maximum retention of equilibrium time. The retention onto nCS1 was about 17 mg of Cr(VI) g-1 of sample at 800 h. In case of binary grafting, the retention time was lower and depended of grafting percentage. Therefore, the Cr(VI) retention reached about 30 mg g-1 at only 3 h (180 min) in BGnCS1 (270 %), while BGnCS1 (184%) retained only 10 mg g-1. This decrease in the equilibrium retention time was due to the grafted chains were swelled too and they put more pressure into network, accelerating the swelling process.
Retention of chromium as a function of the initial concentration
The effect of initial concentration of Cr(VI) on the maximum percentage of retention in samples with different binary grafting percentages can be observed in Table 1.
Table 1 Equilibrium Cr(VI) retention Qe and retention percentage at different chromate concentrations and binary graft percentages.
| K2CrO4
[M] |
Qe (mg g-1) | Retention (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| nCS1 | BGnCS1 184% graft |
nCS3 | BGnCS3 21% graft |
nCS1 | BGnCS1 184% graft |
nCS3 | BGnCS3 21% graft |
|
| 1x10-4 | 9.18 | ---- | 9.19 | 8.58 | 95.09 | ---- | 95.21 | 92.04 |
| 2x10-4 | 15.09 | 16.78 | 16.49 | 17.67 | 78.17 | 89.93 | 97.08 | 95.23 |
| 3.5 x 10-4 | 24.33 | 28.40 | 32.08 | 31.74 | 75.52 | 85.9 | 98.28 | 97.81 |
| 5 x 10-4 | 23.3 | 14.85 | 35.52 | 45.94 | 48.10 | 31.46 | 95.92 | 97.48 |
Maximum Cr(VI) retention (Qe) in net-CS and BGnCS increased with Cr(VI) solution concentration; all samples achieved to retain chromium ions but with different efficiencies (Table 1). Samples based on nCS3 had the best performance, achieving above 95 % efficiency in the range of studied concentrations. Something important is the retention was better at lower grafting percentages such as BGnCS3 (21%) sample. This was attributed to the additional binding affinity via incorporation of NVCL and the DMAAm in the hydrogel network structure. However, the retention capacity decreased when the graft percentage increased because the chains were longer and tangled, which did not allow the functional groups to be available to interact with Cr(VI) ions.
The capacity of the adsorbent can be described by equilibrium sorption isotherms. The sorption isotherms were investigated using two equilibrium models: Freundlich and Langmuir, which give information about the sorption process.
The Freundlich isotherm can be applied to non-ideal adsorption on heterogeneous surfaces as well as multilayer adsorption, and is expressed by the following equation:
where, Qe is the amount of adsorption of Cr(VI) at equilibrium (mg g-1), Ce is the equilibrium concentration (mg L-1), KF and 1/n are the Freundlich constants corresponding to the adsorption capacity and empirical parameter of the intensity of adsorption, respectively. At high KF and 1/n values, the maximum retention capacity is higher and more favorable is the adsorption. If value of 1/n is below one, it indicates a normal adsorption. On the other hand, 1/n being above one indicates cooperative adsorption, where the function has an asymptotic maximum as the concentration is increased [13, 14]. These values are shown in Table 2. For nCS1 and nCS3, KF were found 5.132 and 0.983, and 1/n values of 0.222 and 2.971 respectively. The BGnCS1 and BGnCS3 systems with different grafting percentages showed high values of both parameters with high correlation factors. The values of n less than one, confirms that Freundlich isotherm is valid for the Cr(VI) adsorption in the binary grafted copolymer, which was attributed to a heterogeneous surface structure of the new absorbent.
Table 2 Freundlich and Langmuir isotherm parameters for adsorption of Cr(VI).
| Adsorbent | Freundlich | Langmuir | ||||
|---|---|---|---|---|---|---|
| 1/n | KF | r2 | Qmax (mg g-1) | KL | r2 | |
| nCS1 | 0.222 | 5.132 | 0.997 | 24.63 | 0.528 | 0.988 |
| nCS3 | 2.971 | 0.983 | 0.942 | 55.24 | 1.191 | 0.999 |
| BGnCS1 (184% graft) | 0.841 | 37.367 | 0.981 | 77.52 | 6.45 | 0.996 |
| BGnCS3 (5% graft) | 3.683 | 6.838 | 0.929 | 2.42 | 0.986 | 0.994 |
| BGnCS3 (21% graft) | 3.621 | 1.294 | 0.998 | 142.86 | 0.356 | 0.998 |
The Cr(VI) adsorption on the binary graft copolymers was also tested with Langmuir isotherm (equation 4). This equation is based on monolayer adsorption onto a surface with finite number of identical sites, homogeneously distributed onto the sorbent surface [15].
where, Qe is the amount of Cr(VI) adsorbed per unit of copolymer system at equilibrium concentration (mg g-1) at different initial solution concentration of Cr(VI), Ce is the concentration of chromates solution at equilibrium (mg L-1) with different initial concentration solutions of chromates. Qmax is the maximum capacity of adsorption, corresponding to the total number of interstitial sites in the absorbent and KL is the constant of the Langmuir isotherm. The value of the KL is related to the affinity of the ions to the adsorption sites, the high values imply a greater affinity of the ions studied towards to the adsorbent. Large KL values suggest the stability of sorption complex, which may be the cause of existence of chemical binding forces between chromate anions and surface of the adsorbent. Maximum adsorption capacity obtained for nCS1 and nCS3 was 24.63 and 55.24 mg g-1, respectively. By other side, the retention behavior in binary grafted systems was dependent of grafting percentages; the optimum retention was obtained from BGnCS3 (21% graft) with Qmax 142.86 mg g-1, higher than those values obtained by different researchers [4, 16, 17]. When the percentage was higher, the Cr(VI) retention decreased because of grafted chains hinder the accessibility to the internal sites or block a number of adsorption sites.
The experimental data fits both Freundlich and Langmuir models for all samples since higher correlation coefficients (>0.98), but Langmuir isotherms exhibited a better coefficients. This means that the adsorption process can be described as a monolayer coverage of the Cr(VI) onto a surface with finite number of sites, which are homogeneously distributed over the adsorbent. This outcome is similar to the studies involving Cr(VI) adsorption on chitosan [18].
Characterization Infrared spectroscopy
To confirm the binary graft copolymerization, the IR spectra of different systems were determined, using a Perkin Elmer 100 Instruments, Norwalk USA, with Universal ATR sampling accessory with 16 scans; they are shown in Fig. 5. The characteristic peaks of nCS appear at 3340 cm-1 (O-H stretching), 2923 cm -1 (CH2 stretching vibration of pyranose ring), 1652 cm-1 (C=O streaching) corresponding to amide of N-acetylglucosamine units [16, 17], and 1568 cm-1 (C=N streaching and N-H bending) due to imine bonds formed during the crosslinking reaction between CS and formaldehyde [11]. In the BGnCS3 (21%), only one carbonyl peak was observed at 1638 cm-1 because of the overlap with the carbonyls of DMAAm and NVCL. Once the binary graft system interacted with Cr(VI), the peak at 1636 cm-1 was wider and a little shoulder appeared at 1579 cm-1, indicating that interaction of material and metal ions was carried out by means amine and carbonyl groups.
Thermogravimetric analysis
The thermal stability of different systems was carried out using a TGA Q50 (TA Instruments, New Castle, DE, USA), in nitrogen atmosphere and a heat velocity of 10°C min-1. The thermogram of nCS exhibits a decomposition temperature at 290oC corresponding to dehydratation of saccharide rings, decomposition of acetylated units and depolymerization of CS [11]. The binary graft, BGnCS3 (21%), exhibits two decomposition temperatures, one near 300oC corresponding to decomposition of CS, and the other at 410oC, due to the decomposition of PNVCL and PDMAAm. The Cr(VI) retention in the system increased the first decomposition temperature and decreased the second decomposition temperature due to interaction of NVCL with chromates ions.
Conclusion
This study aimed for a comparative evaluation of the sorption efficiency of Cr(VI) onto net-CS, and NVCL/DMAAm grafted onto nCS using gamma radiation. The characterization showed that nCS has a lower thermal resistance than binary grafted system. Therefore, Cr(VI) sorption capacity was higher for BGnCS3 samples than for nCS3, showing better apparent mechanical properties. The adsorption data was fitted to both Langmuir and Freundlich isotherms, showing good regression correlation coefficients (R2>0.98), but the Langmuir model fitted better. This means that the adsorption process can be described as a monolayer coverage of Cr(VI) onto material surface with finite number of sites, which predicts that chemical sorption occurred. The maximum retention capacity and removal efficiency were dependent of grafting yield, obtaining the best performance for BGnCS3 with 21% graft. This system showed Cr(VI) removal efficiencies over 92% in a wide range of concentrations, and a Qmax of 142.86 mg g-1. Those values were higher than others founded in the literature, and even better than nCS3.











 nueva página del texto (beta)
nueva página del texto (beta)