Introduction
Flavonoids are largely present in our daily life, these phenolic constituents had wide range of biological and physiological roles, mostly attributed to health benefits such as antioxidant, anti-inflammatory, oestrogenic, antiviral, and chemopreventive properties[1]. Flavonoids are a group of chemical entities with a structure based on C6-C3-C6 (two phenyl rings attached through a propane bridge), they are mainly classified as chalcones, flavanones, flavones, flavonols and isoflavones[2]. On the other hand, phenolic acids are phenylpropanoids with an aromatic ring attached to a three carbon side chain; caffeic, ferulic, p-coumaric and hydroxycinnamic acids, are almost common[3]. Flavonoids and phenolic acids have protective role in carcinogenesis, inflammation, atherosclerosis, thrombosis and have relevant antioxidant capacity[3]. Different research have developed flavone scaffolds having various functional groups, and demonstrated their antiproliferative activity[4]. In other reports, flavanone glycosides, flavone O- and C-glycosides, and polymethoxyflavones appear to impact blood leukocytes and microvascular endothelial cells, and it is not surprising that two of the main areas of research on the biological actions of Citrus flavonoids have been inflammation and cancer[5].
In México, a number of Bursera species secrete a solid resin employed profusely in rituals and religious ceremonies as incense since ancestral times and are known with the generic common name of “copal”. Different parts of these plants are also used in the Mexican traditional medicine for treating inflammatory conditions, such as rheumatism[6),(7].
Bursera copallifera (DC) Bullock (Burseraceae), known as “copal ancho”[8], is a small tree that grows principally in the basin of Balsas river[9]. The leaves of this species, prepared as an infusion, have been well documented by folk medicine for treating migraines and bronchopulmonary diseases (e.g., bronchitis and cough)[10]. A previous pharmacological investigation indicated that the chloroform extract from the stems exhibited cytotoxic activity against KB (nasopharyngeal) and MCF-7 (breast) human cancer cell lines[11]. By the other hand, it has been shown that the hexane, ethyl acetate and methanol extracts from the leaves possesses bioinsecticide activity against Spodoptera frugiperda larvae[12].
In our research team, the quantitative phytochemical analysis of the copal resin of B. copallifera resulted in the isolation and characterization of six pentacyclic triterpenes: 3-epilupeol formiate, lupenone, (-amyrin, 3-epilupeol and their acetates. The pharmacological study demonstrated that the anti-inflammatory activity showed by the resin can be attributed to the potent nitric oxide (NO) production inhibitory activity on RAW 264.7 macrophages, together with the moderate inhibitory activity of PLA2 enzyme displayed by all the natural triterpenes, and the COX-2 inhibitory activity showed by (-amyrin and 3-epilupeol [13].
Recently we also described the anti-inflammatory and cytotoxic activities of stems, stem barks and leaves from Bursera copallifera[14]. The results indicated that the dichloromethane-methanol extract (DML) from the leaves showed cytotoxic activity against the cancer cell line MCF-7, this extract also displayed important in vivo anti-inflammatory activity. In a more recent study, the cytotoxic effect of the MeOH extracts obtained from two different populations of leaves of B. copallifera against two breast cancer cell lines MCF7 and MDA-MB-231 (triple negative) was demonstrated, as well as the inhibition of cell migration in the MDA-MB-231 cell line. In this study, hydroxycinnamic acid and flavonols were identified as the main components of the methanolic extract by HPLC-DAD-ESI-MSn analysis [15]. However, the metabolites responsible of these activities are still unknown. In this paper, the first bioassay-guided isolation of five active compounds presents in the dichloromethane-methanol extract obtained from the leaves of B. copallifera is described.
Experimental
General experimental procedures
NMR spectra were acquired on a Varian Unity NMR spectrometer operating at 400 MHz for 1H and 100 MHz for 13C nuclei. Chemical shifts are listed in parts per million (ppm), referenced to CDCl3 and were made on the basis of 1H-1H gCOSY, 1H-1H NOESY, gHSQC and gHMBC spectral analysis as required. NMR experiments were performed in CDCl3 and DMSO-d 6 as solvents. They were referenced to Me4Si (0 ppm). FABMS spectra in a matrix of m-nitrobenzyl alcohol or glycerol were recorded on a JEOL JMX-AX 505 HA mass spectrometer. All reagents and solvents used were analytical grade. Optical rotations were acquired with a Perkin-Elmer 241MC polarimeter (10 cm, 1 mL cell) at the sodium D line.
Plant material, extraction and isolation
The leaves of B. copallifera were collected in Sierra de Huautla (N 18°31'16.5”), Morelos, México, in August 2011. Voucher specimen No. 31809 was deposited at the Herbarium of the University of Morelos (HUMO) in the Centro de Investigación en Biodiversidad y Conservación (CIByC) at the Universidad Autónoma del Estado de Morelos. The air-dried and powdered leaves of B. copallifera (305.5 g) were subjected to exhaustive extractions (5 g of dry tissue/100 mL) using n-hexane (degreased) dichloromethane-methanol (1:1, DML), methanol (Me), and a 70% hydromethanolic solution (HMe) in a maceration process at room temperature for 72 h. Infusion of the leaves (100 g) was performed with water at 100º C for ten minutes. Crude extracts were obtained by following evaporation of the solvents under reduced pressure at 40 °C as described.
DML extract (33 g) was fractionated by percolation on a vacuum liquid chromatography column of silica gel (70-230 mesh-ASTM, Merck, Germany) eluting with n-hexane-ethyl acetate-methanol mixtures of increasing polarity to yield four fractions. BC-1, 2.6 g (80:20:0, 1.5 L), BC-2, 3.3 g (50:50:0, 300 mL) BC-3, 4.6 g (0:70:30, 300 mL), BC-4 12.2 g (0:0:100, 300 mL). The secondary fractionation of BC-3 was performed by gradient elution with hexane-CH2Cl2 followed by a gradient of CH2Cl2 -MeOH (100:0 → 0:100, v/v). This procedure afforded 5 subractions with a volume of 150 mL each one, BC-3-1, 71.6 mg (Hexane:CH2Cl2; 15:85), BC-3-2, 300 mg (Hexane:CH2Cl2→ CH2Cl2:MeOH) (10:90 → 99:01), BC-3-3, 1.12 g (CH2Cl2:MeOH, 98:02 → 96:04), BC-3-4, 779.2 mg (CH2Cl2:MeOH) (95:05 → 94:06) and BC-3-5 with 661.6 mg (CH2Cl2:MeOH (85:15 → 00:100). The triterpenes α-amyrin (3) and 3-epilupeol (4) were obtained from the secondary subfraction BC-3-1 by recrystallization from acetone and were identified by direct comparison by HPLC with authentic samples previously isolated from this plant [13]. Subtraction BC-3-2 (150 mL) afforded by precipitation 9.2 mg of stigmasterol (5), identified by direct comparison with authentic sample from our laboratory.
Purification of subfraction BC-3-4 was performed by vacuum open column chromatography, eluting with a gradient of CH2Cl2:MeOH (100:00 → 60:40) to yield 54 fractions (150 mL). Fractions 32-35 eluted with (CH2Cl2:MeOH, 95:5) afforded 13.8 mg of Luteolin-3’-O-(3’’-O-E-p-coumaroyl)-α-L-rhamnopyranoside (1). Chromatographic purification of fractions 36-38 (eluted with CH2Cl2:MeOH, 95:5) yielded 25 mg of 1 and 46.7 mg of Luteolin-3’-O-(2’’-O-E-p-coumaroyl)-α-L-rhamnopyranoside (2).
Luteolin-3(-O-(3((-O-E-p-coumaroyl)-α-L-rhamnopyranoside (1): Yellow amorphous solid (25 mg); mp 147.5-151.5 °C; [(]D 20 (29 ° (0.5, MeOH); 1H NMR (400 MHz, DMSO-d 6 ) ( 12.93 (1H, s, OH-C-5), 7.77 (1H, d, J = 2.4 Hz, -2'), 7.66 (1H, dd, J = 8.4, 2.4 Hz, H-6'), 7.63 (1H, d, J = 16.0 Hz, H-7'''), 7.56 (2H, d, J =8.8 Hz, H-2''', H-6'''), 7.03 (1H, d, J = 8.4 Hz, H-5'), 6.83 (1H, s, H-3), 6.81 (2H, d, J = 8.8 Hz, H-3''', H-5'''), 6.48 (1H, d, J = 2.0 Hz, H-8), 6.44 (1H, d, J = 16.0 Hz, H-8'''), 6.19 (1H, d, J = 2.0 Hz, H-6), 5.45 (1H, d, J = 1.6 Hz, H-1''), 5.15 (1H, dd, J = 10.0, 4.0 Hz, H-3''), 4.19 (1H, brs, H-2''), 3.95 (1H, dq, J = 9.6, 6.4 Hz, H-5''), 3.62 (1H, m, H-4''), 1.18 (3H, d, J = 6.0 Hz, H-6''); 13C NMR ( 181.7 (C-4), 166.3 (C-9'''), 164.2 (C-7), 163.3 (C-2), 161.4 (C-5), 159.7 (C-4'''), 157.2 (C-9), 152.4 (C-4'), 144.5 (C-7'''), 144.0 (C-3'), 130.1 (C-2''', C-6'''), 125.1 (C-1'''), 122.6 (C-6'), 121.5 (C-1'), 117.8 (C-2'), 116.9 (C-5'), 115.8 (C-3''', C-5'''), 114.7 (C-8'''), 103.6 (C-10), 103.3 (C-3), 99.9 (C-1''), 98.8 (C-6), 93.9 (C-8), 73.7 (C-3''), 69.7 (C-5''), 67.7 (C-2''), 69.0 (C-4''), 17.8 (C-6''); Positive-mode HRFAB-MS m/z: 579.1536 [M+H]+ calcd. for C30H27O12: 579.1503 [M+H]+). Assignments were on the basis of 2D NMR spectra (1H-1H-COSY, 1H-1H-NOESY, HSQC, and HMBC).
Luteolin-3(-O-(2((-O-E-p-coumaroyl)-α-L-rhamnopyranoside (2): Yellow amorphous powder (46.7 mg); mp 174.5-176.5 °C; [(]D 20 (17 ° (0.5 MeOH); 1H NMR (400 MHz, DMSO-d 6 ) ( 12.92 (1H, s, OH-C-5), ( 7.79 (1H, d, J = 2.0 Hz, H-2'), 7.64 (1H, dd, J = 8.0, 2.0 Hz, H-6'), 7.60 (1H, d, J = 16.0 Hz, H-7'''), 7.59 (2H, d, J = 8.8 Hz, H-2''', H-6'''), 7.01 (1H, d, J = 8.4 Hz, H-5'), 6.82 (1H, s, H-3), 6.82 (2H, d, J = 8.4 Hz, H-3''', H-5'''), 6.48 (1H, d, J = 2.0 Hz, H-8), 6.46 (1H, d, J = 16.0 Hz, H-8'''), 6.18 (1H, d, J = 2 Hz, H-6), 5.66 (1H, d, J = 1.6 Hz, H-1''), 5.35 (1H, dd, J = 3.6, 1.6 Hz, H-2''), 4.05 (1H, dd, J = 9.2, 3.6 Hz, H-3''), 3.76 (1H, dq, J = 9.4, 6.4 Hz, H-5''), 3.42 (1H, m, H-4''), 1.21 (3H. d, J = 6 Hz, H-6''); 13C NMR ( 181.7 (C-4), 166.1 (C-9'''), 164.3 (C-7), 163.4 (C-2) , 161.5 (C-5), 159.9 (C-4'''), 157.3 (C-9), 151.6 (C-4'), 145.3 (C-7'''), 143.9 (C-3'), 130.4 (C-2''', C-6'''), 125.1 (C-1'''), 122.1 (C-1'), 121.6 (C-6'), 116.8 (C-5'), 115.8 (C-3''', C-5'''), 115.6 (C-2'), 114.1 (C-8''') , 103.7 (C-10), 103.3 (C-3), 98.9 (C-6), 97.2 (C-1''), 94.0 (C-8), 72.1 (C-4''), 71.8 (C-2''), 69.6 (C-5''), 68.4 (C-3''), 17.8 (C-6''); Positive-mode HRFAB-MS m/z:579.1580. [M+H]+ calcd. for C30H27O12: 579.1503 [M+H]+). Assignments were on the basis of 2D NMR spectra (1H-1H-COSY, 1H-1H-NOESY, HSQC, and HMBC). These compounds were previously described as constituents of B. graveolens and their spectroscopic and spectrometric data match those in the literature [16].
Basic hydrolysis of compounds 1 and 2
3 mg of 1 and 10 mg of 2 were separately stirred at room temperature for 3 h with 2 equiv of LiOH in MeOH (3 mL). Ice was added and the reaction mixture was passed through a Si gel bond elut cartridge (Agilent) eluted with CH2Cl2: MeOH 90:10, to yield the hydrolysis product Luteolin-3’-O-α-L-rhamnopyranoside (6, 98%), 1.8 mg from 1, and 6.6 mg from 2.
Luteolin-3(-O-α-L-rhamnopyranoside (6): Yellow amorphous powder (6.5 mg); mp 260-263 °C; 1H NMR (400 MHz, DMSO-d 6 ) ( 12.94 (1H, s, OH-C-5), 7.73 (1H, d, J = 2.0 Hz, H-2'), 7.63 (1H, dd, J = 8.8, 7.00 (1H, d, J = 8.4 Hz, H-5'), 2.4 Hz, H-6') , 6.80 (1H, s, H-3), 6.46 (1H, d, J = 2.0 Hz, H-8), 6.20 (1H, d, J = 2.0 Hz, H-6), 5.43 (1H, d, J = 1.6 Hz, H-1''), 3.72 (1H, dd, J = 8.0, 4.0 Hz, H-3''), 3.94 (1H, brs, H-2''), 3.67 (1H, dq, J = 9.2, 6.0 Hz, H-5''), 3.29 (1H, m, H-4''), 1.13 (3H, d, J = 6.0 Hz, H-6''); 13C NMR ( 181.6 (C-4), 164.1 (C-7), 163.4 (C-2), 161.4 (C-5), 157.2 (C-9), 151.9 (C-4'), 144.3 (C-3'), 121.5 (C-6'), 122.1 (C-1'), 115.6 (C-2'), 116.7 (C-5'), 103.3 (C-3), 103.6 (C-10), 99.8 (C-1''), 98.8 (C-6), 93.8 (C-8), 70.3 (C-3''), 69.5 (C-5''), 70.0 (C-2''), 71.8 (C-4''), 17.8 (C-6''). This compound was identified by comparison of its physical and spectroscopic data with those reported [16].
Antiinflammatory activity
Animals: Groups of six male mice (CD1) weighing 25-30 g were maintained on a 12:12 h light-dark cycle with food and water available ad libitum. The animals were provided by the Facultad de Medicina at the Universidad Autónoma del Estado de Morelos. The experiments were conducted in accordance with the federal regulations for animal experimentation and care (SAGARPA, NOM-062-ZOO-1999, México). The experimental protocol followed was approved by Comité de Experimentación del Bioterio of the Universidad Autónoma del Estado de Morelos (BIO-UAEM) (Approval number: BIO-UAEM: 009:2013) by the Institutional Animal Care and Use Committee. All of the experiments were performed using groups of six animals each. All of the animals in the study were sacrificed by cervical dislocation.
Mouse model of acute inflammation. The mouse model of acute inflammation that was used in this study was a previously reported procedure [14]. Edema was induced in the right ear of each mouse by the topical application of 2.5 µg of TPA in 20 µL acetone. The effects of the fractions and compounds of B. copallifera on the ear edema were examined by the topical application of the respective extracts to the ears (20 µL/ear, 10 µL on each surface). The mice were sacrificed 4 h later by cervical dislocation. Ear punch biopsies (8 mm in diameter) were obtained and immediately weighed. The weight increase of the ear punches was directly proportional to the degree of inflammation. Indomethacin (INDO) and TPA were dissolved in acetone. The vehicle was the solvent used in the assay, and the negative control was the vehicle with TPA (2.5 µg/ear).
Anti-inflammatory activity against COX-1 and COX-2. The positive control Indomethacine (99%, Sigma-Aldrich Co. St. Louis MO 63103 USA), the fractions, and isolated compounds 1 and 2 from B. copallifera leaves were subjected to a full inhibition curve versus COX-1/COX.2 using a commercially available COX (ovine) Colorimetric Inhibitor Screening Assay Kit (Catalog No. 760111; Cayman Chemical, Ann Arbor, Michigan 48108 USA) according to the manufacturer’s instructions. Positive control and compounds were serially dissolved in DMSO to produce a series of logarithmic final concentrations (400, 40, and 4 μM), the fractions were serially dissolved in DMSO to produce a series of logarithmic final concentrations (200, 20, and 2 μg/mL), which were subsequently assayed.
Antiproliferative assay
Fractions and compounds obtained from the leaves of B. copallifera were tested against breast adenocarcinoma (MCF-7), due to DML extract previously exhibited selective activity against this cancer cell line [14] .The method employed was based on the sulforhodamine B assay, as reported by [17]. All of the stock cultures were grown in T-25 flasks (containing 5 mL of RPMI 1640 medium that was supplemented with L-glutamine, 25 mM HEPES, 0.25% sodium bicarbonate, 10% fetal bovine serum, penicillin, and streptomycin [at 5000 units/mL of medium]). Freshly trypsinized cell suspensions were dispensed in 96-well microplates at densities ranging from 30,000 to 40,000 cells per well together with the plant extracts that were dissolved in DMSO (at a final concentration of 10%) at concentrations of 20-4 µg/mL. Following a 3 days culturing period at 37°C in a humidified atmosphere with 5% CO2, the cells that had adhered to the plastic substratum were fixed with cold trichloroacetic acid (30%). The optical density was measured at 590 nm using a microplate reader (SpectraMaxPlus 384, Molecular Devices). The results were expressed as IC50 values. Podophyllotoxin was included as positive control. According to the National Cancer Institute (NCI) guidelines, the extracts were considered active when the IC50 value were < 20 μg/mL [18].
HPLC analysis
HPLC analysis was performed on a chromatographic system consisted on a Waters Alliance Separation Module (2695, Waters Corporation, Milford, MA) and a photodiode array detector (2996, Waters Corporation), employing Empower 3 software (Waters Corporation). Separation was performed using a Discovery C-18 (25 cm x 4.6 mm, 5μm) HPLC column (Supelco, Bellefonte, PA). The mobile phase consisted on a mixture of trifluoroacetic acid solution (solvent A, 0.5%) and acetonitrile (solvent B) in a gradient system. The changes in this mobile phase were in polarity descendant way as follows: A:B = 100:0 (0-1 min); 95:5 (2-3 min); 70:30 (4-7 min); 50:50 (8-22 min); 20:80 (23 min); 0:100 (24-26 min); 100:0 (27-30 min). The sample injection volume was 10 mL with a 0.9 mL/min flow rate during 30 min. The detection wavelength was 190-600 nm. Flavonoids 1 and 2 which were used as references were detected at 330 nm.
Statistical analysis
Results were expressed as mean ± SD, the data analysis was accomplished using ORIGIN© version 8.0, the IC50 values were obtained by nonlinear regression. The statistical significance was determined via Student’s t-test with P < 0.001, P < 0.01 P < 0.05, considered to be significant.
Copies of the original spectra are obtainable from the corresponding author.
Results and discussion
In a previous research[14], we found potential pharmacologic activities in DML extract from B. copallifera. This extract diminished by 55.5 %, compared with the negative control group (P<0.001), the acute inflammation induced by TPA at the dose of 0.1 mg/ear, and inhibited the COX-1 enzyme activity with IC50 value of 6.5 µg/mL, DML extract did not show considerable activity against COX-2 at the tested concentrations. In the screening evaluation of cancer cell lines, the extract was selectively cytotoxic against MCF-7 cancer cell line (IC50 =19.9 μg/mL)[14]. For that reason, a bioassay-guided fractionation, monitoring by the acute TPA-induced inflammation in mice and the cytotoxic activity against MCF-7 cell line was carried out to get the compounds responsible of these activities.
Different fractions and subfractions obtained from the DML extract of B. copallifera at the dose of 0.1 mg/ear were evaluated in TPA-induced auricular edema in mice, as well as their effect over the activity of the enzymes COX-1 and COX-2. This study showed that fraction BC-3 displayed the highest inhibition of inflammation at the tested concentrations (inhibition of edema 32.6 %, Table 1) with significant differences from the control group (P < 0.001). BC-3 also showed an appreciable inhibition of COX-1 (IC50 = 40.7 µg/mL, r = 0.9966) whereas the positive control (indomethacin) exhibited an IC50 = 4.9 μg/mL (r = 0.9915), however this fraction did not showed activity against COX-2 at the tested concentrations.
Table 1 Bioassay-guided fractionation monitored by mouse model of acute inflammation with TPA.
| Treatment | Edema inhibition % (0.1 mg/ear) |
| Fractions from DML | |
| BC-1 | 7.5** |
| BC-2 | 10.5 |
| BC-3 | 32.6*** |
| BC-4 | 2.0*** |
| Subfractions from BC-3 | |
| BC-3-1 | 37.6*** |
| BC-3-2 | 27.8*** |
| BC-3-3 | 1.2* |
| BC-3-4 | 20.5*** |
| BC-3-5 | 20.3 |
| Compound 1 | 10.5** |
| Compound 2 | NA |
| Indomethacin | 77.8*** |
***P<0.001; **P<0.01; and *P<0.05 (n=6) indicate significant differences compared with the negative control group (vehicle + TPA at 2.5 μg/ear). Significance was determined using Student’s t-test; NA: Not active (i.e., no activity was observed at the evaluated concentration).
Table 2 shows the results of the in vitro antiproliferative activity assay of fractions and isolated compounds on MCF-7 cell line. The highest activity was exhibited by BC-3 fraction with IC50 = 16.8 µg/mL± 0.1; this value is relevant in comparison to podophyllotoxin (IC50 = 0.015 ± 0.004 (M) used as positive control. BC-2 fraction also showed cytotoxic activity against this same cell line, with lower potency but with the same sensitivity (IC50= 31.4 ± 0.66 μg/mL). Fig. 2 shows percentage cell viability of MCF-7 cells through SR assay. BC-3 fraction significantly reduced cell viability above 30 (g/mL concentration. As shown in Fig. 1, there was a decrease in cell viability in a concentration-dependent manner. The highest toxicity of this fraction was observed at 20 (g/mL and cell viability dropped by up to 44.6% compared to controls.
Table 2 In vitro antiproliferative effect of bioassay-guided main fractions and compounds isolated from dichloromethane-methanol extract (DML) of B. copallifera against human cancer cell line MCF7 (Breast adenocarcinoma) using the sulforhodamine B assay.
| Treatment | IC50 /(µg/mL)b (µM) c |
| Fractions from DML | |
| BC-1 | NA |
| BC-2 | 31.4 ± 0.66 b |
| BC-3 | 16.8 ±0.1 b |
| BC-4 | NA |
| Compound 1 | NA |
| Compound 2 | 13.9 ± 0.2c |
| Paclitaxel | 0.016 ± 0.001c |
| Podophyllotoxin | 0.015± 0.004c |
NA: Not active (i.e., no activity was observed at the evaluated concentration);
IC50 inhibitory concentration 50%.
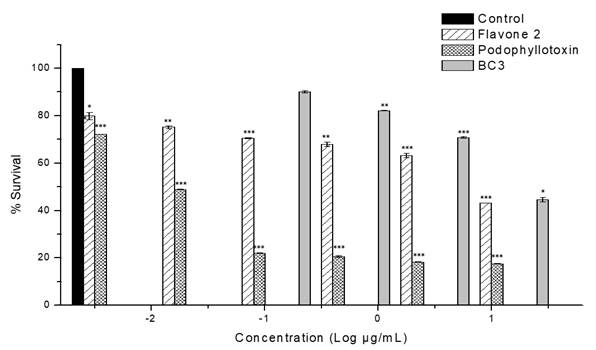
Fig. 1 Effect of fraction BC-3 and flavone 2 on MCF-7 cell proliferation. Graphs showing cell survival rates obtained by adding different concentrations. Podophyllotoxin was used as positive control. The results were normalized against a control treatment with vehicle (DMSO). ***P<0.001; **P<0.01; and *P<0.05 vs. control by unpaired t-test, n=3.
These results suggest that the medium polarity compounds in BC-3, surely are responsible for the antiproliferative activity and the anti-inflammatory effect mediated by arachidonic acid and preventing prostanoid synthesis by inhibition of COX-1. It is also known that TPA stimulates phospholipase A (PLA2) and that consequently a release of arachidonic acid and prostaglandins occurs[19]. By the other hand, considering that one of the criteria that NCI uses to identify a crude extract as promising for further purification is an IC50 value fewer than 20 μg/mL[20]. Based on that criterion, we decided to work with the BC-3 fraction, neverthless BC-2 fraction also represents a promising prospect for further subfraction studies.
Accordingly, a secondary chromatographic fractionation of BC-3 was achieved to yield five sub-fractions. Subfractions BC-3-1, BC-3-2, BC-3-4 and BC-3-5, were also able to reduce inflammation in the previously described mice model, by 37.6, 27.8, 20.5 and 20.3 % respectively at the dose of 0.1 mg/ear (Table 1). These results were significantly (P < 0.05) different from that of the negative control group. Chromatographic purification of the most active BC-3-1 subfraction allowed us to obtain the triterpenes (-amyrin (3) and 3-epilupeol (4), which were previously described as the major anti-inflammatory constituents of the resin of this plant species[13], and were identified by direct comparison (co-chromatography and 1H NMR) with authentic samples. These two triterpenes are well known for their anti-inflammatory properties. α-amyrin (3) has showed topical dose-dependent effect in reducing TPA-induced ear edema, inhibits the LPS-induced NO production in RAW 264.7 macrophages, and is able to decrease the expression of COX-2, levels of PGE2[13]. This pentacyclic triterpene also inhibited the activity of nuclear transcription factors like NF-kB reducing the synthesis of pro- inflammatory proteins[21]. 3-epilupeol, even confers some protection against cartilage degeneration by inhibiting breakdown of the collagenous matrix, and reduce the production of proinflammatory mediators including NO, PGE2 and COX-2[22]. From subfraction BC-3-2, with 27.8 % of edema inhibition, we identified, by direct comparison with an authentic sample available in our laboratory, the phytosterol stigmasterol as the main component which is already known for its anti-inflammatory effect in several models. There are many reports of the anti-inflammatory activity of this compound, and its high percentage of reduction of edema formation induced by TPA in mice, which is associated with a reduction in neutrophil infiltration into inflamed tissues has been described[23]. These results allowed us to attribute the anti-inflammatory activity of leaves of B. copallifera to the presence of α-amyrin (3), 3-epilupeol (4) and stigmasterol (5).
Chemical analysis of subraction BC-3-4 led to the isolation of two acyl glucosyl flavones which were characterized as luteolin-3(-O-(3((-O-E-p-coumaroyl)-α-L-rhamnopyranoside (1) and luteolin-3(-O-(2((-O-E-p-coumaroyl)-α-L-rhamnopyranoside (2) on the basis of their spectroscopic and spectrometric data analysis and comparison with the data previously described in the literature[24], as well as those of their common hydrolysis product (6). Fig. 2 shows the structure of these compounds.
Flavones 1 and 2 were evaluated (0.1 mg/ear) in TPA-induced auricular edema. As illustrated in Table 1, these compounds failed to induce relevant anti-inflammatory activity in this mouse model. Compound 1 reduced 10.5 % the TPA-induced edema with significant differences from the control group (P <0.01). Further, it showed inhibition of COX-1 (IC50 = 93 µM, r = 0.9966), however its isomer (compound 2), which did not show in vivo inhibition of inflammation with TPA, weakly inhibited in vitro the activity of the enzyme COX-1 with an IC50 value of 102 µM (r = 0.8863), but was not active in COX-2 inhibition at the tested concentrations.
However, the effect of COX-1 involved in homoeostatic processes is widely studied, whereas COX-2 is the isoform that plays a major part in the inflammatory process. The anti-inflammatory effect of COX-2 inhibitors on the incidence of vascular diseases requires more examination[25]; these inhibitors could potentially increase the thrombotic risk by blocking the production of PGI2, conversely the role of COX-1 is not only physiological, it has been noted that the anti-inflammatory effect of selective COX-2 inhibitors cannot be seen if the dose is not increased above levels which also inhibit COX-1, suggesting that this isoform may be induced in the inflammation site and has a significant role in the synthesis of proinflammatory prostaglandins[25]. For that reason, the COX-1 inhibitors found in this work represent an interesting alternative.
On the other hand, compound 2 exhibited representative antiproliferative activity against the MCF-7 cell line (IC50 = 13.9 µM) (Table 2); this effect is more potent than that of BC-3 fraction, and as shown in Fig. 1 the effect is also concentration-dependent. However, this activity was less than that displayed by the chemotherapy drug paclitaxel (IC50 = 0.016 ± 1.0-3 (M). It should be noted that subfractions BC-3-1, BC-3-2, BC-3-3 and BC-3-4 were also evaluated and were not active in antiproliferative assay at the tested concentrations against MCF-7 cell line.
On the other side, taken in consideration that the leaves of B. copallifera are popularly prepared as an infusion for the treatment of inflammation, we identified the presence of the active flavones in an infusion obtained from the leaves of this plant species (LI). Using the isolated flavones 1 (Rf= 0.45) and 2 (Rf =0.25) as authentic standards, TLC (Fig. 3) evidenced the presence of these flavones in DML as well as LI, and in the active BC-3 fraction. Similarly, HPLC analysis of these extracts confirm the presence of flavones 1 (Rt = 25.8 min) and 2 (Rt = 27.4 min) (Fig. 4).
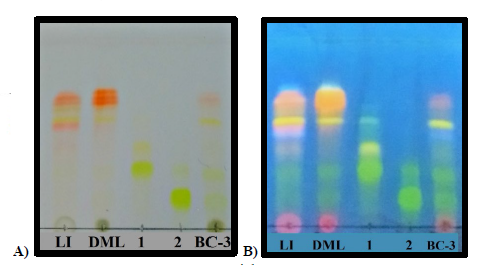
Fig 3 TLC plate. Lanes: LI= Leaf Infusion; DML= Dicloromethane: methanol extract; 1= luteolin-3(-O-(3((-O-E-p-coumaroyl)-α-L-rhamnopyranoside; 2 =luteolin-3(-O-(2((-O-E-p-coumaroyl)-α-L-rhamnopyranoside; BC-3= subfraction BC-3. A) Stained with natural product reagent (NP PEG); B) [Intense fluorescence at UV-365 nm]
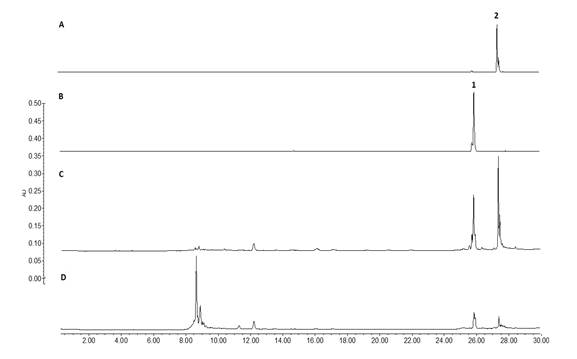
Fig. 4 HPLC chromatogram of Leaf Infusion (D) and BC-3 active fraction (C) showing the flavones 1 (B) and 2 (A) at Rt = 25.8 and 27.4 min respectively, (for HPLC analytical conditions, see text).
A survey in the literature showed that the most numerous glycosylated flavonoids present in plants are flavones/flavonol O-glycosides[26], and the most common flavonoids are those glycosylated at C-3 and C-7, although there are some examples of other sites of glycosylation[27]. In particular, glycosylation at C-3( is not common, and only three acyl 3’-O-luteolin glycosides have been described. For instance, the acyl glycosyl flavones 1 and 2, described in this work, were previously found as constituents of the leaves of B. graveolens, but their biological activity was not determined[16]. On the other hand, luteolin 3'-O-(6''-E-caffeoyl)-β-D-glucopyranoside, isolated from Callicarpa nudiflora, was reported to have anti-platelet aggregation activity[25]. This is the first report on the presence of the compounds 1-5 in the leaves of B. copallifera and the first time that the anti-inflammatory and antiproliferative activities of the acyl glycosyl flavones 1 and 2 respectively is described
Conclusions
In the present study, we report the bioassay-guided phytochemical study of the antiproliferative and anti-inflammatory dichloromethane-methanol extract (DML) of B. copallifera leaves. This study allowed the isolation and characterization of luteolin-3(-O-(3((-O-E-p-coumaroyl)-α-L-rhamnopyranoside (1), luteolin-3(-O-(2((-O-E-p-coumaroyl)-α-L-rhamnopyranoside (2), (-amyrin (3), 3-epilupeol (4) and stigmasterol (5). The antiproliferative activity was related to the presence of the acyl glycosyl flavone 2. In addition, flavone 1, together with the triterpenes 3 and 4 were the responsible for inhibiting TPA-induced inflammation by inhibiting COX-1 enzyme. This is the first report on the bioactivity of the flavones 1 and 2.
This study provides scientific evidence for the traditional claim of the plant for the treatment of inflammation and its potencial activity against cancer.











 nueva página del texto (beta)
nueva página del texto (beta)



