Introduction
Semiconductor photocatalysis has recently attracted great attentions for environmental remediation and purification through advanced oxidation processes [1,2]. Heterogeneous semiconductor photocatalysis was reported as a suitable approach to detoxification from both industrial and biological pollutants in many researches [3,4]. Among the semiconductors investigated, anatase TiO2 is one of the most commonly used materials for photocatalytic degradation of chemical and microbiological pollutants due to its non-toxic nature, low cost, abundance, UV-driven high activity, photo and thermal stability [5,6]. Nevertheless, the photocatalytic activity of TiO2 is constrained by two main problems which are the wide band gap energy (Ebg) that limit its application to ultra-violet region and the fast recombination of photogenerated electrons and holes [7,8]. Both of these drawbacks have been studied to resolving by using several approaches such as doping the TiO2 with metallic or nonmetallic elements, dye sensitization and surface coupling with other semiconductors to form a heterojunction [9-11], that cause of improving the photocatalytic activity of TiO2 for degradation of organic pollutants by shifting the light absorption of TiO2 to visible-light region and retarding the recombination of electron and hole [9,12]. Moreover, the affinity interaction between the catalyst TiO2 and pollutants should be paid more attention to enhance the photocatalytic efficiency. Since aromatic compounds are common pollutants, it has been proven that surface modification of TiO2 with aromatic organic acid compounds as surface modifiers can improve not only adsorption efficiency of aromatic pollutants, but also lead to an increase in the light utilization [13-15]. These modifiers can enhance the surface coverage of the pollutants on TiO2 through phenyl group interaction. Wang et al. found that surface modification of TiO2 nanotubes/Ti with salicylic acid can be carried out in saturated solution of salicylic acid through an ester-like bond between -COOH group and the surface -OH groups of TiO2[16]. They concluded that salicylic acid-modified TiO2 can enhance photoactivity of TiO2 nanotubes/Ti in degradation of p-nitrophenol due to improved surface adsorption properties and light utilization. Moreover, it has been known that the coupling of Ag nanoparticles (AgNPs) with TiO2 can spread the light absorption spectrum of TiO2 toward the visible-light region and promote the separation of photogenerated electron-hole (e--h+) pairs to facilitate the electron transfer from TiO2 to AgNPs [17-19]. According to the mentioned reasons, it seems that the fabrication of salicylic acid-Mod-Ag/TiO2 ternary composite can be investigated as a good approach to merge the photocatalytic advantages of salicylic acid-Mod-TiO2 and AgNPs-TiO2 composites and therefore improve the photocatalytic activity of TiO2 for photocatalytic degradation.
In the present study, we described an experimental investigation of simple surface chemical co-modification of TiO2 nanotubes/Ti plates with salicylic acid and AgNPs. Methylene Orange (MO) dye was chosen as the model pollutant to investigate the photocatalytic activity of the modified plates in the presence of UV light irradiation.
Experimental
Catalyst preparation
Titanium dioxide nanotubes arrays (TiO2NTs) were growth on pure Ti plates by potansiostatic anodic oxidation process according to our previous work [20]. Very briefly, Ti plates (purity 99%) with geometric area of 3 cm2 were mechanically polished with different emery papers and then ultrasonically washed in acetone and deionized water bath. The anodizing process was performed in a mixed solution containing of glycerol/water (75:25, vol.%) + 0.5 wt.% NH4F under magnetic stirring in two electrode configuration, where cleaned Ti plate and Pt sheet were used as anode and cathode at an applied voltage of 20 V in 25 ºC. The TiO2NTs/Ti plates were modified by immersing of the plates in the mixture of saturated solution of salicylic acid and 0.3 M AgNO3 at 45 oC for the duration of 45 min. Finally, the prepared plates were directly calcined at 450 °C for 2 h. The yellow colored, homogeneous and adherent coatings were successfully obtained on the TiO2NTs/Ti plates. The yellow color is due to esterification between salicylic acid and -OH groups on surface of TiO2NTs/Ti plate according to following mechanism (Fig. 1) reported in [13]. These chemically modified plates were denoted as salicylic acid(mod)-Ag /TiO2NTs/Ti plate.
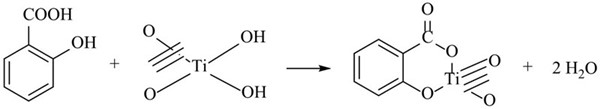
Fig. 1 Suggested mechanism of esterification between salicylic acid and -OH groups on TiO2 surface in [13].
Characterization and electrochemical studies
The surface morphology of the plates was characterized with a scanning electron microscope (Philips, Model XL30). FT-IR spectrometry was conducted using a Fourier transform infrared spectrophotometer (Nexus 670, Thermo Nicolet USA). Electrochemical measurements were carried by Autolab PGSTAT302N potentiostat.
Photocatalytic degradation of Methylene Orange (MO)
The photocatalytic activities of the bare and modified TiO2NTs/Ti/Ti plates were tested by the degradations of MO dye as a chemical pollutant under UV light irradiation. In a general photocatalytic measurement, four plates (geometric area of 3 cm2) were put around the beaker (500 mL) containing aqueous solution of MO (10 ppm) and irradiated under magnetic stirring in a photoreactor set-up equipped with a 250-W high-pressure mercury lamp. The photoreactor setup was explained in our previous reports [20]. The initial pH of MO solution (10 ppm) was at about 6.2 and all photocatalytic tests were done in this pH. Prior to the irradiation, the adsorption efficiency for MO removal was tested. In this regard, the MO suspensions including the plates were magnetically stirred in the dark for 30 min to establish the adsorption/desorption equilibrium and subsequently, the reaction was started. At a given time interval (15 min), 5 mL of solution was withdrawn periodically and MO concentration was determined by measuring the UV-Vis absorption.
Results and Discussion
Structural and morphological characteristics
SEM micrograph of TiO2 nanotubes layer electrochemically grown on Ti plate by applying a constant voltage of 20 V are shown in Fig. 2a. From the top-view, it can be observed that the diameter of the nanotubes is about 70 nm and the wall thickness is around 10 nm. Fig. 2b, shows the TiO2NTs/Ti after surface chemical modification process with salicylic acid. As can be seen from Figure, esterification between salicylic acid and -OH groups on TiO2 surface has been led to formation of uniform deposits on the walls of TiO2NTs/Ti plates where the walls are almost closed. Fig. 2c, presents the microstructure of the salicylic acid(mod)-Ag composite coated on TiO2NTs/Ti plate. As can be seen from the figure, the composite has completely covered the entire surface of TiO2NTs/Ti plate, where nanotubular structure is disappeared. In order to probe the chemical composition, the plate material was further characterized by EDX. Fig. 2d, shows the EDX spectrum of the salicylic acid(mod)-Ag/TiO2NTs/Ti plate that confirms the presence of carbon and silver on TiO2NTs/Ti.
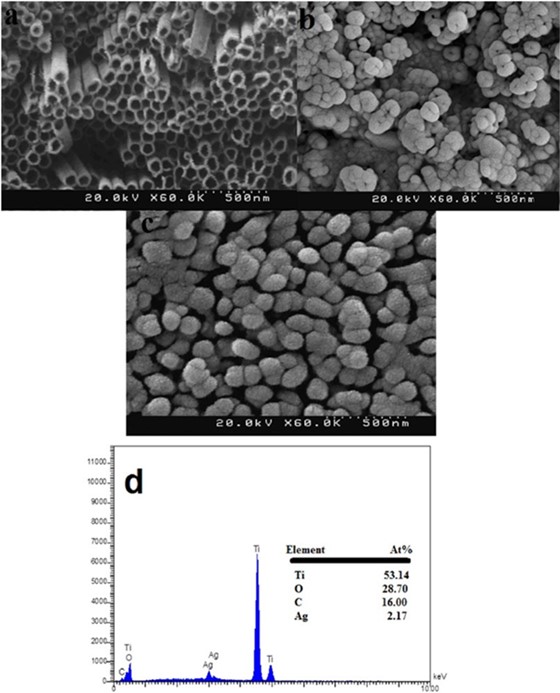
Fig. 2 SEM images of bare TiO2-NTs/Ti plate (a), Salicylic acid(mod)/TiO2NTs/Ti plate (b), Salicylic acid(mod)-Ag/TiO2NTs/Ti plate (c) and EDX spectra obtained of salicylic acid(mod)-Ag/TiO2NTs/Ti plate (d).
The FT-IR spectra of the TiO2NTs/Ti and salicylic acid(mod)/TiO2NTs/Ti plate are shown in Fig. 3. There are obvious differences in the FT-IR spectra of the TiO2 nanotubes and salicylic acid modified TiO2NTs/Ti plates. In contrast to FT-IR spectrum of the bare TiO2 nanotubes, the spectrum of the salicylic acid(mod)/TiO2NTs/Ti consists of absorption bands at ~2351 cm-1 (attributed adsorbed CO2 molecule), ~1650 cm-1 (C=O stretching) and ~1450 cm-1 (C=C stretching in the aromatic rings) [13,14]. All these results clearly reveal the esterification between salicylic acid and -OH groups on surface of TiO2NTs/Ti plate.
Photoelectrochemical performance
Photocurrent response is an important parameter for studying the separation efficiency and transfer performance of the photoinduced charge carriers of the fabricated plates. Thereby, the photocurrent response of fabricated plates was analyzed according to Fig. 4, by several on-off cycles of intermittent irradiation (50 s) at -0.2 V versus Ag/AgCl in 0.1 M Na2SO4 solution. The dark currents of the all plates are almost zero. In presence of UV light, the photocurrent of salicylic acid(mod)-Ag/TiO2NTs/Ti is about 500 µA which is approximately 1.5 times higher than Ag/TiO2NTs/Ti and salicylic acid(mod)/TiO2NTs/Ti plates, indicating that the photogenerated electron transfer efficiency on salicylic acid(mod)-Ag/TiO2NTs/Ti plate was higher than that of other plates under similar condition [21,22].
Photocatalytic activity
The photocatalytic activity of TiO2NTs/Ti and modified plates was investigated during MO degradation under UV light irradiation in aqueous solution. As can be seen from Fig. 5a, the photodegradation efficiency of the salicylic acid(mod)/TiO2NTs/Ti plate is higher compared to bare TiO2NTs/Ti plate. The formation of ester with stabile six-member ring could avail the esterification between salicylic acid and -OH groups on TiO2 surface which can lead to formation of weak charge transfer between the aromatic system and Ti4+ center [13]. In addition, the higher photocatalytic activity of the salicylic acid(mod)/TiO2NTs/Ti plate can be attributed to improved surface adsorption properties between MO and the plate. In case of Ag/TiO2NTs/Ti, silver can trap the excited electrons from TiO2NTs under UV light irradiation and leave the holes for the degradation of pollutants, improving the charge carrier separation and therefore photocatalytic activity [17,19]. The salicylic acid(mod)-Ag/TiO2NTs/Ti plate has maximum photocatalytic performance compared to the other plates in MO degradation. It seems that the fabrication of salicylic acid(mod)-Ag/TiO2NTs ternary hybrid integrate the photocatalytic advantages of salicylic acid(mod)-TiO2 and Ag-TiO2 hybrids and therefore maximize the photocatalytic activity. Fig. 5b, shows the absorbent spectrums of MO degradation with various fabricated TiO2NTs/Ti plates under UV light illumination for 15 min. As seen, the decrease amount of MO concentration for salicylic acid(mod)-Ag/TiO2NTs/Ti is higher compared to other TiO2NTs/Ti plates. The synergetic effect among salicylic acid(mod), Ag and TiO2NTs motivate a fast charge separation, a slow electron-hole recombination and improved surface adsorption, thus increasing the photocatalytic performance of the salicylic acid(mod)-Ag/TiO2NTs/Ti plate. The comparison of the photodegradation efficiency of the salicylic acid(mod)-Ag/TiO2NTs/Ti plate with previously reported photocatalysts for degradation of MO is summarized in Table 1. It is clear that the photodegradation efficiency of final modified TiO2NTs/Ti plate is comparable with values reported in previous literatures.
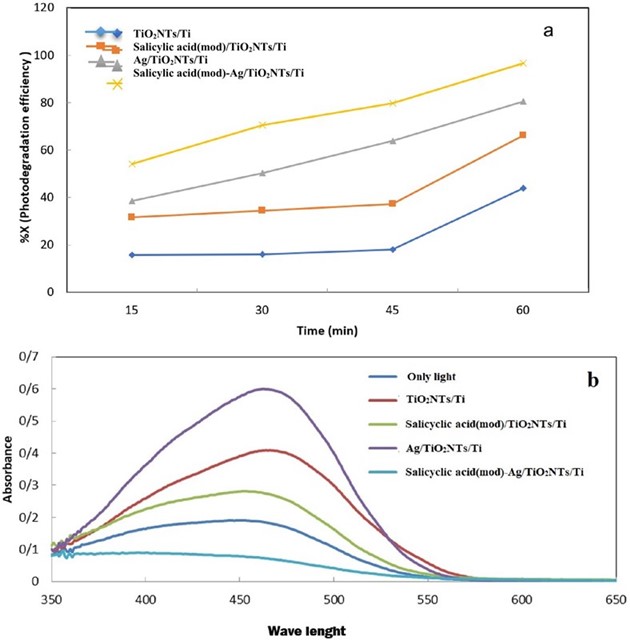
Fig. 5 The degradation efficiency of MO under UV light illumination for the salicylic acid(mod)-Ag/TiO2NTs/Ti, Ag/TiO2NTs/Ti, salicylic acid(mod)/TiO2NTs/Ti and TiO2NTs/Ti plates (a) and Typical absorbent spectra of the MO solution illuminated by UV light (b).
Table 1 The comparison of photodegradation efficiency of the salicylic acid(mod)/TiO2NTs/Ti plate with previously reported photocatalysts for degradation of MO.
| Photo-Catalyst | Pollutant | Lamp Source | Photodegradation Efficiency (%) | Time (min) | Refrence |
|---|---|---|---|---|---|
| TiO2/Cu2+ | Methyl Orange | UVc lamp | 81 | 90 | [23] |
| TiO2/TENA | Methyl Orange | UVc lamp | 76 | 120 | [24] |
| TiO2/Zeolite | Methyl Orange | UVc lamp | 86.2 | 30 | [25] |
| TiO2/Ce | Methyl Orange | UVc lamp | 78 | 240 | [26] |
| Salicylic acid (mod)-Ag/TiO2NTs/Ti | Methyl Orange | UVc lamp | 96.6 | 60 | Present study |
Kinetic study of photocatalysis
Fig. 6a, shows plotting the graph of ln(Co/Ct) versus time(min) based on first-order model for all fabricated TiO2NTs/Ti plates. According to Eq. 1, rate constant (kapp) can be obtained for MO photocatalytic degradation from plotting the graph of ln (Co/Ct) versus t. For more clarifications, the obtained results for kapp are demonstrated for all fabricated plates in Fig. 6b. As can be seen from Fig. 6b, the salicylic acid(mod)-Ag/TiO2NTs/Ti shows maximum rate constant compared to other plates, attributing to synergetic effect between Ag, salicylic acid and TiO2 for photocatalytic degradation of MO.
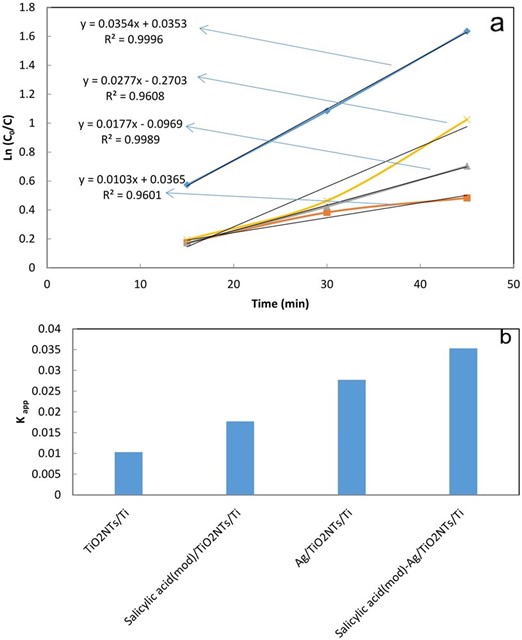
Fig. 6 Linear-log plot under UV light irradiation (a) and Rate constant for the degradation of MO under UV light irradiation (b) with various fabricated TiO2NTs/Ti plates.
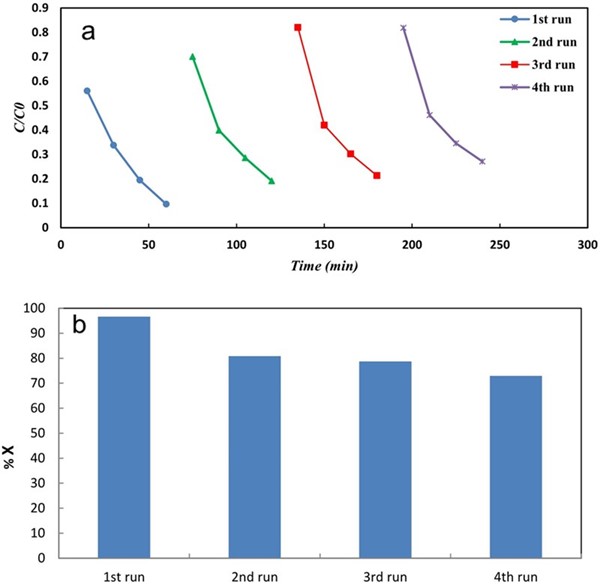
The stability of the salicylic acid(mod)-Ag/TiO2NTs/Ti was evaluated in four consecutive experiments by using fresh aqueous solution of MO (10 ppm). Between each experiment, the photocatalyst plate was removed, then washed with distilled and used. According to results of Fig. 7a and 7b, during the 4 cycle experiments, modified TiO2NTs/Ti shown a slightly decrease in degradation efficiency from 96.6% in first cycle to 72.9% in fourth cycle experiment, showing modified TiO2NTs/Ti plates have high chemical and photochemical stability in dye degradation under UV light illumination.
Conclusions
Synthesis and characterization of salicylic acid (mod)-Ag/TiO2NTs/Ti plate as a photocatalyst in the photodegradation of methylene orange (MO) under UV light irradiation were carried out. Synthesis was conducted by immobilization of TiO2NTs/Ti plates in the saturated solution of AgNO3 and salicylic acid at 45 oC. The results showed the photochemically modified plates possess excellent photocatalytic performance for MO degradation under UV light irradiation when compared with that of the TiO2NTs/Ti plate. These new plates may have potential applications in environmental area.











 nueva página del texto (beta)
nueva página del texto (beta)