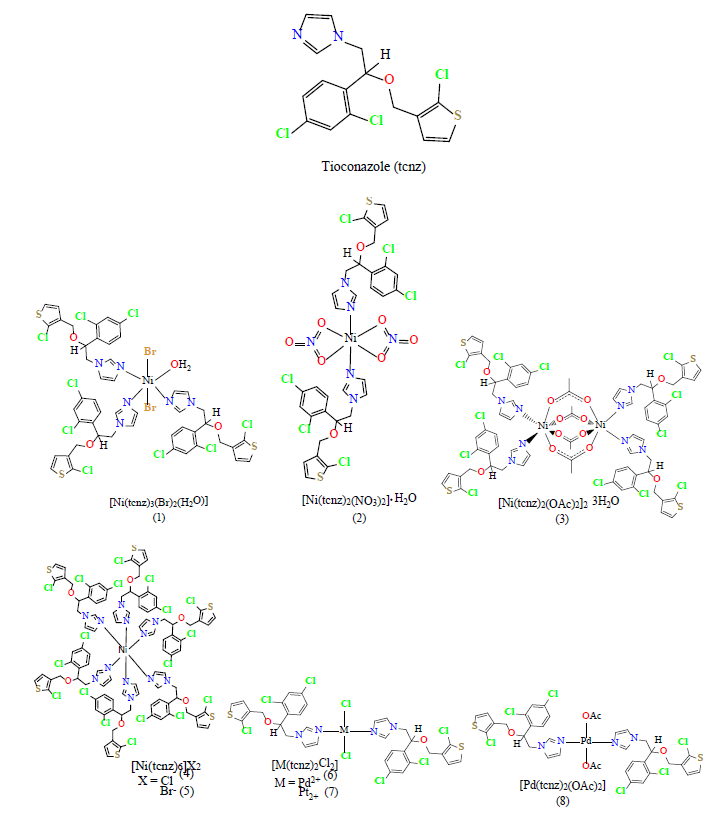
Introduction
The discovery of active sites containing nickel centers in metalloproteins, [1),(2),(3),(4] has stimulated the study of its coordination compounds and their biological activity, such as antitumor, anticonvulsants or antiepileptic, antibacterial and antifungal.[5),(6] Additionally, palladium and platinum, also from group 10, have shown anticancer activity.[7),(8),(9),(10] Platinum(II) compounds, as cisplatin, oxaliplatin and carboplatin, are the more effective anticancer drugs used in chemotherapy.[11),(12]
Cisplatin, used for the testicular and ovarian cancer treatment, is one of the most widely used antitumor drugs in the world.[13),(14),(15] However, it has side effects such as nephrotoxicity, drug tolerance, limited solubility and intravenous administration.[14),(16),(17),(18),(19] Due to the fact that the cancer is the second leading cause of death,[16),(20),(21] the development of improved metal based drugs is currently of interest.
When designing new antitumor agents, the similarity in the chemistry of the PtII and PdII compounds has led to the study of palladium antitumor drugs with high activity.[22),(23),(24),(25),(26)] Some of them are stabilized by chelates, as Schiff bases, or by voluminous ligands with monodentate nitrogen atoms,[22] which presented activity in HeLa cell line. It has been reported that trans-Pd compounds with Schiff base ligands have better activity than the cis-Pd compounds.[18),(22] Additionally, a series of coordination compounds whit benzylamine and PdII were studied on MCF-7 and MDA-MB-231 breast cancer cell lines, indicating an effective anticancer potential. [17]
When combining metal ions with an established biological activity of the ligands, an enhancement or a modification of their pharmacological properties has been observed, as it is the case of ticonazole, clotrimazole and miconazole, with antifungal properties, which their organometallic ruthenium(II) compounds showed antiparasite activity.[27] Transition metal coordination compounds with Shiff base derivatives, (CoII, NiII, CuII, ZnII) have proved to enhance the antimicrobial and antifungal activity of the free ligands.[28]
In a previous study, we synthesized and characterized CuII and ZnII coordination compounds with tioconazole (tcnz) and their cytotoxic activity in HCT-15 and HeLa cell lines was studied. The octahedral [Cu(tcnz)4Cl2] compound showed promising activity in the HCT-15 cell line. While the tetrahedral [Zn(tcnz)2Br2] compound had cytotoxic activity in HeLa cell lines.[29]
Continuing our work in this field, NiII, PtII and PdII compounds were synthesized and characterized (Fig. 1) as their biological activity as possible anticancer agents in HCT-15, HeLa, MCF-7 and PC-3 cell lines, was studied.
Experimental
Materials and methods
All reagents and solvents were purchased and used without any further purification: tioconazole 98% (Aldrich, Co); NiCl2∙6H2O, NiBr2∙3H2O, Ni(NO3)2∙6H2O, Ni(OAc)2∙4H2O (J.T. Baker); PdCl2, PtCl2 and Pd(OAc)2 (Aldrich, Co); solvents (Merck). The complexes [PdCl2(CH3CN)2] and [PtCl2(CH3CN)2] were synthesized according with the reported procedure.[30] FT IR spectra in the range 4000-400 cm-1 were collected in a Perkin Elmer FT-IR Spectrum 400 spectrophotometer with an universal ATR sampling accessory at 298K. Mass spectra (MS-ESI+) were determined in an Esquire 6000 mass spectrometer (Fig. S1-S6). Elemental analyses for carbon, hydrogen, nitrogen and sulfur were carried out with a Fisons EA 1108 analyzer. Magnetic susceptibility measurements at room temperature of powdered samples were obtained on a Johnson-Matthey DG8 5HJ balance, using the Gouy method. NMR spectra were obtained at room temperature on a 300 MHz Bruker-Avance Unity spectrometer. 1H and 13C {1H} NMR spectra were obtained using DMSO-d 6 and CDCl3 (Fig. S7-S15). Electronic spectra were measured over the range 40000-5000 cm-1 by the diffuse reflectance method on a Cary-5000 Varian spectrophotometer at 298 K (Fig. S16).
Synthesis of the coordination compounds
Coordination compounds form the NiII salts were synthesized by similar procedures. A solution of one equivalent of the corresponding transition metal salt in acetone was added to a solution of one equivalent of tioconazole in acetone, with exception of the [Ni(tcnz)2(OAc)2]2∙3H2O 3 compound where ethanol was used as solvent. For the compounds [Ni(tcnz)6]Cl2 4 and [Ni(tcnz)6]Br2 5 a 1:3 ratio was used. The reaction mixture was heated under reflux with constant stirring during 24 h. PdII and PtII compounds were synthesized using a 1:2 ratio (metal salt:tcnz) in acetone. The solvent was evaporated at RT and the products were washed with water and ethanol and dried under vacuum overnight.
Synthesis of [Ni(tcnz)3Br2(H2O)] (1)
NiBr2∙3H2O (0.07 g, 0.25 mmol) was added to a solution of tioconazole (0.1g, 0.25 mmol) in acetone (15mL), the obtained dark blue solution was left to stand at RT, a bright blue solid precipitated. UV-Vis-NIR ((cm-1): (1 = 8665, (2 = 14993 and (3 = 24918. FT-IR (ATR, (cm-1): ((O-H) 3324, ((C=N) 1589, ((C-O-C) 1088, ((C-S) 736. MS-ESI+ (m/z) 1301 [C48H39Cl9BrN6O3S3Ni]+. Anal. Found: C, 41.08; H, 2.51; N, 6.10; S, 5.99%. Calc. for C48H41Cl9Br2N6O4S3Ni: C, 41.19; H, 2.95; N, 6.00; S, 6.87%. µeff = 3.40 BM. Yield: (0.19 g, 92%).
Synthesis of [Ni(tcnz)2(NO3)2]∙H2O (2)
Ni(NO3)2∙6H2O (0.074 g, 0.25 mmol) was added to a solution of tioconazole (0.1g, 0.25 mmol) in acetone (15mL). A dark green solution was observed, a green solid was obtained. UV-Vis-NIR ((cm-1): (1 = 8936, (2 = 15553 and (3 = 25477. FT-IR (ATR, ( cm-1): ((O-H) 3344, ((C=N) 1589, (as(NO3) 1468, (s(NO3) 1306, ((N=O) 1235, ((C-O-C) 1087, ((C-S) 736. MS-ESI+ (m/z) 896 [C32H26Cl6N5O5S2Ni]+. Anal. Found: C, 39.36; H, 2.90; N, 8.45; S, 6.26%. Calc. for C32H28Cl6N6O9S2Ni: C, 39.37; H, 2.89; N, 8.61; S, 6.57%. µeff = 3.50 BM. Yield: (0.11g, 81%).
Synthesis of [Ni(tcnz)2(OAc)2]2∙3H2O (3)
Ni(OAc)2∙4H2O (0.064 g, 0.25 mmol) was added to a solution of tioconazole (0.1g, 0.25 mmol) in ethanol (15mL). An emerald green solution was observed, a bright green solid precipitated. UV-Vis-NIR ((cm-1): (1 = 8936, (2 = 15417 and (3 = 25409. FT-IR (ATR, ( cm-1): ((O-H) 3128, ((C=N) 1586, (as(COO) 1558, (s(COO) 1408, ((C-O-C) 1084, ((C-S) 732. MS-ESI+ (m/z) 1456 [C54H47Cl9N6O9S3Ni2]+. Anal. Found: C, 42.45; H, 3.01; N, 5.70; S, 5.35%. Calc. for C36H38Cl6N4O9S2Ni: C, 42.97; H, 3.81; N, 5.57; S, 6.37%. µeff = 3.20 BM. Yield: (0.11g, 90%.).
Synthesis of [Ni(tcnz)6]Cl2 (4)
NiCl2∙6H2O (0.07 g, 0.30 mmol) was added to a solution of tioconazole (0.34 g, 0.90 mmol) in acetone (15mL), the obtained blue solution was left to stand at RT. After two weeks purple crystals suitable for X-ray diffraction were isolated. UV-Vis-NIR ((cm-1): (1 = 10880, (2 = 17550 and (3 = 24330. FT-IR (ATR, ( cm-1): ((C=N) 1589, ((C-O-C) 1078, ((C-S) 738. Due to its insolubility in common solvents it was not possible to obtain its MS-ESI+ (m/z). Anal. Found: C, 7.70; H, 3.33; N, 6.58; S 7.70%. Calc. for C96H78Cl20N12O6S6Ni: C, 46.94; H, 3.20; N, 6.84; S, 7.83%. µeff = 3.14 BM. Yield: (0.24g, 33%).
Synthesis of [Ni(tcnz)6]Br2 (5)
NiBr2∙3H2O (0.07 g, 0.25 mmol) was added to a solution of tioconazole (0.3 g, 0.75 mmol) in acetone (15mL), the obtained blue solution was let to stand at RT. After a week purple crystals suitable for X-ray diffraction were isolated. UV-Vis-NIR ((cm-1): (1 = 10880, (2 = 17550 and (3 = 24330. FT-IR (ATR, ( cm-1): ((C=N) 1589, ((C-O-C) 1079, ((C-S) 737. Due to its insolubility in common solvents it was not possible to obtain its MS-ESI+ (m/z). Anal. Found: C, 44.47; H, 3.21; N, 7.54; S, 6.59%. Calc. for C96H78Cl18Br2N12O6S6Ni: C, 45.31; H, 3.09; N, 7.56; S, 6.60%. µeff = 3.01 BM. Yield: (0.25g, 36%).
Synthesis of [Pd(tcnz)2Cl2] (6)
[PdCl2(CH3CN)2] (0.05 g, 0.19 mmol was added to a solution of tioconazole (0.14g, 0.38 mmol) in acetone (15mL) during 24h at RT a light yellow solid precipitated from the solution. UV-Vis-NIR ((cm-1): (1=25595. FT-IR (ATR, ( cm-1): ((C=N) 1587, ((C-O-C) 1084, ((C-S) 735. 1H NMR (DMSO-d6, 300MHz): 8.08 (2H, s, CH, Ha), 7.67 (2H, d, CH, Hf), 7.51 (2H, dd, CH, Hh), 7.43 (2H, s, CH, Hj), 7.41 (2H, s, CH, Hc), 7.17 (2H, s, CH, Hg), 7.11 (2H, s, CH, Hb), 6.89 (2H, d, CH, Hk), 4.95 (2H, dd, CH, He), 4.37-4.26 (8H, m, CH2, Hi,d).13C NMR (DMSO-d6, 75MHz assignments by HSQC ): 139.79 (C1), 134.44 (C6), 134.06 (C7), 133.73 (C9), 133.20 (C13), 129.36 (C14), 128.99 (C8) , 128.63 (C10), 127.98 (C15), 127.91 (C11), 126.50 (C16), 124.56 (C3), 119.92 (C2), 75.59 (C5), 63.08 (C12), 50.96 (C4). MS-ESI+ (m/z) 917 [C32H26Cl7N4O2S2Pd]+. Anal. Found: C, 40.84; H, 2.38; N, 5.88; S 6.73%. Calc. for C32H26Cl7N4O2S2Pd : C, 40.34; H, 2.75; N, 5.88; S, 6.73%. Yield: (0.12g, 68%).
Synthesis of [Pt(tcnz)2Cl2]∙2H2O (7)
[PtCl2(CH3CN)2] (0.05 g, 0.10 mmol) was added to a solution of tioconazole (0.082g, 0.20 mmol) in mixture of acetone and acetonitrile (15 mL) under reflux during 4h at RT a yellow solid precipitated from the solution. UV-Vis-NIR ((cm-1): (1=27024. FT-IR (ATR, ( cm-1): ((O-H) 3444, ((C=N) 1588, ((C-O-C) 1083, ((C-S) 732. 1H NMR (CDCl3, 300MHz): 8.24 (2H, s, CH, Ha), 7.65 (2H, d, CH, Hf), 7.47-7.38 (4H, m, CH, Hc,h,j), 7.23 (2H, m, CH, Hg), 7.17 (2H, m, CH, Hb), 6.88 (2H, d, CH, Hk), 4.97 (2H, dd, CH, He), 4.33 (2H, d, CH2, Hi), 4.28 (4H, m, CH2, Hd), 2.06 (6H, s, CH3, Hl).13C NMR (CDCl3, 75MHz assignments by HSQC): 139.84 (C1), 134.88 (C7), 134.36(C6), 134.16 (C9), 134.65 (C13), 129.77 (C8), 129.64 (C14), 129.46 (C10), 128.40 (C11), 128.26 (C15), 127.02 (C16) , 125.06 (C3), 121.37 (C2), 75.78 (C5), 63.53 (C12), 51.43 (C4). MS-ESI+ (m/z) 970 [C32H26Cl6N4O2S2Pt]+. Anal. Found: C, 35.72; H, 2.06; N, 5.48; S 5.17%. Calc. for C32H30Cl8N4O4S2Pt : C, 35.67; H, 2.81; N, 5.20; S, 5.95%. Yield: (0.07g, 61%).
Synthesis of [Pd(tcnz)2(OAc)2] (8)
[Pd(OAc)2] (0.026 g, 0.12 mmol) was added to a solution of tioconazole (0.1g, 0.25 mmol) in acetone (15mL) during 24h at RT a beige solid precipitated from the solution. UV-Vis-NIR ((cm-1): (1=27683. %. FT-IR (ATR, ( cm-1): ((C=N) 1588, (as(COO) 1632, (s(COO) 1354 ((C-O-C) 1089, ((C-S) 733. 1H NMR (CDCl3, 300MHz): 7.67 (2H, s, CH, Ha), 7.42 (2H, d, CH, Hf), 7.35-7.30 (4H, m, CH, Hh,j), 7.17 (2H, d, CH, Hc), 6.96 (2H, m, CH, Hg), 6.77 (2H, d, CH, Hk), 6.73 (2H, m, CH, Hb), 4.85 (2H, dd, CH, He), 4.40 (2H, d, CH2, Hi), 4.24 (4H, d, CH2, Hi), 4.09-3.88 (4H, m, CH2, Hd), ), 1.94 (6H, s, CH3, Hl).13C CDCl3, 75MHzassignments by HSQC):178.09 (C17), 138.55 (C1), 135.29 (C6), 133.64(C7), 133.42 (C9), 133.35 (C13), 129.79 (C8), 128.73 (C16) ,128.63 (C14), 128.53 (C10), 128.53 (C11), 127.71 (C15), 124.03 (C3), 119.57 (C2), 75.89 (C5), 63.75 (C12), 52.44 (C4), 23.66 (C18). MS-ESI+ (m/z) 940 [C34H29Cl6N4O4S2Pd]+. Anal. Found: C, 43.35; H, 2.81; N, 5.99; S 5.77%. Calc. for C36H32Cl6N4O6S2Pd: C, 43.24; H, 3.23; N, 5.60; S, 6.41. Yield: (0.089g, 69%).
X-ray crystallographic study
Diffraction intensity patterns from single crystals of compounds 4 and 5 were collected on a SMART APEX I diffractometer (Bruker AXS) equipped with a CCD-detector and using graphite monochromated Mo K( ((= 0.71073 Å) radiation source. APEX2 v2012.10.0 (Bruker, 2012) package was used for data collection and data integration. Absorption corrections were applied using analytical procedure. The structures were solved by direct methods using the package SHELXS-2012 and refined with an anisotropic approach for non-hydrogen atoms using the SHELXL-2014/7 program. All hydrogen atoms attached to C atoms were positioned geometrically as riding on their parent atoms, with
C-H = 0.93-0.99 A and Uiso(H) = -1.2 Ueq(C) for aromatic and methylene groups.[31),(32),(33] A summary for data collection and refinements is given in Table 1.
Table 1 Crystallographic data and refinement parameters of compounds [Ni(tcnz)6]Cl2 4 and [Ni(tcnz)6]Br2 5.
| Compound | 4 | 5 |
|---|---|---|
| Empirical formula | C96 H78Cl20N12NiO12S6 | C96 H78Br2Cl18N12NiO6S6 |
| Formula weight (g mol-1) | 2551.77 | 2640.69 |
| Crystal size (mm) | 0.249 x 0.217 x 0.168 mm | 0.357 x 0.240 x 0.234 mm |
| Crystal color | Purple | Purple |
| Crystal system | Trigonal | Trigonal |
| Space group | R-3 | R-3 |
| Unit cell dimensions | ||
| a (Å) | 24.6475(9) | 24.655(4) |
| b (Å) | 24.6475(9) | 24.655(4) |
| c (Å) | 15.8565(6) | 15.943(3) |
| ( (() | 90 | 90 |
| ( (() | 90 | 90 |
| ( (() | 120 | 120 |
| V (Å3) | 8342.3(7) | 8393(3) |
| Z | 3 | 3 |
| Dcalc (g/cm3) | 1.524 | 1.567 |
| ( (mm-1) | 0.831 | 1.492 |
| F(000) | 3894 | 3858 |
| Temp (K) Completeness | 298(2) 99.7 % | 298 99.6 % |
| Rint | 0.0641 | 0.0729 |
| R (I>2σ(I)) | 0.0906 | 0.0785 |
| Rw (I>2σ(I)) | 0.2764 | 0.2213 |
| S | 1.027 | 1.025 |
In vitro cytotoxic activity determination
Cell culture
HCT-15 (colon), HeLa (cervix uterine), MCF-7 (breast) and PC-3 (prostate) human carcinoma cell lines were acquired from ATCC (American Tissue Culture Collection) and maintained in incubation at 310 K and 5% CO2 with RPMI (GIBCO®, Invitrogen corporation) supplemented with 10% BFS (GIBCO®, Invitrogen corporation), 1% L-glutamine and 1% penicillin/streptomycin. Experiments were performed with cells within at least 5 passages from each other. All cells were split when around 80-95% confluence was reached using 0.25% trypsin/EDTA.
In vitro growth inhibition assay
After placing 2 x 104 cells/well in 96-well microplate (Costar®) with 300 lL capacity and allowed to attach incubating at 310 K for 48 h, HCT-15 (colon), HeLa (cervix-uterine), MCF-7 (breast) and PC-3 (prostate) human carcinoma cells were treated with the NiII, PdII, and PtII complexes. The metal complexes (cisplatin and tioconazole control was added to the plates to act as a positive and comparative control) were tested in 5% DMSO and in a physiological solution to 0.9% NaCl to give a 1 mM stock solution. Two rows free of drug solution acted as the 100% cell survival control. Sonication was sometimes used to facilitate complete dissolution. Serial dilutions were carried out to give final screening, concentrations of the coordination compounds of 400, 200, 20, 2 and 0.2 µM (final concentration of DMSO of 0.5% (v/v)). Aliquots of 50 µL of these solutions were added to the wells (in triplicate) already containing 150 µL of media, so that the final concentrations were 0.01, 0.1, 1, 10, and 100 μg/mL (final concentration of DMSO of 0.125% (v/v)). The cells were exposed to the complex for 24 h, which then was removed and the cells washed with washing media followed by the addition of 200 µL of fresh RPMI media. Then the cells were incubated for 72 h of recovery time. The remaining biomass was then estimated by the sulforhodamine B assay [34),(35),(36] (SRB assay). The four screening concentrations were used in an initial test of activity. The selected complexes were then tested for half maximal inhibitory concentration (IC50) values determination. Each assay was done in triplicate. IC50 values were obtained from plots of % cell survival against log of the drug concentration.
Results and discussion
A series of coordination compounds of tioconazole with nickel(II), palladium(II) and platinum(II) were synthesized. The geometry depends of the metal ion, with nickel(II) the octahedral compounds 1-5 were obtained, for palladium and platinum(II) compounds 6-8 square planar arrangement is preferred. The structures of the coordination compounds shown in Fig. 1 are based on their spectroscopic characterization, elemental analyses, molar conductivity, magnetic susceptibility and by X-ray diffraction, when suitable crystals of the coordination compound were obtained.
Spectroscopic characterization
The IR spectra of the complexes present a characteristic band of the ((C=N) vibration from the tioconazole ligand, which is shifted to 1586-1589 cm-1, compared with free ligand (1562 cm-1), indicating that the metal ion is coordinated through the imidazolic nitrogen atom. The band associated to the ((C-S) vibration in the tcnz, at 733 cm-1, remains in the same region, 733-737 cm-1, as the sulphur atom does not participate as coordination site. Compound 1 present a broad band at 3324 cm-1 associated to the ((O-H) vibration of the coordinated water molecule, while for compounds 2, 3 and 7 a broad band in the region 3128-3444 cm-1 was assigned to the water molecules of crystallization. The nitrato compound 2 showed three bands associated to the NO3 group, at 1468 cm-1 (as(NO3), 1306 cm-1 (s(NO3) and 1235 ((N=O) cm-1, where the ((((as-(s) = 162 cm-1 indicating a bidentate coordination mode.[37] Compound 3 presents two intense bands at 1558 cm-1, (as(COO), and at 1408 cm-1 (s(COO), with a ((((as-(s) = 150 cm-1, characteristic of a bridging coordination mode, [37),(38),(39),(40] while for compound 8, (as(COO) was assigned at 1632 cm-1 and (s(COO) at 1354 cm-1, with a ((((as-(s) = 278 cm-1, characteristic of a monodentate carboxylate coordinated to the metal ion.
The solid state electronic spectra (UV-Vis-NIR) for the nickel(II) compounds correspond to an octahedral geometry for the metal atom. For the [Ni(tcnz)3Br2(H2O)] 1, [Ni(tcnz)2(NO3)2]∙H2O 2 and [Ni(tcnz)2(OAc)2]2∙3H2O 3 compounds, the corresponding electronic transitions were observed: (1 3T2(F)←3A2g(F) (8665, 8936 and 8970 cm-1), (2 3T1g(F)←3A2g(F) (14993, 15553 and 15417 cm-1) and (3 3T1g(P)←4A2g(F) (24918, 25477 and 25409 cm1), for 1-3 respectively. Compounds 4 and 5, [Ni(tcnz)6]X2 (X= Cl, Br), presented similar spectra, with (1 3T2(F)←3A2g(F) at 11060-11000 cm-1, (2 3T1g(F)←3A2g(F) at 17557-17550 cm-1 and (3 3T1g(P)← 4A2g(F) at 25550-25450 cm-1, with a larger 10Dq (ca. 11030 cm-1) than those of compounds 1-3 (ca. 8900 cm-1) due to the coordination of six tcnz ligands. For compounds [Pd(tcnz)2Cl2] 6, [Pt(tcnz)2Cl2]∙2H2O 7 and [Pd(tcnz)2(OAc)2] 8 a broad band centered on 25595, 27024 and 27683 cm-1 respectively, was assigned to the electronic transition 1A2g← 1A1g, for these metal ions in a square planar environment, (Table 2, Fig. SI 16). [39]
1 H and 13 C { 1 H} NMR spectroscopy
The 1H, 13C and HSQC NMR data (Fig. SI 7-15) supported the coordination mode of the tioconazole to the metal ion, where chemical shifts of the signals was observed compared to free ligand, confirming the proposed trans-square planar structures for the diamagnetic coordination compounds 6-8.
The 1H NMR spectra of the coordination compounds showed a shift for the (C2-H) imidazole proton Ha, between the two nitrogen atoms to 8.08 ppm 6, 8.24 ppm 7 and 7.67 ppm 8, the free ligand present this signal in 7.55 ppm [29], confirming the proposed coordination mode via N3 to the metal ion. The compound 8 presented one singlet in 1.94 ppm due to the acetate.
13C NMR {1H} spectra of coordination compounds exhibited sixteen signals corresponding to tioconazole, the compound 8 presented two more signals C17 in 23.66 ppm and C18 in 178.09 ppm for acetate. The C1 signal was observed in 137.81 ppm for the free ligand, the shifting in coordination compounds were 139.79, 139.84 and 138.55 ppm for 6, 7 and 8, respectively.
Magnetic susceptibility and conductivity
All the effective magnetic moments (µeff) for the nickel(II) complexes are in the range 3.20-3.50 BM, these values are within the expected range for this metal center in a 2+ oxidation state.[42] The conductivity was measured for all compounds, showing good agreement for the neutral compounds with coordinated halides, nitrates or acetates, compounds 1-3 and 6-8, while the cationic compounds 4 and 5 presented a 1:2 conductivity, Table 2.
Table 2 Electronic transitions, magnetic moments and molar conductivity of tcnz coordination compounds with NiII, PdII and PtII.
| Coordination compound | (eff (BM) | (M a | (1(cm-1) | (2(cm-1) | (3(cm-1) | |
| 1 | [Ni(tcnz)3Br2(H2O)] | 3.40 | 33.9 | 8665 | 14993 | 24918 |
| 2 | [Ni(tcnz)2(NO3)2]∙H2O | 3.50 | 13.2 | 8936 | 15553 | 25477 |
| 3 | [Ni(tcnz)2(OAc)2]∙3H2O | 3.20 | 10.4 | 8970 | 15417 | 25409. |
| 4 | [Ni(tcnz)6]Cl2 | 3.14 | 231.3* | 11060 | 17557 | 25550 |
| 5 | [Ni(tcnz)6]Br2 | 3.01 | 225.1* | 11000 | 17550 | 25450 |
| 6 | [Pd(tcnz)2Cl2] | - | 4.26 | 25595 | - | - |
| 7 | [Pt(tcnz)2Cl2]∙2H2O | - | 1.55 | 27024 | - | - |
| 8 | [Pd(tcnz)2(OAc)2] | - | 1.71 | 27683 | - | - |
a(M , molar conductance (µS cm-1) for 1x10-3 M solutions in acetone (no-electrolyte = 0-99 µS cm-1), and acetonitrile* (electrolyte 1:2 = 220-300 µS cm-1) [43] at 293 K.
X-ray diffraction analysis
[Ni(tcnz)6]Cl2 4 and [Ni(tcnz)6]Br2 5
Crystals of [Ni(tcnz)6]Cl2 4 and [Ni(tcnz)6]Br2 5 were grown from a saturated acetone solution at room temperature. The compounds are isostructural and crystallized in a trigonal system with an R-3 spatial group. The unit cell consists of three molecules. In both compounds the metal ion presents an octahedral geometry, with six tioconazole molecules in the coordination sphere (Fig. 2). The N3-Ni coordination bond length is 2.12(1) Å. The molecules are highly symmetrical with N-Ni-N angles in the range of 89.14 - 90.82° for cis-positions and 179.93° for trans-positions, and. The nickel(II) atom presents a regular octahedral geometry, despite the fact that ticonazole is a bulky ligand.

Fig. 2 ORTEP diagram of the [Ni(tcnz)6]Br2 5 compound. Displacement ellipsoids are drawn at 30% probability. H atoms were omitted for clarity.
Compounds 4 and 5 present intermolecular hydrogen bonding between the chloro atoms from the thiophen moiety and the methylene hydrogen atoms from neighboring molecules, C(9)-H(9B)∙∙∙Cl(2), 2.763(4) Å (Fig. 3).

Fig. 3 Intermolecular hydrogen bonding C(9)-H(9B)∙∙∙Cl(2) in compound 5, giving place to a 3D supramolecular arrangement.
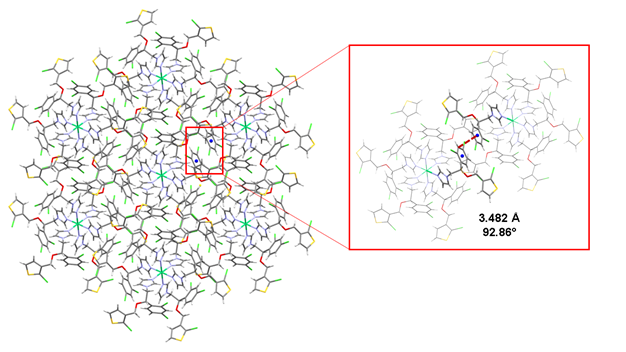
The tioconazole ligands are accommodated as a propeller, occupying the six octahedral coordination sites of the nickel(II) atom, giving place to a 3D supramolecular arrangement, stabilized through intermolecular hydrogen bonding and (∙∙∙( stacking interactions between the benzene rings of neighboring moelcules, with a distance of ca. C(17)∙∙∙( of 3.482 Å, from the ring centroid to the C(17) aromatic carbon, as shown in Fig. 4, [44].
Cancer cell growth inhibition
The in vitro cytotoxic activity of the coordination compounds in the human cancer cell lines; HCT-15 (colon adenocarcinoma), HeLa (breast adenocarcinoma), MCF-7 (breast) and PC-3 (prostate) was investigated, using cisplatin as reference. The IC50 value ((g/mL) indicates the amount of drug necessary to inhibit 50% of the growth of cancer cells, after 24 h of exposition (table 2). The free ligand was not active under these conditions.
In previous work with coordination compounds of imidazole and benzimidazole derivatives it was found that the copper(II) tetrahedral compounds, with coordinated halides, were the most active.[45),(46),(47] With the analogous ligand clotrimazole, the tetrahedral nickel(II) coordination compounds presented moderate cytotoxic activity in vitro and the octahedral complexes were not active.[47] Interestingly, for the tioconazole copper(II) octahedral compound a significant cytotoxic activity was observed.[29]
In the present work the nickel(II) octahedral compounds presented cytotoxic activity. The [Ni(tcnz)6]Cl2 4, without halogens in the coordination sphere, showed moderate activity against HCT-15, HeLa, and PC-3; while the [Ni(tcnz)2(NO3)2]∙H2O 2 compound, presented the best activity in HeLa and MCF-7 cell lines, and the [Ni(tcnz)3Br2(H2O)] 1 compound showed moderate activity in PC-3, followed by compounds 2, 4 and 5. The palladium(II) and platinum(II) compounds did not presented any significant activity, Table 2. The stability of the studied compounds was determined in DMSO solution and all of them were stable up to 24 hours (Fig. S17).
Table 3 Cell-growth inhibitory assay results. IC50 value (µg/mL) for NiII, PdII and PtII. tioconazole coordination compounds (1-3 and 6-8).
| Human carcinoma cell line | |||||
| Coordination compound | HCT-15 | HeLa | MCF-7 | PC-3 | |
| 1 | [Ni(tcnz)3Br2(H2O)] | 46.35 | 12.15 | 14.26 | 11.43 |
| 2 | [Ni(tcnz)2(NO3)2]∙H2O | 18.50 | 7.39 | 12.53 | 16.74 |
| 3 | [Ni(tcnz)2(OAc)2]∙3H2O | 323.19 | 32.70 | NA | 31.15 |
| 4 | [Ni(tcnz)6]Cl2 | 14.02 | 12.33 | 101.12 | 11.67 |
| 5 | [Ni(tcnz)6]Br2 | 303.27 | 10.12 | 15.36 | 16.15 |
| 6 | [Pd(tcnz)2Cl2] | NA | 15.67 | 51.27 | NA |
| 7 | [Pt(tcnz)2Cl2]∙2H2O | ND | ND | ND | ND |
| 8 | [Pd(tcnz)2(OAc)2] | NA | NA | NA | NA |
| Cisplatin | 8.25 | 5.55 | 1.3 | 3.83 | |
ND = not determined; NA = not active
Conclusions
Coordination compounds with tioconazole and NiII, PdII, and PtII were synthesized and fully characterized. The coordination compounds stabilized octahedral (1-5) and square planar (6-8) geometries, depending on the metal ion. Despite that tioconazole is a bulky ligand it can be accommodated in a propeller arrangement, occupying the six octahedral coordination sites of a nickel(II) atom. The crystallographic arrangements of compounds 4 and 5 were stabilized through hydrogen bonding and (∙∙∙( stacking interactions.
The octahedral nickel(II) compounds showed moderate cytotoxic activity (HeLa), the IC50 increased upon coordination to the metal ion when compared to the inactive free ligand, which can be related to the nature and the geometry of the metal ion, as the square planar platinum(II) and palladium(II) complexes did not presented any significant activity.











 nueva página del texto (beta)
nueva página del texto (beta)